Hans Journal of Agricultural Sciences
Vol.
09
No.
12
(
2019
), Article ID:
33586
,
7
pages
10.12677/HJAS.2019.912167
Regulation of Ca2+ in Plant Response Mechanisms under Cold Stress
Wenjuan Wang1, Juanjuan Fu2
1Ningxia Technical College of Wine and Desertification Prevention, Yinchuan Ningxia
2College of Grassland Agriculture, Northwest A&F University, Yangling Shaanxi
Received: Nov. 30th, 2019; accepted: Dec. 17th, 2019; published: Dec. 24th, 2019

ABSTRACT
Cold stress presents one of the major limitations for plant growth, development and yield worldwide, as well as distribution, especially in areas of north and high-latitude regions. Worldwide, the annual crop economic losses due to the low temperature above 0˚C is amount to hundreds billions of yuan. So an investigation of responses mechanism of cold stress in plant and improvement of its resistance has significant important value. Ca2+ acts as the second messenger coupling of extracellular signals and intracellular physiological response. It plays an important role in mediating plant growth and development, as well as involved in the regulation of various abiotic stresses. The review discusses the molecular mechanism of Ca2+ regulating cold stress in plant.
Keywords:Ca2+ Signaling, Cold Stress, Plant, Regulatory Mechanism

钙信号调控植物低温胁迫的分子机制研究进展
王文娟1,付娟娟2
1宁夏葡萄酒与防沙治沙职业技术学院,宁夏 银川
2西北农林科技大学草业与草原学院,陕西 杨凌
收稿日期:2019年11月30日;录用日期:2019年12月17日;发布日期:2019年12月24日

摘 要
低温冷害是最为严重的自然灾害之一。低温冷害抑制植物的生长发育,全世界范围内每年因0℃以上的低温冷害造成的作物经济损失达数千亿元。因此研究植物的低温胁迫适应机理,提高其低温抗性不仅具有十分重要的科学意义,而且有着更为重要的现实价值。Ca2+作为重要的第二信使不仅参与调控植物的生长发育过程,还参与调控各种生物与非生物胁迫应答。本文重点论述了Ca2+调控植物低温胁迫应答的分子机制。
关键词 :钙信号,低温胁迫,植物,应答机制

Copyright © 2019 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
低温作为重要的非生物逆境被认为是世界最为严重的自然灾害之一。全世界范围内每年因零度以上的低温造成的作物经济损失达数千亿元 [1],在我国每年因低温冷害造成的作物生产损失可达3~5亿吨 [2]。因此,研究植物的低温胁迫应答机制,提高其低温抗性具有重要的意义 [1]。Ca2+除了是植物必需的元素以外,还起到植物细胞膜保护剂的作用,能够稳定植物细胞膜和膜蛋白等;另外,Ca2+也作为植物细胞内重要的第二信使,在植物的各种生物与非生物胁迫应答中起到信号转导的作用 [2]。已经有许多研究报道Ca2+信号在植物低温应答过程中发挥重要的作用。本文综述了Ca2+信号在植物低温胁迫下的响应及Ca2+参与植物低温应答的转导途径(图1),并对Ca2+信号与CBF依赖的低温应答通路的调控关系进行展望。
2. 低温胁迫下植物钙信号的响应
