Advances in Clinical Medicine
Vol.
11
No.
06
(
2021
), Article ID:
43017
,
6
pages
10.12677/ACM.2021.116371
白细胞介素6可能通过Jak2诱导平滑肌细胞成骨样分化及钙化
郭欣欣,曲一丹,赵旋
青岛大学附属医院,山东 青岛
收稿日期:2021年5月7日;录用日期:2021年5月25日;发布日期:2021年6月9日

摘要
目的:探讨IL6诱导平滑肌细胞钙化及成骨样分化的机制。方法:体外培养平滑肌细胞,分别给予IL6、IL6 + CP690550干预细胞,设空白对照。免疫印迹法(Western blot)检测钙化相关分子TNAP、转录因子Runx2、Jak2的表达,邻甲酚酞法(O-cresolphthalein)检测不同时间的钙盐含量。结果:IL6组较空白组,钙盐含量明显高于对照组,同时TNAP、Runx2、Jak2的表达明显上调。给予Jak2抑制剂后,较空白组TNAP、Runx2表达明显下调。结论:IL6可能通过Jak2诱导平滑肌细胞成骨样分化及钙化。
关键词
白细胞介素6,平滑肌细胞,钙化,成骨样分化,Jak2
Interleukin 6 May Induce Osteogenic Differentiation and Calcification of Vascular Smooth Muscle Cells through Jak2
Xinxin Guo, Yidan Qu, Xuan Zhao
The Affiliated Hospital of Qingdao University, Qingdao Shandong
Received: May 7th, 2021; accepted: May 25th, 2021; published: Jun. 9th, 2021

ABSTRACT
Objective: To investigate the mechanism of IL6 inducing calcification and osteogenic differentiation of vascular smooth muscle cells. Methods: Vascular smooth muscle cells were cultured in vitro, IL6 and IL6 + CP690550 were given to the intervention cells respectively, and a blank control was set. Western blotting was used to detect the expression of calcification-related molecules TNAP, transcription factor Runx2 and Jak2, and the o-cresolphthalein method was used to detect the calcium salt content at different times. Results: Compared with the blank group, the IL6 group had significantly higher calcium content than the control group, and the expressions of TNAP, Runx2 and Jak2 were significantly up-regulated. After adding Jak2 inhibitor, the expressions of TNAP and Runx2 were significantly down-regulated compared with the blank group. Conclusion: IL6 may induce osteogenic differentiation and calcification of vascular smooth muscle cells through Jak2.
Keywords:Interleukin 6, Vascular Smooth Muscle Cells, Calcification, Osteogenic Differentiation, Jak2

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
血管钙化(Vascular Calcification, VC)常见于高血压、慢性肾脏病、糖尿病、类风湿性关节炎等疾病,是血管病变的风险因素和预后不良的指标 [1] [2]。血管平滑肌细胞(Vascular Smooth Muscle Cell, VSMC)在炎症或氧化应激等因素的刺激下会出现成骨样分化的特征,如骨相关因子runt相关转录因子(Runt-related Transcription Factor 2, Runx2)以及组织非特异性碱性磷酸酶(Tissue Nonspecific Alkaline Phosphatase, TNAP)的上升从而诱导血管钙化 [3]。慢性肾脏病(Chronic Kidney Disease, CKD)的患者和接受慢性透析的患者中,高磷酸盐血症是血管钙化的最重要病因之一,而高水平的氧化应激,血清C反应蛋白(CRP),炎性细胞因子等被称为心血管疾病的危险因素 [4] [5] [6]。
