Advances in Analytical Chemistry
Vol.
11
No.
03
(
2021
), Article ID:
44866
,
17
pages
10.12677/AAC.2021.113022
新型电极材料的研究进展
汪永霞1,王敏敏1,2*
1南通大学化学化工学院,江苏 南通
2南通智能与新能源材料重点实验室,江苏 南通

收稿日期:2021年7月28日;录用日期:2021年8月12日;发布日期:2021年8月30日

摘要
电解水是大规模制氢的重要途径,为了降低阳极阴极过电位以节约能耗,研究开发低过电位、高催化活性的电极材料具有重要的意义。影响电极材料催化活性的主要因素有结构因素和能量因素。本文主要简述了电解水的基本原理,以及电极材料的研究现状,期待未来能够开发出具有低过电位、高催化活性和高稳定性的新型电解水电极材料。
关键词
电解水,析氧电极材料,析氢电极材料,结构,催化活性

Recent Advanced Study of Novel Electrode Materials
Yongxia Wang1, Minmin Wang1,2*
1School of Chemistry and Chemical Engineering, Nantong University, Nantong Jiangsu
2Nantong Key Laboratory of Intelligent and New Energy Materials, Nantong Jiangsu

Received: Jul. 28th, 2021; accepted: Aug. 12th, 2021; published: Aug. 30th, 2021

ABSTRACT
Electrocatalysis water splitting is an important way for large-scale hydrogen production. In order to reduce the anode and cathode overpotential to save energy consumption, it is of great significance to research and develop electrode materials with low overpotential and high catalytic activity. The main factors affecting the catalytic activity of electrode materials are structural factors and energy factors. In this paper, the basic principle of electrolysis of water and the research status of electrode materials are summarized, and new electrolysis hydroelectric electrode materials with low over-potential, high catalytic activity and high stability are expected to be developed in the future.
Keywords:Electrolytic Water, Oxygen Evolution Electrode Materials, Hydrogen Evolution Electrode Materials, Structure, Catalytic Activity

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
能源、材料和信息是近代社会得以繁荣和发展的三大支柱。其中,能源更是人类社会赖以生存和发展的基础,在国民经济中具有特别重要的战略地位 [1]。比较于现有的化石能源,氢能有以下主要优点:1) 氢的资源丰富,是二次可再生能源。2) 氢气的能量密度高,导热性能好。3) 氢气对环境友好,清洁无污染。4) 氢气具有可储、可运性。除具有以上优点外,氢还是和平能源。氢气来源广泛又可再生,有丰富的资源,不会像化石燃料那样因资源分布的不合理而引起能源的争夺或战争。
虽然氢元素几乎存在于世界各地,但极少有自然氢存在。因此,氢气必须通过含氢物质的分解才能得到,但是如何实现高效大规模廉价制氢,依然是制约氢能开发和利用的关键问题。现有的制氢技术主要有:矿物燃料制氢、化学氢化物制氢、核能制氢、生物质制氢、太阳能光解水制氢和电解水制氢等。
水电解法制氢即利用外加电能对水进行电解来产生氢气。与其他的制氢技术相比,电解水制氢技术是技术比较成熟的制氢方法,生产技术安全可靠,制备工艺简单,产品分离操作简单、纯度高;自动化程度高,工艺设备维护方便;污染小,可以实现自动化控制 [2]。电解水制氢的研究对目前的能源危机和环境污染意义重大,虽然耗电量很大,但电能为可再生能源,很多清洁能源如太阳能、风能、生物能源等都可以被用来发电,因此电解水制氢是最有应用前景的一种方法,也是目前广泛采用的一种较廉价的理想制氢方法,也实现了大规模的生成技术。此外,氯碱工业、氢能汽车产业以及许多精细化工、冶金、浮法玻璃等行业都会用到电解水技术。电解水过程中必然伴随着阴极析氢和阳极析氧的同时发生,两者是相互联系、密不可分的,研究降低过电位的阴极材料和阳极材料对于电解水制氢都是至关重要的。
2. 电解水制氢基本原理
电解水是指利用电能将液态水分解为氢气和氧气的反应。作为一项氢能经济的基石性技术,在能量的转化和储存方面起着不可或缺的作用,吸引了众多科研工作者的关注。整个电解水反应是在电场存在下的气/液/固三相界面上进行的,分为阳极析氧反应和阴极析氢反应 [3],(如图1为碱性电解水电解槽 [3] )反应方程式如下所示:
在酸性溶液中:
阴极反应:4H++4e→2H2
阳极反应:2H2O→4H++4e+O2
在碱性溶液中:
阴极反应:4H2O + 4e→2H2 + 4OH−
阳极反应:4OH−→2H2O + 4e + O2
总反应为:2H2O→2H2 + O2