植物在正常生长条件下,其细胞内的Ca2+含量维持在较低的水平,一旦植物遭遇到不良环境胁迫后,细胞内的Ca2+水平在短时间内骤增,从而产生和放大钙信号 [3],在Ca2+信号转导过程中,Ca2+能够与其下游的钙调素等钙结合蛋白结合,激活一系列的钙依赖的蛋白激酶,这些激活的蛋白激酶能够进一步通过调控下游的胁迫应答相关的基因表达,从而调控植物体内各种生理代谢响应 [4]。对拟南芥和紫花苜蓿的研究发现,低温诱导的基因转录水平同低温诱导的钙离子流存在正相关关系 [5]。低温胁迫一方面诱导Ca2+从质膜流入细胞内,另一方面从中央液泡释放到细胞内最终导致细胞内Ca2+浓度上升 [6]。采用微电极法分析胞外Ca2+水平变化,发现低温胁迫导致植物根组织中Ca2+流从胞外进入胞内,从而导致细胞内Ca2+升高 [7]。低温胁迫会抑制质膜上Ca2+通道的活性。在Ca2+信号转导过程中,Ca2+跨膜运输离不开钙离子通道的调控作用。钙离子通道定位于质膜与细胞内膜,当植物遭遇外界不良环境刺激时,通道打开,胞外或胞内的Ca2+进入到细胞质中,导致胞内Ca2+水平瞬间升高,从而激活一系列防御响应 [2]。细胞内Ca2+浓度升高可以诱导磷酸酯酶C和D活性增强,导致三磷酸肌醇(IP3)和磷脂酸积累。IP3能够通过其调控的钙离子通道进一步放大Ca2+信号。拟南芥fry1突变体同野生型相比积累更多的IP3,提高了CBFs和COR (cold responsive genes)基因的表达,具有更高的抗寒性 [8]。定位在液泡膜上的Ca2+/H+反向转运体CAX1具有将细胞质中的Ca2+转运到液泡中的作用。cax1功能缺失突变体在非冷驯化条件下没有明显的表型,但是经过冷驯化以后,表现出明显的抗冻表型。这一研究表明,CAX1通过向外转移细胞质中Ca2+流,从而负向调控植物的抗冻性 [9]。另外,在高等植物之中还存在另一类环核苷酸钙离子通道CNGC (cyclic nucleotide gated calcium channel),它能够通过调控Ca2+内流而提高植物的各种生物与非生物抗性 [10]。Nawaz [11] 在水稻发现16个CNGC基因,其中10个是由低温诱导上调表达。近年来,在水稻根细胞研究中发现低温受体蛋白COLD1 (Chilling-tolerance Divergence 1)可与G蛋白互作,激活钙离子通道,导致根细胞中的钙离子内流加快,并改变膜电信号,从而激活钙离子信号通路,最终增强植物的耐寒性 [12]。以上研究都表明,Ca2+浓度迅速升高和钙离子通道开关在植物响应低温胁迫的过程中起到非常重要的作用。
3. 低温胁迫下钙信号的应答机制
3.1. Ca2+转运系统
植物的细胞内Ca2+信号产生后,需要经过一个解码的过程,Ca2+信号首先由Ca2+感受元件(Calcium sensor)感知,之后将Ca2+信号逐级放大,最终引发一系列的生理代谢响应 [13]。钙–钙感受器–靶蛋白–关键基因是调控植物响应逆境胁迫的关键途径。目前研究比较深入地植物对环境信号的感知和传递的钙信使系统,主要包括钙离子–钙调蛋白(Ca2+-CaM)、钙离子–钙调磷酸酶B类似蛋白(Ca2+-Calcineurin B-Like protein (CBL))和钙离子–钙依赖性蛋白激酶(Ca2+-CDPK) [14]。近年来,CBL-CBL-interacting protein kinases (CIPKs)信号在调控植物胁迫应答方面受到国内外学者的普遍关注 [15] [16]。CIPK信号是在高等植物中特有的一类丝氨酸/苏氨酸蛋白激酶,它能与CBLs特异性互作。CBL-CIPK信号通路在植物Ca2+信号传递过程中起着重要作用。
3.2. Ca2+参与植物低温应答的转导途径
CBLs在检测到植物细胞产生的Ca2+信号后,通过激活下游CIPKs的激酶活性来实现Ca2+信号的转导 [16] [17]。许多研究表明,植物CIPKs基因受多种逆境胁迫诱导表达,CBL-CIPK信号网络在植物逆境应答过程中起关键作用 [18] [19] [20] [21]。然而,关于CBL-CIPK信号调控植物低温胁迫应答的报道较少。在水稻中的研究表明,低温胁迫诱导OsCIPK3 (OsCK1)表达,并且OsCIPK3 (OsCK1)可能通过Ca2+信号依赖的方式调控植物的低温胁迫应答 [22]。Xiang等 [23] 研究发现,过表达OsCIPK03可以提高水稻的抗寒/冻性。在拟南芥中的研究发现,CBL1与CIPK7互作共同调控植物的低温应答 [24]。Deng等 [25] 研究发现,异源表达小麦TaCIPK14基因能够提高番茄的抗寒性。