研究表明,血清白细胞介素6 (Interleukin 6, IL6)的血清水平随肾功能障碍的恶化而增加,血清IL6较高的患者心血管死亡率较高 [7]。Caselli等证实血清IL6升高可能是冠状动脉疾病导致死亡的危险因素,而IL-6是开始透析治疗的患者死亡率的独立预测因子 [8]。Hénaut L等发现IL6是VSMC成骨转化和矿化的著名诱导剂 [9],在慢性肾脏病患者中,IL6较其他炎症标记物(如C反应蛋白,白蛋白或TNF-α)能更好地预测死亡风险。同时,体外研究已经证明IL6增强钙化相关基因的表达和钙化血管平滑肌细胞 [10] [11]。但是,IL-6对VSMCs转录调控的机制仍不清楚。
本文通过体外培养平滑肌细胞,重组人IL6体外诱导细胞钙化,探讨IL6诱导平滑肌细胞钙化及成骨样分化的机制。
2. 实验材料与方法
2.1. 材料
DMEM、胎牛血清、胰胰蛋白酶(美国Gibco公司),IL6 (美国RD Systems),成骨诱导剂(50 μM抗环血酸、10 μM β-甘油磷酸钠、0.1 μM地塞米松,广州赛业生物科技有限公司),一抗β-actin (美国Cell Signal公司)、Runx2 (美国Abcam公司),Jak2 (美国Cell Signal公司)组织非特异性碱性磷酸酶(TNAP,美国Santa Cruz Biotechnology公司),二抗羊抗兔IgG、羊抗鼠IgG (美国Abbkine公司),FITC羊抗兔(Elabscience公司),钙含量检测试剂盒(碧云天生物技术有限公司),Triton X-100、DAPI溶液、山羊封闭血清、青–链霉素。
2.2. 平滑肌细胞的培养及干预条件
取青岛大学附属医院健康新生胎儿脐带,无菌分离脐动脉,用眼科剪将血管中膜剪成约0.5~1 mm3的小块,用弯头吸管,将血管小块移入25 cm2塑料培养瓶内,以5块/cm2密度均匀种植于培养瓶底部,加入4 ml含双抗DMEM (20% FBS),将培养瓶于37℃、5% CO2培养箱(湿度100%)内静置培养,待组织块贴附后,将培养瓶缓慢翻转平放培养。3 d后换新鲜培养液,以后每3 d换液1次,每次只换2/3量,保留1/3的原液,待细胞融合到80%,加入0.25%胰蛋白酶消化1:3传代。取第3~5代细胞进行实验
2.3. 蛋白质印迹法检测钙化及成骨分化相关蛋白以及Jak2表达水平
采用常规培养、100 ng/ml IL6、IL6 + CP690550干预细胞,裂解实验干预后的细胞,每个样本每孔加入20 μg蛋白液,经十二烷基碘酸钠–聚丙烯酰胺(SDS-PAGE)胶电泳后转膜,以5% BSA溶液室温封闭1 h,β-actin、Runx2、Jak2 (1:1000)、TNAP (1:500)一抗工作液4℃孵育过夜,TBST洗涤10 min × 3次,分别加入羊抗兔或鼠IgG (H + L)抗体(1:10,000)室温下摇床孵育60 min,TBST洗涤10 min × 3次,ECL显色、曝光、显影、定影。凝胶成像系统分析并拍照,以蛋白条带的强度代表蛋白的表达量。
2.4. 钙盐含量的测定
采用常规培养、100 ng/ml IL6干预细胞24 h、48 h、72 h,裂解实验干预后的细胞,取其上清用钙含量检测试剂盒检测钙含量,并用BCA法检测其蛋白含量。用蛋白浓度校正钙浓度。
2.5. 统计学处理
数据采用SPSS 23.0统计软件进行分析,计量资料以 表示,组间比较采用单因素方差分析,两两比较采用LSD-t检验,以P < 0.05为差异有统计学意义。
3. 实验结果
3.1. IL6诱导平滑肌细胞钙化
将细胞以5 × 104/L接种于6孔细胞培养板中,常规给予DMEM + 成骨诱导培养。用100 ng/ml IL6干预细胞24 h、48 h、72 h,检测钙盐含量,结果显示,较空白对照组IL6组钙盐含量明显升高,差异具有统计学意义(*P < 0.05),见图1。

Figure 1. IL6 induces smooth muscle cell calcification
图1. IL6诱导平滑肌细胞钙化
3.2. IL6通过上调Runx2诱导平滑肌细胞成骨样分化及钙化
将细胞以5 × 104/L接种于6孔细胞培养板中,常规给予DMEM + 成骨诱导培养。用100 ng/ml IL6干预细胞24 h,检测钙化相关蛋白NATP、Runx2的表达,结果显示,较空白对照组IL6组NATP、Runx2蛋白的表达明显升高,见图2。

Figure 2. IL6 induces osteogenic differentiation of smooth muscle cells by up-regulating Runx2
图2. IL6通过上调Runx2诱导平滑肌细胞成骨样分化
3.3. IL6可以上调平滑肌细胞Jak2的表达
将细胞以5 × 104/L接种于6孔细胞培养板中,常规给予DMEM + 成骨诱导培养。用100 ng/ml IL6干预细胞24 h,检测Jak2的表达,结果显示,较空白对照组IL6组Jak2的表达明显升高,见图3。

Figure 3. IL6 can up-regulate the expression of smooth muscle cells Jak2
图3. IL6可以上调平滑肌细胞Jak2的表达
3.4. 抑制Jak2的表达可以下调平滑肌细胞成骨样分化
将细胞以5 × 104/L接种于6孔细胞培养板中,常规给予DMEM + 成骨诱导培养。用100 ng/ml IL6、IL6 + CP690550干预细胞24 h,检测钙化相关蛋白NATP、Runx2的表达,结果显示,较空白对照组IL6组NATP、Runx2蛋白的表达明显降低,见图4。
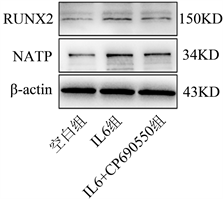
Figure 4. Inhibition of Jak2 expression can down-regulate osteogenic differentiation of smooth muscle cells