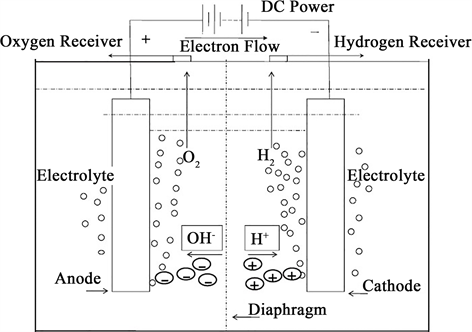
Figure 1. Schematic diagram of electrolytic water principle
图1. 电解水原理示意图
从理论上讲,水电解产生氢气所需的理论电压仅为1.23 V,但实际上,制备所消耗电量远远大于理论值,这主要是由于电解液、电解装置的电阻及反应中过电位的存在导致反应的外加电压增大,甚至高达1.8 V以上。在不改变电解槽装置的情况下,电极材料是影响电解水效率和能耗的重要因素,电解水的能耗有相当一部分为析氢过电位和析氧过电位。为了降低能耗高效电解水制氢,研究开发新型电极材料(阳极析氧材料和阴极析氢材料)降低过电位 [4],提高能量转化效率具有非常重要的实际意义。
为了提高电解水的电化学效率,降低过电位,可以从三方面考虑:1) 提高电解槽的电解温度;根据能斯特方程,温度增加,会降低过电位,但过高的温度使设备更容易损坏而不可避免的增加了仪器成本,经济效益降低。2) 通过结构优化、化学功能化修饰和化学组分的掺杂增强电极材料的催化性能,构建新型低温、高效稳定的电解水电极材料。3) 向电解液中加入少量的添加剂(主要是表面活性剂)构建新型电解液;研究人员 [5] 认为添加剂的加入降低了溶剂水分子的重组活化能,进而降低过电位。目前,开发研制新型电极材料提高现有电极材料的电催化性能来降低过电位,不仅对设备要求低,且效率高,经济成本低,是解决问题的最佳途径,也是目前研究热点 [6]。
3. 电极材料的研究现状
3.1. 析氧电极材料的研究现状
为了降低能耗高效电解水制氢,在电解水反应过程中,阳极析氧反应因为涉及复杂的四电子转移过程,要比阴极析氢反应困难许多,其反应过电位也高很多,为了提高电解水的电化学效率,寻找性能优越的阳极析氧催化剂尽可能地减小阳极析氧反应的过电位,是电解水研究的难点 [7]。目前析氧材料主要分为以下几种:
3.1.1. 贵金属合金及其氧化物材料
贵金属及其氧化物一直是研究的热点,如贵金属Pt、Ru、Ir、Pd等 [8] 及相应的RuO2、IrO2、RhO2等氧化物 [9] [10] 具有较高析氢催化活性。
Reier Tobias小组 [11] 系统的比较了酸性条件下碳支撑的Ru、Ir、Pt体相材料和纳米材料的催化活性,如图2。作者发现相比于体相材料,纳米材料的循环伏安电化学特征并未明显改变,但是其析氧反应有了明显不同体相材料的催化活性明显优于纳米粒子,且Ru > Ir > Pt。尽管体相Ru显示了最高的催化活性,但其稳定性不如Ir纳米粒子,综合考虑更适合析氧反应工业化应用的是Ir纳米粒子,即显示高的析氧催化活性又显示了高的稳定性。
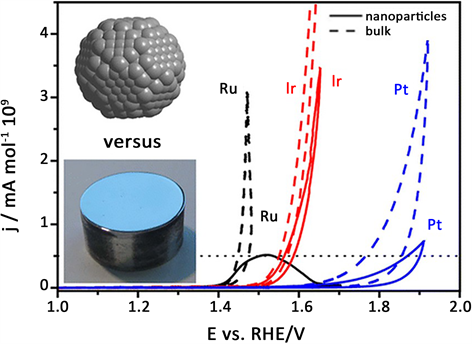
Figure 2. Catalytic activity diagram of Ru, Ir, Pt bulk materials and nano materials for oxygen evolution (scan rate 6 mV/s, 0.1 M HClO4)
图2. Ru、Ir、Pt体相材料和纳米材料析氧催化活性图(扫速6 mV/s,0.1 M HClO4)
在酸性介质中,贵金属单质及其氧化物具有导电性高、热力学稳定、催化活性好的特点 [12]。但是在强碱性条件下,无论贵金属单质还是其氧化物,阳极极化现象严重,稳定性差,进而导致析氧活性降低 [13]。因此,许多研究者在贵金属、贵金属氧化物中掺杂其他金属或氧化物来增加电极的稳定性和活性,如添加一定量的惰性氧化物 [14]。
为了促进贵金属的应用,科研人员 [15] 致力于开发酸性和碱性条件下均具有高催化活性和稳定性的阳极材料。最近,根据密度泛函理论,人们预测金红石相的RuO2可能会具有比IrO2更高的催化活性。Yang Shao-Horn小组 [16] 构建了大约粒径6 nm的金红石相RuO2和IrO2并在酸性和碱性条件下测试了其析氧催化活性(如图3)。作者发现,无论酸碱条件下,金红石相的RuO2显示了略高于IrO2的催化活性,且二者均显示要优于商业的Ru/C和Ir/C的高催化活性和稳定性。
贵金属及其氧化物电极价格较高、资源有限,限制了其大规模应用。为此科研人员将贵金属和其他材料复合,构建了具有高催化活性和稳定性的阳极析氧材料 [17]。
Alexis T. Bell小组 [18] 将钴氧化物沉积于贵金属(Au,Pd,Pt等)基底上,并在碱性条件下测试了其析氧催化反应。作者发现,当钴氧化物为0.4层时,其催化活性是体相钴氧化物的40倍,并且发现基底不同其催化活性也有不同(Au > Pt > Pd),推测其原因是电化学氧化过程中形成了高催化活性物种的Co4+。
3.1.2. 过渡金属合金及其简单氧化物、过渡金属复合物材料
除贵金属以外,过渡金属Ni、Co、Mn、Zr、Nb等析氧催化活性较高。其中镍耐碱性较强、成本也相对较低应用最广。为了提高过渡金属电极的析氧催化性能,研究致力于提高其表面积的方法,例如Raney Ni,
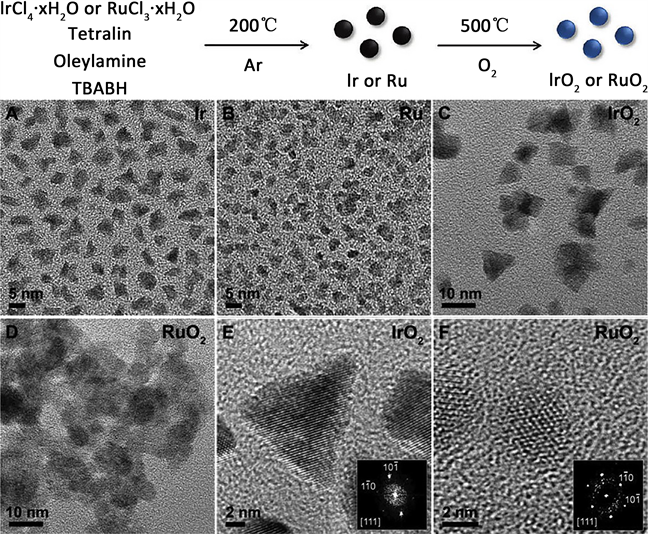
Figure 3. Synthesis schematics of RuO2 and IrO2 in rutile phase and corresponding transmission electron microscopy (TEM) images
图3. 金红石相的RuO2和IrO2的合成示意图及其对应的透射电子显微镜(TEM)照片
由于其隧道状孔结构和细裂纹使它具有很高的表面积和很高的稳定性,且电催化活性方面有很大的提高 [19]。相比过渡金属电极,过渡合金电极具有更低的析氧过电位,比如Ni-Fe、Ni-Cu、Ni-Mo、Ni-Ir、Co-Fe等。Fazle Kibria等 [20] 研究了Ni-30 at%Cu电极在30wt% KOH中的析氧行为,发现该电极的交换电流密度比Cu、NiCo2O4、Ni电极都要大,这表明Cu的存在能明显提高Ni电极的析氧催化活性,降低析氧过电位。
Hongjie Dai小组 [21] 构建了超薄碳纳米管支撑的层状镍铁氢氧化物(NiFe-LDH/CNT)析氧阴极材料(如图4),通过碱性条件下测试,其析氧催化活性高且稳定性强,优于商业Ir催化剂的性能。