植物遭受低温等外界刺激后,其细胞膜流动性和结构发生改变,导致细胞膜上离子通道打开,从而引起细胞质中Ca2+水平瞬间激增,细胞内瞬间增加的Ca2+流被下游的钙信号感受器(CRLK1,CPKs和CIPKs)所感知,进一步将Ca2+信号向下游传递,从而调控低温应答基因响应 [17]。Yang等 [26] 报道,低温逆境下,CRLK1与MEKK1互作,从而激活MAPK级联响应。Teige等 [27] 在拟南芥中的研究发现,低温激活MAP2K/MKK2信号从而调控下游的COR基因表达。MKK2作为MAPK3/MEKK1的下游信号,而作为MPK4和MPK6的上游信号参与调控植物的低温应答响应 [28]。在拟南芥中,低温胁迫诱导升高的[Ca2+]cyt与CaMs结合,进一步在蛋白修饰水平调控下游的类受体激酶(RLKs)的活性以及激活MEKK1-MKK2-MPK4级联信号,从而诱发植物的低温胁迫应答 [29]。低温胁迫诱导的[Ca2+]cyt浓度的升高还能通过调节CBF2和CAMTA3的表达,进而激活低温胁迫响应通路中的转录因子 [30]。综上,低温首先降低细胞膜的流动性,细胞膜的这种改变被定位于质膜的钙离子通道或蛋白所感知,导致钙离子流的激活以及CPKs、CIPKS、CRLK1等钙响应蛋白激酶和MAPK级联响应,最终调控低温响应基因COR表达。低温胁迫下植物Ca2+信号转导途径见图1。
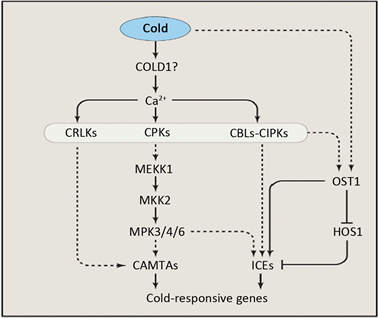
Figure 1. Ca2+ signaling transduction pathway under cold stress
图1. 低温胁迫下Ca2+信号转导途径 [18]
4. 钙信号与CBF信号的潜在调控关系
低温诱导植物体内的冷响应基因(CORs)表达,CORs基因编码一些保护性的蛋白质,如脱水素,这类蛋白质能够保护植物免受低温伤害。CBFs/DREBs (C-repeat (CRT)-binding factors/dehydration-responsive element (DRE) binding factors)是作用于CORs基因上游的一类重要的转录因子 [31]。截止目前,CBF信号通路是公认的低温信号转导途径。低温胁迫下植物的CBF转导途径见图2。CBF通过结合COR、LT1 (Low temperature Induced)、KIN (Cold-induced)及RD (Responsive to Dehydration)等类型低温调节基因启动子区域的CRT (C-repeat)/DRE (Dehydration Response Element)顺式作用元件上,诱导其表达,从而提高植物的抗寒性 [32] [33] [34]。研究发现,苹果bHLH转录因子MdCIbHLH1 (Cold-Induced bHLHl)可以通过正调控MdCBF2表达,提高了苹果植株的抗寒性。同时,该基因也可以结合到拟南芥AtCBF3启动子上的顺式调控元件 [35]。Zhao等 [36] 研究发现香蕉MaMYC2转录因子可以与MalCEl互作,进一步调节MaCBF1、MaCBF2、MaCORl、MaKIN2、MaRD2及MaRD5等基因的表达,提高香蕉果实的抗寒性。Shi等 [37] 通过对拟南芥乙稀突变体的分析,证实EIN3也可以负调控CBFs基因的表达。近期的研究发现,低温诱导SnRK2.6/OST1上调表达,这一诱导的SnRK2.6蛋白进一步磷酸化ICE1,从而激活CBF-COR依赖的低温信号应答途径 [38] [39]。Liu等 [40] 研究表明,定位于质膜的冷响应蛋白激酶1 (cold-responsive protein kinase 1, CRPK1)磷酸化14-3-3蛋白,磷酸化的14-3-3蛋白进入细胞核与CBF蛋白互作调控其稳定性,从而实现CRPK1-14-3-3模块精细地调控植物的低温应答响应。Ca2+信号感受器可能通过某种未知机制调控ICE1,CBF的转录水平及蛋白稳定性,从而进一步调控植物的低温抗性。另外,Li等 [41] 研究发现,低温激活的蛋白激酶MPK3和MPK6能够磷酸化ICE1,降低其转录活性及蛋白稳定性,抑制CBF基因表达,从而负调控植物的低温抗性。
5. 展望
目前,虽然关于钙离子调控植物低温应答的机理研究已取得很大的进展,然而对于植物感受低温信号的受体激酶,以及该受体是如何将低温信号传递到次级信使Ca2+尚未有定论。因此,在以后的研究中,Ca2+信号是如何与低温信号感受器作用,并将低温信号进一步放大将会是一个非常重要的研究领域。尤其是Ca2+信号通路与CBF低温应答通路的关键互作蛋白的研究尤为重要。综上所述,深入研究Ca2+
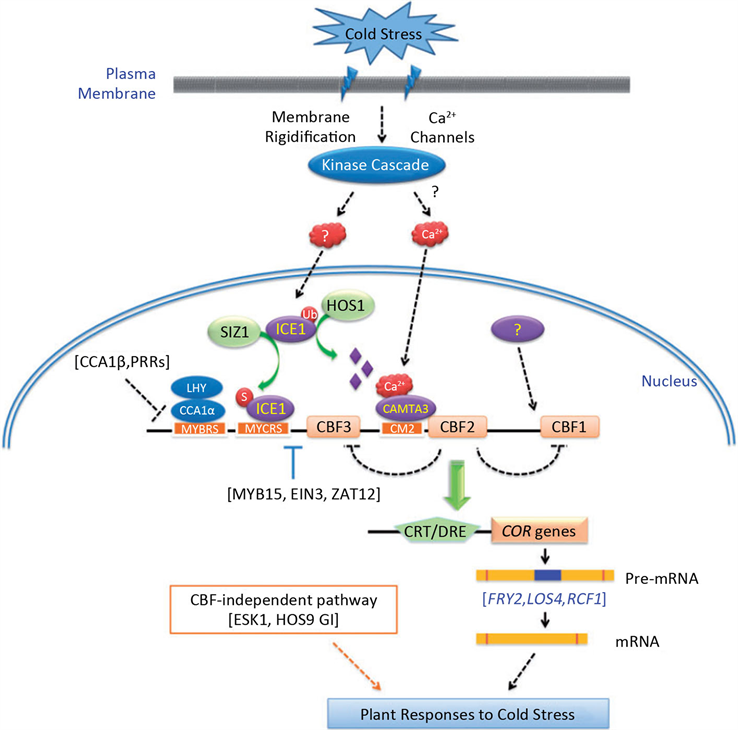
Figure 2. The signaling transduction pathway of CBF-regulated response to stress in plants
图2. 植物CBF冷信号转导途径 [35]
依赖的低温信号转导机制将有助于揭示植物低温胁迫应答的机理,同时也为选育耐寒作物及牧草新品种提供理论支撑。
基金项目