图4. 抑制Jak2的表达可以下调平滑肌细胞成骨样分化
4. 讨论
研究结果显示,IL6刺激细胞可以诱导平滑肌细胞钙化,较空白组IL6组钙盐含量明显升高,同时TNAP的表达明显升高,TNAP是成骨细胞表型和成骨细胞分化的典型蛋白产物,其活性增加代表成骨细胞生成的早期典型标志,是血管钙化的关键酶。研究显示TNAP可能作为血管钙化治疗的潜在靶标 [12]。在此基础上我们发现IL6上调重要信号通路分子Runx2的蛋白表达。加入CP690550抑制Jak2的表达,可以抑制平滑肌细胞TNAP的表达,同时抑制Runx2的表达。
IL6是VSMC成骨样分化及钙化的重要诱导剂。在体外实验中发现,IL-6通过诱导热休克蛋白70(HSP70)表达减少MGP对骨形态发生蛋白2 (Bone Morphogenic Protein 2, BMP2)活性的抑制作用,从而促进了VSMC钙化 [13]。有趣的是,IL-6可以直接诱导在非钙化条件下培养的VSMC表达BMP2,TNAP和骨桥蛋白(Osteopontin, OPN),从而引起血管钙化 [11]。IL-6是核因子κ-B配体(RANKL)受体激活剂的强诱导剂,抑制IL-6的表达从而减少RANKL诱导的TNAP、Runx2的表达,并且在体外抑制了Pi诱导的VSMCs钙化的RANKL依赖性扩增 [14]。
IL6介导的信号通路主要有三种:1) Jak2/Stat3途径。IL6/IL6R/mgp130复合物使Jak2磷酸化,然后使Stat3发生磷酸化,从而将信号传递到细胞核,在肝细胞内启动CRP急性相关蛋白的表达,同时还参与MSCs和淋巴细胞的增殖。2) Ras-MAPK途径,gp130的同源二聚化反应,使含有SH2结构域的蛋白酪氨酸发生磷酸化,从而激活Ras/Raf/MAPK信号通路,参与细胞的生长调节。3) PI3K/AKT途径,该途径主要发挥抗细胞凋亡的作用 [15]。
Jak/Stat途径主要是干扰素(Interferon, IFN)-γ和IL-6家族成员的受体激活途径 ,在免疫调节、免疫细胞增殖方面发挥关键的作用。IL6通过与IL6受体结合,诱导gp130的同源二聚化反应,从而激活Jak2并使其磷酸化,磷酸化的Jak2活化Stat3并使其磷酸化,p-Stat3与SH2结合形成二聚体,并将其转运至细胞核内与DNA的特定反应原件结合从而激活靶基因的转录 [16] [17]。IL6/Jak2/Stat3信号通路参与许多重要的生物学过程,其中包括细胞增殖、分化、凋亡、免疫调节和造血作用等过程 [18]。最新研究发现,Jak/2Stat3信号通路在骨骼的发育、代谢和愈合过程中发挥重要作用,炎症细胞因子可以通过激活Jak2/Stat3信号通路对关节内外的破骨细胞和成骨细胞发挥作用干扰正常的骨骼重塑 [19] [20]。CP690550 (Tofacitinib,托法替尼)是美国FDA在2012年批准的首个Jak抑制剂,用于治疗对甲氨蝶呤反应不佳的患者的中度至重度活动性RA ,研究显示托法替尼抑制JAK3,JAK2,JAK1的IC 50值分别为1 nM,20 nM和112 nM,而Jak3主要在造血细胞、平滑肌细胞表达,并不参与骨代谢 [21] [22]。所以我们用CP690550抑制Jak2的表达,可以抑制平滑肌细胞TNAP的表达,同时抑制Runx2的表达。
5. 结论
综上所述,IL6可能是通过Jak2信号转导通路诱导人平滑肌细胞成骨样分化及钙化。
文章引用
郭欣欣,曲一丹,赵 旋. 白细胞介素6可能通过Jak2诱导平滑肌细胞成骨样分化及钙化
Interleukin 6 May Induce Osteogenic Differentiation and Calcification of Vascular Smooth Muscle Cells through Jak2[J]. 临床医学进展, 2021, 11(06): 2576-2581. https://doi.org/10.12677/ACM.2021.116371
参考文献
- 1. Reaven, P.D. and Sacks, J. (2005) Coronary Artery and Abdominal Aortic Calcification Are Associated with Cardiovascular Disease in Type 2 Diabetes. Diabetologia, 48, 379-385. https://doi.org/10.1007/s00125-004-1640-z
- 2. Okuno, S., Ishimura, E., Kitatani, K., Fujino, Y., et al. (2007) Presence of Abdominal Aortic Calcification Is Significantly Associated with All-Cause and Cardiovascular Mortality in Maintenance Hemodialysis Patients. American Journal of Kidney Diseases, 49, 417-425. https://doi.org/10.1053/j.ajkd.2006.12.017
- 3. Shao, S., Cai, J. and Towler, D.A. (2006) Molecular Mechanisms of Vascular Calcification: Lessons Learned from the Aorta. Arteriosclerosis, Thrombosis, and Vascular Biology, 26, 1423-1430. https://doi.org/10.1161/01.ATV.0000220441.42041.20