除了研究开发新型催化剂外,科研人员对镍铁氧化催化活性增强的机理进行了研究 [22] [23],探究了铁引入对性能的影响。
Alexis T. Bell小组 [24] 采用电沉积技术将镍铁氧化物薄膜修饰于基底,而后测试了其析氧催化活性并采用原位拉曼技术监测了析氧反应过程中镍的变化(如图5),结合电化学实验,作者发现当镍铁催化剂中铁的含量为40%时其催化活性是单纯沉积镍膜的两倍,同时作者发现铁的引入使得Ni(OH)2/NiOOH的氧化还原电势发生了正移,其其析氧反应活性更强。
除了镍铁析氧的相关研究,钴、铈、铜等的二元和三元氧化物也是常用的析氧催化剂材料 [25]。此外,Co3O4和NiCo2O4等 [22] 尖晶石型结构材料,由于具有电催化活性高、耐蚀性好、价格低廉易得等优点,被认为是很有前景的阳极材料,成为研究热点 [26]。钴基尖晶石氧化物因在析氧电位之前,在活性组分表面生成了高活性的中间态钴化合物,使阳极析氧过电位降低。增大析氧电极的比表面积和电极表面的粗糙度可以有效降低真实电流密度,可以降低过电位。

Figure 4. Construction schematic diagram of NiFe-LDH/CNT and corresponding TEM photographs and performance comparison diagram with commercial Ir catalyst
图4. NiFe-LDH/CNT构建示意图及对应的TEM照片及与商业Ir催化剂性能对比图
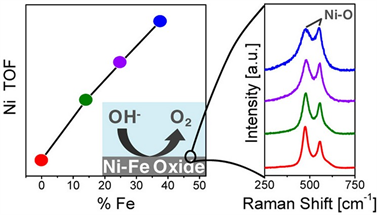
Figure 5. Oxygen evolution reaction properties and Raman monitoring diagram of Ni-Fe Oxide thin films
图5. 镍铁氧化物薄膜析氧反应性质及拉曼监测图
通过构建掺杂镍的介孔NixCo3−xO4纳米线阵列并探讨了其析氧催化活性 [27],如图6。作者发现,掺杂的镍在阵列中主要分布在表面,促进了材料的电子传导性,且材料具有大的粗糙因子,相比于Co3O4纳米线阵列,掺杂镍的NixCo3−xO4纳米线阵列显示了高析氧催化活性。
近年来,关于过渡金属化合物构建的析氧阳极材料性能提升的机理研究也一直是一个热点,如对NiOx [28]、Nickel-Borate薄膜 [29] 等进行的关于镍化合价的改变引起性能提升的探讨。
Vittal K. Yachandra和Daniel G. Nocera小组 [30] 合作利用X射线吸收光谱探讨了Nickel-Borate (Ni-Bi)薄膜阳极活化后析氧催化活性增强的原因,如图7。作者发现,活化后镍的价态变成了+3.6,也就是在薄膜中有相当部分的镍以四价形式存在,同时镍的桥联结构发生了改变。
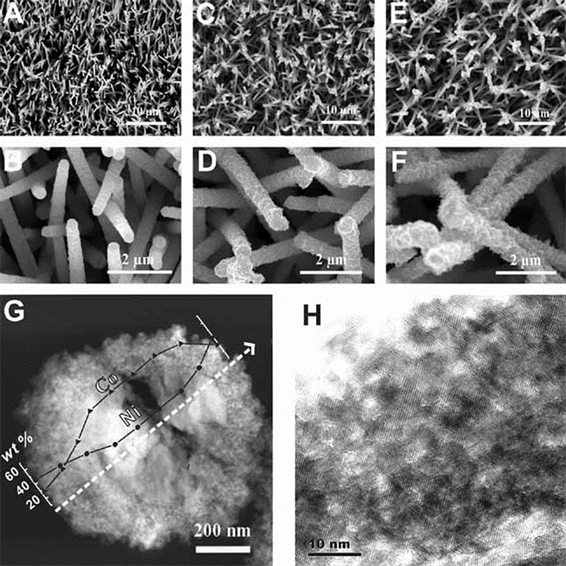
Figure 6. SEM images: (A) (B) Co3O4 nanowire array; (C) (D) (E) (F) NixCo3−xO4 nanowire arrays doped with different nickel contents; (G) STEM image of NixCo3−xO4 nanowire array; (H) HRTEM image of NixCo3−xO4 nanowire array
图6. SEM照片:(A) (B) Co3O4纳米线阵列;(C) (D) (E) (F) 掺杂不同镍含量的NixCo3−xO4纳米线阵列;(G) NixCo3−xO4纳米线阵列STEM照片;(H) NixCo3−xO4纳米线阵列HRTEM照片

Figure 7. Schematic diagram of enhanced catalytic performance of anode activation for oxygen evolution
图7. 阳极活化析氧催化性能增强示意图
除了镍的相关催化机理研究之外,科研人员对钴基材料的催化活性机理也进行了探讨 [31],如Leonardo Guidoni小组 [32] 研究了cobalt-based catalyst (CoCat)的相关反应路径,如图8。
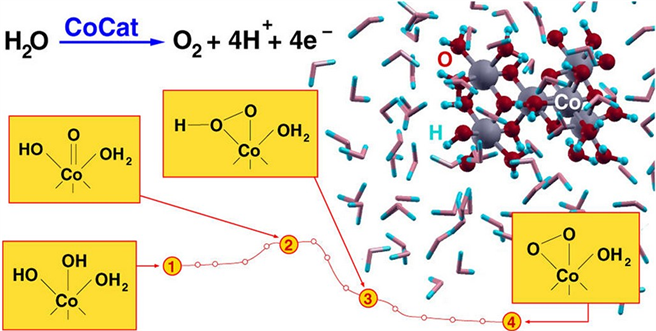
Figure 8. Schematic diagram of the reaction path of cobalt-based catalyst (CoCat)
图8. Cobalt-based catalyst (CoCat)的反应路径示意图
3.1.3. 钙钛矿型复合金属氧化物材料
钙钛矿型(ABO3)过渡金属氧化物也是一类重要的析氧催化剂材料 [33]。ABO3钙钛矿型复合金属氧化物,是一种既含稀土元素(A:Ln,Ca,Sr等),又含过渡金属元素(B:Ni,Co,Fe,Mn等过渡金属)的复合氧化物(如图9)。ABO3钙钛矿型复合金属氧化物B(尤其是Ni、Fe、Co、Mn、Cr等)内部的氧空穴使其具有很好的吸附和脱附氧的功能,因此被广泛作为析氧电极材料。LaNiO3、LaCoO3等 [34] 镧系钙钛矿氧化物,具有良好的导电性和电催化性能,如果再在其中添加一种或多种元素,有利于产生高浓度的氧空穴,能更好的提高其催化活性。Singh等人 [35] 发现,Ni和Fe的低取代都可以将La0.8Sr0.2CoO3的几何因素和电子因素进行改善,然而当Ni的取代含量太高时,会降低La0.8Sr0.2CoO3的析氧活性,而Fe的高含量取代则会由于增加了膜的表面积使电极的催化活性得以提高。
Hubert A.Gasteiger小组 [36] 构建了系列钙钛矿型析氧反应催化材料,并用分子轨道理论对其析氧催化反应进行解释,同时采用火山图归属了各种钙钛矿性析氧催化剂的催化活性,如图10。
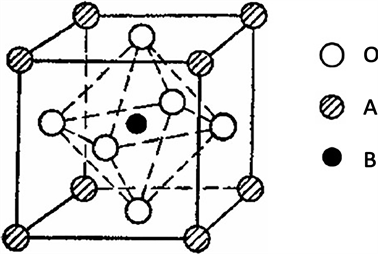
Figure 9. Structure of perovskite composite oxide
图9. 钙钛矿复合氧化物结构