宁夏科技厅自然科学基金项目[NZ1620],宁夏自治区财政林业新技术引进及推广项目《宁夏沙区特种植物砂生槐展示区综合建设》。
文章引用
王文娟,付娟娟. 钙信号调控植物低温胁迫的分子机制研究进展
Regulation of Ca2+ in Plant Response Mechanisms under Cold Stress[J]. 农业科学, 2019, 09(12): 1173-1179. https://doi.org/10.12677/HJAS.2019.912167
参考文献
- 1. Chinnusamy, V., Zhu, J.K. and Sunkar, R. (2010) Gene Regulation during Cold Stress Acclimation in Plants. In: Sunkar, R., Ed., Plant Stress Tolerance, Springer, Heidelberg, 39-55. https://doi.org/10.1007/978-1-60761-702-0_3
- 2. Zhang, Z.Y., Li, J.J., Pan, Y.H., Li, J.L., Zhou, L., Shi, H.L., Zeng, Y.W., Guo, H.F., Yang, S.M., Zheng, W.W., Yu, J.P., Sun, X.M., Li, G.L., Ding, Y.L., Ma, L., Shen, S.Q., Dai, Y.L., Zhang, H.L., Yang, S.H., Guo, Y. and Li, Z.C. (2017) Natural Variation in CTB4a Enhances Rice Adaptation to Cold Habitats. Nature Communications, 8, Article No. 14788. https://doi.org/10.1038/ncomms14788
- 3. Dodd, A.N., Kudla, J. and Sanders, D. (2010) The Language of Calcium Signaling. Annual Review of Plant Biology, 61, 593-620. https://doi.org/10.1146/annurev-arplant-070109-104628
- 4. Reddy, A.S., Ali, G.S., Celesnik, H., et al. (2011) Coping with Stresses: Roles of Calcium- and Calcium/Calmodulin-Regulated Gene Expression. Plant Cell, 23, 2010-2032. https://doi.org/10.1105/tpc.111.084988
- 5. Kleist, T.J. and Luan, S. (2016) Constant Change: Dy-namic Regulation of Membrane Transport by Calcium Signalling Networks Keeps Plants in Tune with Their Environment. Plant Cell and Environment, 39,467-481. https://doi.org/10.1111/pce.12599
- 6. Nordin, H.K. and Trewavas, A. (2003) The Effect of Short-Term Low-Temperature Treatments on Gene Expression in Arabidopsis Correlates with Changes in Intracellular Ca2+ Levels. Plant, Cell & Environment, 26, 485-496. https://doi.org/10.1046/j.1365-3040.2003.00979.x
- 7. Wilkins, K.A., Matthus, E., Swarbreck, S.M., et al. (2016) Calcium-Mediated Abiotic Stress Signaling in Roots. Fronts in Plant Science, 7, 1296. https://doi.org/10.3389/fpls.2016.01296
- 8. Sulaiman, Y., Knight, M.R. and Kataky, R. (2012) Non-Invasive Monitoring of Temperature Stress in Arabidopsis thaliana Roots, Using Ion Amperometry. Analytical Methods, 4, 1656-1661. https://doi.org/10.1039/c2ay05747f