- 4. Shioi, A., Taniwaki, H., Jono, S., Okuno, Y., et al. (2001) Mönckeberg’s Medial Sclerosis and Inorganic Phosphate in Uremia. American Journal of Kidney Diseases, 38, S47-S49. https://doi.org/10.1053/ajkd.2001.27396
- 5. Massy, Z.A., Stenvinkel, P. and Drueke, T.B. (2009) The Role of Oxidative Stress in Chronic Kidney Disease. Seminars in Dialysis, 22, 405-408. https://doi.org/10.1111/j.1525-139X.2009.00590.x
- 6. Buturović-Ponikvar, J. (2003) Renal Replacement Therapy in Slovenia: Annual Report 2001. Nephrology Dialysis Transplantation, 5, v53-v55. https://doi.org/10.1093/ndt/gfg1048
- 7. Barreto, D.V., Barreto, F.C., Liabeuf, S., et al. (2010) European Uremic Toxin Work Group (EUTox). Plasma Interleukin-6 Is Independently Associated with Mortality in Both Hemodialysis and Pre-Dialysis Patients with Chronic Kidney Disease. Kidney International, 77, 550-556. https://doi.org/10.1038/ki.2009.503
- 8. Caselli, C., De Graaf, M.A., Lorenzoni, V.D., et al. (2015) HDL Cholesterol, Leptin and Interleukin-6 Predict High Risk Coronary Anatomy Assessed by CT Angiography in Patients with Stable Chest Pain. Atherosclerosis, 241, 55-61. https://doi.org/10.1016/j.atherosclerosis.2015.04.811
- 9. Hénaut, L. and Massy, Z.A. (2018) New Insights into the Key Role of Interleukin 6 in Vascular Calcification of Chronic Kidney Disease. Nephrology Dialysis Transplantation, 33, 543-548. https://doi.org/10.1093/ndt/gfx379
- 10. Zickler, D., Luecht, C., Willy, K., et al. (2018) Tumour Necrosis Factor-Alphain Uraemic Serum Promotes Osteoblastic Transition and Calcification of Vascular Smooth Muscle Cells via Extracellular Signal-Regulated Kinases and Activator Protein1/c-FOS-Mediated Induction of Interleukin 6 expression. Nephrology Dialysis Transplantation, 33, 574-585. https://doi.org/10.1093/ndt/gfx316
- 11. Sun, M., Chang, Q., Xin, M., et al. (2017) Endogenous Bone Morphogenetic Protein 2 Plays a Role in Vascular Smooth Muscle Cell Calcification Induced by Interleukin6 in Vitro. International Journal of Immunopathology and Pharmacology, 30, 227-237. https://doi.org/10.1177/0394632016689571
- 12. Azpiazu, D., Gonzalo, S. and Villa-Bellosta, R. (2019) Tissue Non-Specific Alkaline Phosphatase and Vascular Calcification: A Potential Therapeutic Target. Current Cardiology Reviews, 15, 91-95. https://doi.org/10.2174/1573403X14666181031141226