Figure 10. Volcanic diagram of perovskite-type oxygen evolution catalyst
图10. 钙钛矿型析氧催化剂火山图
3.2. 析氢电极材料的研究现状
相比于析氧反应,电解析氢过程主要涉及到溶剂化的质子在表面放电形成吸附氢的过程(电化学Volmer反应),以及吸附氢的脱附过程(电化学脱附Heyrovsky反应或者复合脱附Tafel反应),基本反应过程如下 [37]:
电化学步骤(Volmer反应):H3O++e+M→MH+H2O (酸性介质)
或H2O + e + M→MH + OH− (中性或碱性介质)
电化学脱附步骤(Tafel反应):MH + H3O+ + e→H2 + M + H2O (酸性介质)
或MH + H2O + e→H2 + M + OH− (中性或碱性介质)
复合脱附步骤(Heyrovsky反应):MH + MH→H2 + 2M
一般析氧反应为Volmer-Heyrovsky机理或Volmer-Tafel机理,而根据转化中速度控制步骤的不同,两机理又可以分为迟缓放电理论,电化学脱附理论和复合脱附理论。在析氢反应中,关于析氢反应速度和机理的影响因素有很多,为了降低过电位,加速电解水制氢,常用的析氢电极材料有以下几种:
3.2.1. 贵金属及贵金属基复合材料 [33] [34]
不同的金属表现出不同的催化析氢性能,通过对比相同反应电流密度情况下的过电位大小,可以将金属材料大致分为三种:
(a) 高析氢过电位金属:主要有Pb,Hg,Zn,Sn等。
(b) 中析氢过电位金属:包括铁族元素,W,Cu等。
(c) 低析氢过电位金属:主要包括Pt,Pd,Ru等贵金属。
图11是经典的析氢活性的“火山图” [35]。从图中可以看出,随着金属与氢之间的表面吸附键能增强,材料的催化析氢活性表现出先增强后降低的特性。Pt等贵金属与氢原子之间的吸附能处在一个比较适中的能量区间,在析氢电极中,显示了优异的电化学催化活性和较低的析氢过电位,是很好的析氢电极材料 [38]。
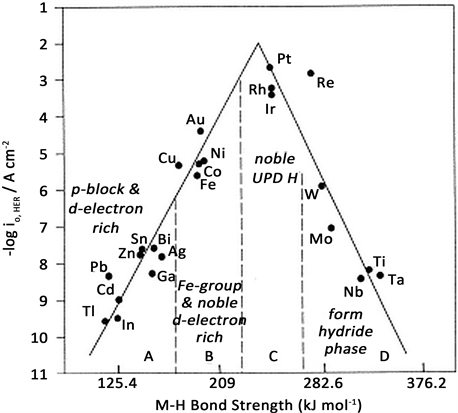
Figure 11. Volcanic curve of hydrogen evolution material based on M-H bond energy as logi0 value
图11. 根据M-H键能作为logi0值的析氢材料火山曲线
2015年Katherine E. Ayers小组 [39] 构建了Ru@Pt核壳纳米晶,其Pt负载量极低(< 50 μg/cm2),但是其导电性高,且相比于Pt负载量达到3 mg/cm2 Pt black电极,其显示了高的析氢催化活性和稳定性。
众所周知,Pt,Ru等贵金属因储量有限,价格昂贵而无法在工业上广泛运用,为了促进其应用,科研人员研究将其和其他廉价原料构建成高性能的复合催化剂,进一步增强电解水析氢反应动力学,降低过电位减少能耗 [40]。
2014年XiongYujie小组 [41],基于催化剂表面状态影响表面催化活性的考虑,构建了可控Pt壳层厚度的Pt-Pd-Graphene复合结构析氢材料,如图12。经研究发现随着Pt壳层厚度的减小其析氢催化反应活性逐步增大,作者采用表面极化机制对其进行了解释。作者认为随着Pt层厚度的变化,其表面功涵不同,功涵的不同又导致了Pt表面的极化不同,进而使得表层的状态不同,影响了材料的析氢反应催化活性 [42]。
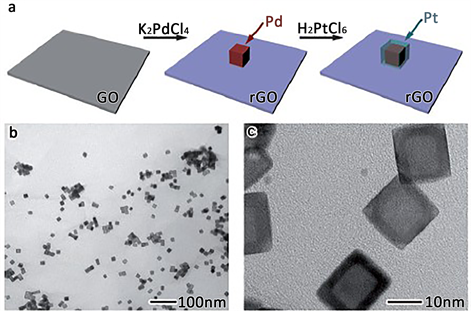
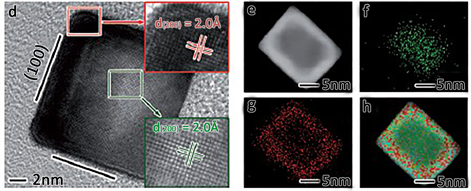
Figure 12. Construction diagram of Pt-Pd-Graphene material and corresponding TEM analysis images
图12. Pt-Pd-Graphene材料构建示意图及对应的TEM分析照片
2015年,Nenad M. Markovic小组 [43] 通过界面效应的调控构建了一个高活性的Li+-Ni(OH)2-Pt复合析氢催化剂,如图13。相比于Pt(111)电极,将Ni(OH)2 (高0.7 nm,宽为8到10 nm)修饰到Pt islands/Pt (111)电极表面,其催化性能提升了八倍,其原因是Ni(OH)2活化了水分子,促使其HO-H分解,方便了Pt捕获分离的氢形成Pt-Hadsorption,进而形成氢气。当把Li+引入到体系中后,处于Ni(OH)2-Pt表层的Li+进一步促进了水的解离,使其催化活性提升到十倍。
3.2.2. 镍基材料
根据Brewer-Engel价键理论 [44],当合金是由两种或两种以上的过渡金属(其中一类存在空的或半充满的d轨道,另一类存在成对的但不容易成键的d电子)形成时,将会显示出很好的电催化协同作用,对析氢反应的进行非常有利。镍储量丰富、价格较低、且在碱性条件下耐蚀性优良,因而成为研究热点。
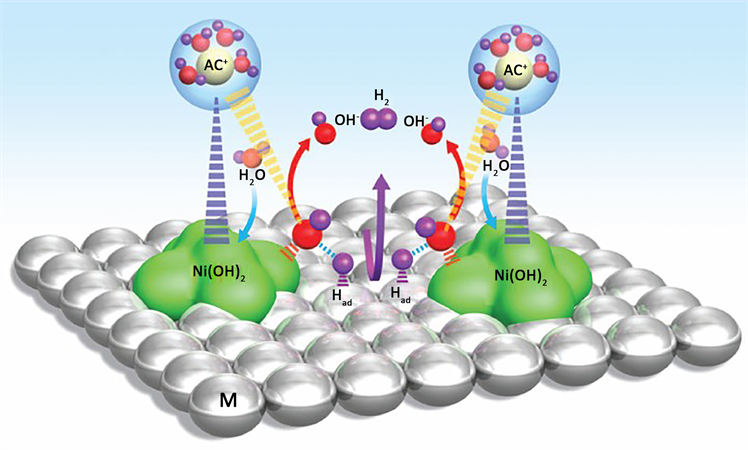
Figure 13. Schematic diagram of catalytic hydrogen production mechanism of Li+-Ni(OH)2-Pt composite hydrogen evolution catalyst
图13. Li+-Ni(OH)2-Pt复合析氢催化剂催化产氢机理示意图
镍作为析氢催化电极材料,应用最广泛的是Raney-Ni电极。早在上世纪20年代,M. Raney等人就发现Ni-Al或Ni-Zn合金在碱液中溶去Al或Zn元素后形成的Raney-Ni是相当好的析氢反应活性合金 [45]。Raney Ni由于多孔的骨架结构而具有较大的比表面积,在析氢反应过程中,当以较高的电流密度进行电解时,局部的电流密度能够显著的降低,具有很好的电催化活性。但由于此类电极存在机械强度差、易脱落、结构松弛等缺点,在长期连续电解后,多孔结构会出现塌陷,活性位点也会相应的减少。为了进一步改善Raney Ni的性能,研究者 [46] 在制备过程中进行添加Mo、Co等金属形成RaneyNi-Mo合金,发现电极的析氧催化活性以及稳定性都得到了明显的改善,目前研究较多的析氢反应镍基合金和镍基非金属材料如表1所示:
Table 1. Nickel-based alloys and nickel-based non-metallic materials commonly used for hydrogen evolution reaction
表1. 常用析氢反应的镍基合金和镍基非金属材料
2015年Jiao Feng和Chen Jingguang G.合作 [47] 构建了具有高催化高稳定析氢性能的多孔Cu-Ti双金属合金催化剂,其性能是Pt/C的两倍,如图14。尽管Cu和Ti的析氢性能不好,但作者将Cu和Ti结合构建的Cu-Ti合金具有Cu-Cu-Ti活性位点,其具有和Pt相类似的氢吸附能,此外因其大量的孔隙 [48] 提供了大量的活性表面,方便了溶液等的传输。
除了合金外,镍的一些磷化物,硒化物及其和其他材料形成的复合物也具有高的析氢反应活性,并且已成为热点研究 [49]。

Figure 14. (a) Bimetallic active site; (b) TEM images of Cu-Ti alloys and its corresponding surface scanning energy spectra; (c, d) SEM images of Cu-Ti alloy
图14. (a) 双金属活性位;(b) Cu-Ti合金TEM照片及其对应的面扫描能谱图;(c, d) Cu-Ti合金SEM照片
3.2.3. 钼基材料
钼基材料引起了人们的广泛关注,尤其硫化钼。2007年ChorkendorffIb小组 [50] 构建了不同纳米粒径的MoS2,将其负载到Au基底,研究探讨了其析氢催化活性位点位于材料的边缘位置 [51],如图15。
 (a) (b) (c)
(a) (b) (c)
Figure 15. Scanning tunneling microscope image of MoS2 based on Au: quenching temperature (a) 440˚C, (b) (c) 550˚C
图15. Au基底MoS2的扫描隧道显微镜照片:(a) 440℃;(b) (c) 550℃
为了开发高催化活性高稳定性的析氢催化剂材料,除了硫化钼外 [52] [53],科研人员还研究了钼基碳化物 [54]、氮化物 [55]、磷化物、硒化物 [56] [57] 等,以及与铜、镍、钴等形成的氧化物,及其和其他材料形成的复合杂化材料 [48] [53] - [61],其这些材料均表现出了高的催化性能和稳定性 [58]。
Thomas F. Jaramillo小组 [59] 将硫引入到磷化钼(MoP)表面,构建了MoP|S催化剂,并测试了其析氢催化活性。经实验测试,作者发现,相比于纯的磷化钼,MoP|S催化剂显示了更高的催化活性和稳定性,作者推测硫与磷的协同作用对析氢催化活性有很大影响。
Lain-Jong Li和Jiaxing Huang小组 [60] 合作将MoSx修饰与褶皱石墨烯功能化的碳布表面(如图16和图17),相比于未石墨烯功能化的碳布支撑的MoSx材料,修饰后的材料表现了强的析氢催化活性和稳定性 [61],且更易形成形貌一致化的MoSx,当电势为0.3 V (vs. RHE)时,修饰后的石墨烯功能化材料电流强度可以达到220 mA/cm2。

Figure 16. (a) Schematic diagram of MoSx formation supported by carbon cloth, (b) schematic diagram of MoSx formation supported by graphene functionalized carboncloth
图16. (a) 碳布支撑的MoSx形成示意图,(b) 石墨烯功能化碳布支撑的MoSx形成示意图
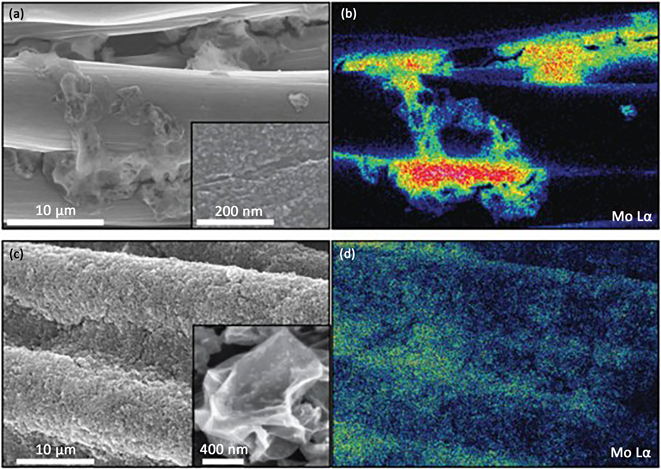
Figure 17. (a) (b) SEM images of MoSx supported by carbon cloth and corresponding EDS spectrum, (c) (d) SEM images of MoSx supported by graphene functionalized carbon cloth and corresponding EDS spectrum
图17. (a) (b) 碳布支撑的MoSx SEM照片及对应的EDS能谱,(c) (d) 石墨烯功能化碳布支撑的MoSx SEM照片及对应的EDS能谱
4. 结论
能源作为人类赖以生存、不可或缺的部分,与人类生活息息相关,构成了国民经济的命脉。传统能源(煤、石油、天然气)的大量使用,造成了严重的环境问题和日益突出的能源危机。氢能作为一种可再生的二次绿色能源,在未来能源结构布局中占有重要的位置。水电解制氢是实现工业化廉价制备氢气的重要手段,但该技术存在的最大问题是电能消耗大,使得生产成本偏高,造成电能消耗大的主要原因是电解电极的过电位过高,反应动力学缓慢,因此研究如何降低过电位来降低电解能耗加快反应动力学尤为重要。目前用于电解水的电极材料存在价格昂贵、比表面不大、电催化活性不高等缺点,严重制约了电解水制氢技术的发展,期待未来能够开发出具有低过电位、高催化活性和高稳定性的新型电解水电极材料。
基金项目
江苏省高校自然科学研究基金(NO. 19KJB430030)和南通市科技项目(NO. JC2020130, JC2020133, JC2020134)资助。
文章引用
汪永霞,王敏敏. 新型电极材料的研究进展
Recent Advanced Study of Novel Electrode Materials[J]. 分析化学进展, 2021, 11(03): 200-216. https://doi.org/10.12677/AAC.2021.113022
参考文献
- 1. Meng, N., Leung, D.Y. and Leung, M.K. (2006) An Overview of Hydrogen Production from Biomass. Fuel Processing Technology, 87, 461-472.
https://doi.org/10.1016/j.fuproc.2005.11.003 - 2. Fujishima, A. and Honda, K. (1972) Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature, 238, 37-38.
https://doi.org/10.1038/238037a0 - 3. Zeng, K. and Zhang, D. (2010) Recent Progress in Alkaline Water Electrolysis for Hydrogen Production and Applications. Progress in Energy and Combustion Science, 36, 307-326.
https://doi.org/10.1016/j.pecs.2009.11.002 - 4. Wei, Z., Ji, M. and Sun, C. (2007) Water Electrolysis on Carbon Electrodes Enhanced by Surfactant. Electrochimica Acta, 52, 3323-3329.
https://doi.org/10.1016/j.electacta.2006.10.011 - 5. Reier, T., Oezaslan, M. and Strasser, P. (2012) Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catalysis, 2, 1765-1772.
https://doi.org/10.1021/cs3003098 - 6. Diaz-Morales, O., Calle-Vallejo, F., Munck, C. and Koper, M.T.M. (2013) Electrochemical Water Splitting by Gold: Evidence for an Oxide Decomposition Mechanism. Chemical Science, 4, 2334-2343.
https://doi.org/10.1039/C3SC50301A - 7. Lee, Y., Suntivich, J., May, K.J. and Shao-Horn, Y. (2012) Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. The Journal of Physical Chemistry Letters, 3, 399-404.
https://doi.org/10.1021/jz2016507 - 8. Ten, C.W. and Hu, C. (1992) Hydrogen and Oxygen Evolutions on Ru-Ir Binary Oxides. Journal of the Electrochemical Society, 139, 2158-2163.
https://doi.org/10.1149/1.2221195 - 9. Terezo, A.J. and Pereira, E.C. (2000) Fractional Factorial Design Applied to Investigate Properties of Ti/IrO2-Nb2O5 Electrodes. Electrochimica Acta, 45, 4351-4358.
https://doi.org/10.1016/S0013-4686(00)00540-5 - 10. Yeo, B.S. and Alexis, T.B. (2011) Enhanced Activity of Gold-Supported Cobalt Oxide for the Electrochemical Evolution of Oxygen. Journal of the American Chemical Society, 133, 5587-5593.
https://doi.org/10.1021/ja200559j - 11. Balej, J., Divisek, J., Schmitz, H. and Mergel, J. (1992) Preparation and Properties of Raney Nickel Electrodes on Ni-Zn Base for H2 and O2 Evolution from Alkaline Solutions Part I: Electrodeposition of Ni-Zn Alloys from Chloride Solutions. Materials Chemistry and Physics, 22, 705-710.
https://doi.org/10.1007/BF01027497 - 12. Fazle, A.K.M. and Tarafdar, S.A. (2002) Electrochemical Studies of a Nickel-Copper Electrode for the Oxygen Evolution Reaction (OER). International Journal of Hydrogen Energy, 27, 879-884.
https://doi.org/10.1016/S0360-3199(01)00185-9 - 13. Landon, J., Demeter, E., İnoğlu, N., Keturakis, C., Wachs, I.E., Vasić, R., et al. (2012) Spectroscopic Characterization of Mixed Fe-Ni Oxide Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Electrolytes. ACS Catalysis, 2, 1793-1801.
https://doi.org/10.1021/cs3002644 - 14. Trotochaud, L., Young, S.L., Ranney, J.K. and Boettcher, S.W. (2014) Nickel-Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. Journal of the American Chemical Society, 136, 6744-6753.
https://doi.org/10.1021/ja502379c - 15. Trotochaud, L., Ranney, J.K., Williams, K.N. and Boettcher, S.W. (2012) Solution-Cast Metal Oxide Thin-Film Electrocatalysts for Oxygen Evolution. Journal of the American Chemical Society, 134, 17253-17261.
https://doi.org/10.1021/ja307507a - 16. Louie, M.W. and Bell, A.T. (2013) An Investigation of Thin-Film Ni-Fe Oxide Catalysts for the Electrochemical Evolution of Oxygen. Journal of the American Chemical Society, 135, 12329-12337.
https://doi.org/10.1021/ja405351s - 17. Smith, R.D.L., Prévot, M.S., Fagan, R.D., Trudel, S. and Berlinguette, C.P. (2013) Water Oxidation Catalysis: Electrocatalytic Response to Metal Stoichiometry in Amorphous Metal Oxide Films Containing Iron, Cobalt, and Nickel. Journal of the American Chemical Society, 135, 11580-11586.
https://doi.org/10.1021/ja403102j - 18. Gong, M., Li, Y., Wang, H., Liang, Y., Wu, J.Z., Zhou, J., et al. (2013) An Advanced Ni-Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. Journal of the American Chemical Society, 135, 8452-8455.
https://doi.org/10.1021/ja4027715 - 19. Smith, R.D.L., Prévot, M.S., Fagan, R.D., Zhang, Z., Sedach, P.A., Siu, M.K.J., et al. (2013) Photochemical Route for Accessing Amorphous Metal Oxide Materials for Water Oxidation Catalysis. Science, 340, 60-63.
https://doi.org/10.1126/science.1233638 - 20. Song, F. and Hu, X. (2014) Exfoliation of Layered Double Hydroxides for Enhanced Oxygen Evolution Catalysis. Nature Communications, 5, Article No. 4477.
https://doi.org/10.1038/ncomms5477 - 21. McCrory, C.C.L., Jung S, Peters, J.C. and Jaramillo, T.F. (2013) Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. Journal of the American Chemical Society, 135, 16977-16987.
https://doi.org/10.1021/ja407115p - 22. Yu, X., Sun, Z., Yan, Z., Xiang, B., Liu, X. and Du, P. (2014) Direct Growth of Porous Crystalline NiCo2O4Nanowire Arrays on a Conductive Electrode for High-Performance Electrocatalytic Water Oxidation. Journal of Materials Chemistry A, 2, 20823-20831.