- 13. Yao, Y., Watson, A.D., Ji, S., et al. (2009) Heat Shock Protein 70 Enhances Vascular Bone Morphogenetic Protein-4 Signaling by Binding Matrix Gla Protein. Circulation Research, 105, 575-584. https://doi.org/10.1161/CIRCRESAHA.109.202333
- 14. Wang, Z., Castresana, M.R. and Newman, W.H. (2001) NF-kappaB Is Required for TNF-Alpha-Directed Smooth Muscle Cell Migration. FEBS Letters, 508, 360-364. https://doi.org/10.1016/S0014-5793(01)03109-X
- 15. Moshapa, F.T., Riches-Suman, K. and Palmer, T.M. (2019) Therapeutic Targeting of the Proinflammatory IL-6-JAK/STAT Signalling Pathways Responsible for Vascular Restenosis in Type 2 Diabetes Mellitus. Cardiology Research and Practice, 2019, Article ID: 9846312. https://doi.org/10.1155/2019/9846312
- 16. Heinrich, P.C., Behrmann, I., Haan, S., et al. (2003) Principles of Interleukin (IL)-6-Type Cytokine Signalling and Its Regulation. Biochemical Journal, 374, 1-20. https://doi.org/10.1042/bj20030407
- 17. Hirano, T., Ishihara, K. and Hibi, M. (2000) Roles of STAT3 in Mediating the Cell Growth, Differentiation and Survival Signals Relayed through the IL-6 Family of Cytokine Receptors. Oncogene, 19, 2548-2556. https://doi.org/10.1038/sj.onc.1203551
- 18. Bolli, R., Dawn, B. and Xuan, Y.T. (2003) Role of the JAK-STAT Pathway in Protection against Myocardial Ischemia/Reperfusion Injury. Trends in Cardiovascular Medicine, 13, 72-79. https://doi.org/10.1016/S1050-1738(02)00230-X
- 19. Bellido, T., Borba, V.Z., Roberson, P., et al. (1997) Activation of the Janus Kinase/STAT (Signal Transducer and Activator of Transcription) Signal Transduction Pathway by Interleukin-6-Type Cytokines Promotes Osteoblast Differentiation. Endocrinology, 138, 3666-3676. https://doi.org/10.1210/endo.138.9.5364
- 20. Gaber, T., Brinkman, A.C., Pienczikowski, J., et al. (2020) Impact of Janus Kinase Inhibition with Tofacitinib on Fundamental Processes of Bone Healing. International Journal of Molecular Sciences, 21, 865. https://doi.org/10.3390/ijms21030865
- 21. Kawalec, P., Mikrut, A., Wiśniewska, N., et al. (2013) The Effectiveness of Tofacitinib: A Novel Janus Kinase Inhibitor, in the Treatment of Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Clinical Rheumatology, 32, 1415-1424. https://doi.org/10.1007/s10067-013-2329-9
- 22. Verbsky, J.W., Bach, E.A., Fang, Y.F., et al. (1996) Expression of Janus Kinase 3 in Human Endothelial and Other Non-Lymphoid and Non-Myeloid Cells. Journal of Biological Chemistry, 271, 13976-13980. https://doi.org/10.1074/jbc.271.24.13976