https://doi.org/10.1039/C4TA05315J - 23. Zou, X., Su, J., Silva, R., Goswami, A., Sathe, B.R. and Asefa, T. (2013) Efficient Oxygen Evolution Reaction Catalyzed by Low-Density Ni-Doped Co3O4 Nanomaterials Derived from Metal-Embedded Graphitic C3N4. Chemical Communications, 49, 7522-7524.
https://doi.org/10.1039/C3CC42891E - 24. Ratcliff, E.L., Meyer, J., Steirer, K.X., Garcia, A., Berry, J.J., Ginley, D.S., et al. (2011) Evidence for Near-Surface NiOOH Species in Solution-Processed NiOx Selective Interlayer Materials: Impact on Energetics and the Performance of Polymer Bulk Heterojunction Photovoltaics. Chemical Materials, 23, 4988-5000.
https://doi.org/10.1021/cm202296p - 25. Bediako, D.K., Lassalle-Kaiser, B., Surendranath, Y. and Daniel, G.N. (2012) Structure-Activity Correlations in a Nickel-Borate Oxygen Evolution Catalyst. Journal of the American Chemical Society, 134, 6801-6809.
https://doi.org/10.1021/ja301018q - 26. McAlpin, J.G., Surendranath, Y., Dincǎ, M., Stich, T.A., Stoian, S.A., Casey, W.H., et al. (2010) Evidence for Co(IV) Species Produced During Water Oxidation at Neutral pH. Journal of the American Chemical Society, 132, 6882-6883.
https://doi.org/10.1021/ja1013344 - 27. Mattioli, G., Giannozzi, P., Amore Bonapasta, A. and Guidoni, L. (2013) Reaction Pathways for Oxygen Evolution Promoted by Cobalt Catalyst. Journal of the American Chemical Society, 135, 15353-15363.
https://doi.org/10.1021/ja401797v - 28. Gardner, G.P., Go, Y.B., Robinson, D.M., Smith, P.F., Hadermann, J., Abakumov, A., et al. (2012) Structural Requirements in Lithium Cobalt Oxides for the Catalytic Oxidation of Water. Angewandte Chemie International Edition, 51, 1616-1619.
https://doi.org/10.1002/anie.201107625 - 29. Singh, R.N., Tiwari, S.K., Singh, S.P., Singh, N.K., Poillerat, G. and Chartier, P. (1996) Synthesis of (La, Sr)CoO3 Perovskite Films Via a Sol-Gel Route and Their Physicochemical and Electrochemical Surface Characterization for Anode Application in Alkaline Water Electrolysis. Journal of the Chemical Society, Faraday Transactions, 92, 2593-2597.
https://doi.org/10.1039/FT9969202593 - 30. Suntivich, J., May, K.J., Gasteiger, H.A., Goodenough, J.B. and Shao-Horn, Y. (2011) A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science, 334, 1383-1385.
https://doi.org/10.1126/science.1212858 - 31. Grimaud, A., May, K.J., Carlton, C.E., Lee, Y.-L., Risch, M., Hong, W.T., et al. (2013) Double Perovskites as a Family of Highly Active Catalysts for Oxygen Evolution in Alkaline Solution. Nature Communications, 4, Article No. 2439.
https://doi.org/10.1038/ncomms3439 - 32. Laursen, A.B., Kegnaes, S., Dahl, S. and Chorkendorff, I. (2012) Molybdenum Sulfides-Efficient and Viable Materials for Electro- and Photo Electrocatalytic Hydrogen Evolution. Energy & Environmental Science, 5, 5577-5591.
https://doi.org/10.1039/C2EE02618J - 33. Wang, J., Zhang, Y., Capuano, C.B. and Ayers, K.E. (2015) Ultralow Charge-Transfer Resistance with Ultralow Pt Loading for Hydrogen Evolution and Oxidation Using Ru@PtCore-Shell Nanocatalysts. Scientific Reports, 5, Article No. 12220.
https://doi.org/10.1038/srep12220 - 34. Subbaraman, R., Tripkovic, D., Strmcnik, D., Chang, K.-C., Uchimura, M., Paulikas, A.P., et al. (2011) Enhancing Hydrogen Evolution Activity in Water Splitting by Tailoring Li+-Ni(OH)2-Pt Interfaces. Science, 334, 1256-1260.
https://doi.org/10.1126/science.1211934 - 35. Conway, B.E. and Jerkiewicz, G. (2000) Relation of Energies and Coverages of Underpotential and Overpotential Deposited H at Pt and Other Metals to the ‘Volcano Curve’ for Cathodic H2 Evolution Kinetics. Electrochimica Acta, 45, 4075-4083.
https://doi.org/10.1016/S0013-4686(00)00523-5 - 36. Greeley, J., Nørskov, J.K., Kibler, L.A., El-Aziz, A.M. and Kolb, D.M. (2006) Hydrogen Evolution over Bimetallic Systems: Understanding the Trends. ChemPhysChem, 7, 1032-1035.
https://doi.org/10.1002/cphc.200500663 - 37. Mukherjee, S., Libisch, F., Large, N., Neumann, O., Brown, L.V., Cheng, J., et al. (2012) Hot Electrons Do the Impossible: Plasmon-Induced Dissociation of H2 on Au. Nano Letters, 13, 240-247.
https://doi.org/10.1021/nl303940z - 38. Esposito, D.V., Hunt, S.T., Stottlemyer, A.L., Dobson, K.D., McCandless, B.E., Birkmire, R.W., et al. (2010) Low-Cost Hydrogen-Evolution Catalysts Based on Monolayer Platinum on Tungsten Monocarbide Substrates. Angewandte Chemie International Edition, 49, 9859-9862.
https://doi.org/10.1002/anie.201004718 - 39. Bai, S., Wang, C., Deng, M., Gong, M., Bai, Y., Jiang, J., et al. (2014) Surface Polarization Matters: Enhancing the Hydrogen-Evolution Reaction by Shrinking Pt Shells in Pt-Pd-Graphene Stack Structures. Angewandte Chemie International Edition, 53, 12120-12124.
https://doi.org/10.1002/anie.201406468 - 40. Chen, Z., Ye, S., Wilson, A.R., Ha, Y.-C. and Wiley, B.J. (2014) Optically Transparent Hydrogen Evolution Catalysts Made from Networks of Copper-Platinum Core-Shell Nanowires. Energy & Environmental Science, 7, 1461-1467.
https://doi.org/10.1039/C4EE00211C - 41. Yang, X., Koel, B.E., Wang, H., Chen, W. and Bartynski, R.A. (2012) Nanofaceted C/Re(112̅1): Fabrication, Structure, and Template for Synthesizing Nanostructured Model Pt Electrocatalyst for Hydrogen Evolution Reaction. ACS Nano, 6, 1404-1409.
https://doi.org/10.1021/nn204615j - 42. Danilovic, N., Subbaraman, R., Strmcnik, D., Chang, K.-C., Paulikas, A.P., Stamenkovic, V.R., et al. (2012) Enhancing the Alkaline Hydrogen Evolution Reaction Activity Through the Bifunctionality of Ni(OH)2/Metal Catalysts. Angewandte Chemie International Edition, 51, 12495-12498.
https://doi.org/10.1002/anie.201204842 - 43. Jakšić, M.M. (1984) Electrocatalysis of Hydrogen Evolution in the Light of the Brewer-Engel Theory for Bonding in Metals and Intermetallic Phases. Electrochimica Acta, 29, 1539-1550.
https://doi.org/10.1016/0013-4686(84)85007-0 - 44. Lee, H.K., Jung, E.E. and Lee, J.S. (1998) Enhancement of Catalytic Activity of Raney Nickel by Cobalt Addition. Materials Chemistry and Physics, 55, 89-93.
https://doi.org/10.1016/S0254-0584(98)00126-6 - 45. Tasić, G.S., Lačnjevac, U., Tasić, M.M., Kaninski, M.M., Nikolić, V.M., Žugić, D.L., et al. (2013) Influence of Electrodeposition Parameters of Ni-W on Ni Cathode for Alkaline Water Electrolyser. International Journal of Hydrogen Energy, 38, 4291-4297.
https://doi.org/10.1016/j.ijhydene.2013.01.193 - 46. Yüce, A.O., Döner, A. and Kardaş, G. (2013) NiMn Composite Electrodes as Cathode Material for Hydrogen Evolution Reaction in Alkaline Solution. International Journal of Hydrogen Energy, 38, 4466-4473.
https://doi.org/10.1016/j.ijhydene.2013.01.160 - 47. Lu, Q., Hutchings, G.S., Yu, W., Zhou, Y., Forest, R.V., Tao, R., et al. (2015) Highly Porous Non-Precious Bimetallic Electrocatalysts for Efficient Hydrogen Evolution. Nature Communications, 6, Article No. 6567.
https://doi.org/10.1038/ncomms7567 - 48. Liao L., Wang S., Xiao J., Bian, X., Zhang, Y., Scanlon, M.D., et al. (2014) A Nanoporous Molybdenum Carbide Nanowire as an Electrocatalyst for Hydrogen Evolution Reaction. Energy & Environmental Science, 7, 387-392.
https://doi.org/10.1039/C3EE42441C - 49. Jaramillo, T.F., Jørgensen, K.P., Bonde, J., Nielsen, J.H., Horch, S., Chorkendorff, I., et al. (2007) Identification of Active Edge Sites for Electrochemical H2Evolution from MoS2Nanocatalysts. Science, 317, 100-102.
https://doi.org/10.1126/science.1141483 - 50. Wang, H., Lu, Z., Xu, S., Kong, D., Cha, J.J., Zheng, G., et al. (2013) Electrochemical Tuning of Vertically Aligned MoS2 Nanofilms and its Application in Improving Hydrogen Evolution Reaction. Proceedings of the National Academy of Sciences of the United States of America, 110, 19701-19706.
https://doi.org/10.1073/pnas.1316792110 - 51. Wang, H., Lu, Z., Kong, D., Sun, J., Hymel, T.M. and Cui, Y. (2014) Electrochemical Tuning of MoS2 Nanoparticles on Three-Dimensional Substrate for Efficient Hydrogen Evolution. ACS Nano, 8, 4940-4947.
https://doi.org/10.1021/nn500959v - 52. Chang, Y., Lin, C., Chen, Z., Hsu, C.-L., Lee, Y.-H., Zhang, W., et al. (2013) Highly Efficient Electrocatalytic Hydrogen Production by MoSx Grown on Graphene-Protected 3D Ni Foams. Advanced Materials, 25, 756-760.
https://doi.org/10.1002/adma.201202920 - 53. Chen, W.F., Iyer, S., Iyer, S., Sasaki, K., Wang, C.-H., Zhu, Y., et al. (2013) Biomass-Derived Electrocatalytic Composites for Hydrogen Evolution. Energy & Environmental Science, 6, 1818-1826.
https://doi.org/10.1039/C3EE40596F - 54. Kong, D., Wang, H., Cha, J.J., Pasta, M., Koski, K.J., Yao, J., et al. (2013) Synthesis of MoS2 and MoSe2Films with Vertically Aligned Layers. Nano Letters, 13, 1341-1347.
https://doi.org/10.1021/nl400258t - 55. Chen, W.F., Sasaki, K., Marinkovic, N., Marinkovic, N., Xu, W., Muckerman, J.T., et al. (2013) Highly Active and Durable Nanostructured Molybdenum Carbide Electrocatalysts for Hydrogen Production. Energy & Environmental Science, 6, 943-951.
https://doi.org/10.1039/C2EE23891H - 56. Kibsgaard, J. and Jaramillo, T.F. (2014) Molybdenum Phosphosulfide: An Active, Acid-Stable, Earth-Abundant Catalyst for the Hydrogen Evolution Reaction. Angewandte Chemie International Edition, 126, 14661-14665.
https://doi.org/10.1002/ange.201408222 - 57. Xiao, P., Thia, L., Alam Sk, M., Ge, X., Lim, R.J., Wang, J.-Y., et al. (2014) Molybdenum Phosphide As an Efficient Electrocatalyst for Hydrogen Evolution Reaction. Energy & Environmental Science, 7, 2624-2629.
https://doi.org/10.1039/C4EE00957F - 58. Smith, A.J., Chang, Y., Raidongia, K., Chen, T.-Y., Li, L.-J. and Huang, J. (2014) Molybdenum Sulfide Supported on Crumpled Graphene Balls for Electrocatalytic Hydrogen Production. Advanced Energy Materials, 4, Article ID: 1400398.
https://doi.org/10.1002/aenm.201400398 - 59. Chen, W., Sasaki, K., Ma, C., Frenkel, A.I., Marinkovic, N., Muckerman, J.T., et al. (2012) Hydrogen-Evolution Catalysts Based on Non-Noble Metal Nickel-Molybdenum Nitride Nanosheets. Angewandte Chemie International Edition, 51, 6131-6135.
https://doi.org/10.1002/anie.201200699 - 60. Cao, B., Veith, G.M., Neuefeind, J.C., Adzic, R.R. and Khalifah, P.G. (2013) Mixed Close-Packed Cobalt Molybdenum Nitrides as Non-Noble Metal Electrocatalysts for the Hydrogen Evolution Reaction. Journal of the American Chemical Society, 135, 19186-19192.
https://doi.org/10.1021/ja4081056 - 61. Tran, P.D., Nguyen, M., Pramana, S.S., Bhattacharjee, A., Chiam, S.Y., Fize, J., et al. (2012) Copper Molybdenum Sulfide: A New Efficient Electrocatalyst for Hydrogen Production from Water. Energy & Environmental Science, 5, 8912-8916.
https://doi.org/10.1039/C2EE22611A