- 9. Xiong, L., Lee, B., Ishitani, M., et al. (2001) FIERY1 Encod-ing an Inositol Polyphosphate 1-Phosphatase Is a Negative Regulator of Abscisic Acid and Stress Signaling in Ara-bidopsis. Gene Development, 15, 1971-1984. https://doi.org/10.1101/gad.891901
- 10. Catala, R., Santos, E., Alonso, J.M., et al. (2003) Mutations in the Ca2+/H+ Transporter CAX1 Increase CBF/DREB1 Expression and the Cold-Acclimation Response in Arabidopsis. Plant Cell, 15, 2940-2951. https://doi.org/10.1105/tpc.015248
- 11. Jha, S.K., Sharma, M. and Pandey, G.K. (2016) Role of Cyclic Nucleotide Gated Channels in Stress Management in Plants. Current Genomics, 17, 315-329. https://doi.org/10.2174/1389202917666160331202125
- 12. Nawaz, Z., Kakar, K.U., Saand, M.A., et al. (2014) Cyclic Nucleotide-Gated Ion Channel Gene Family in Rice, Identification, Characterization and Experimental Analysis of Expression Response to Plant Hormones, Biotic and Abiotic Stresses. BMC Genomics, 15, 853. https://doi.org/10.1186/1471-2164-15-853
- 13. Ma, Y., Dai, X., Xu, Y., et al. (2015) COLD1 Confers Chilling Tolerance in Rice. Cell, 160, 1209-1221. https://doi.org/10.1016/j.cell.2015.01.046
- 14. Hashimoto, K. and Kudla, J. (2011) Calcium Decoding Mechanisms in Plants. Biochimie, 93, 2054-2059. https://doi.org/10.1016/j.biochi.2011.05.019
- 15. Ormancey, M., Thuleau, P., Mazars, C., et al. (2017) CDPKs and 14-3-3 Proteins: Emerging Duo in Signaling. Trends in Plant Science, 22, 263-272. https://doi.org/10.1016/j.tplants.2016.11.007
- 16. Luan, L. (2009) The CBL-CIPK Network in Plant Calcium Sig-naling. Trends in Plant Science, 14, 37-42. https://doi.org/10.1016/j.tplants.2008.10.005
- 17. Sanyal, S.K., Rao, S., Mishra, L.K., et al. (2016) Plant Stress Responses Mediated by CBL-CIPK Phosphorylation Network. Enzymes, 40, 31-64. https://doi.org/10.1016/bs.enz.2016.08.002
- 18. Zhu, J.K. (2016) Abiotic Stress Signaling and Responses in Plants. Cell, 167, 313-324. https://doi.org/10.1016/j.cell.2016.08.029
- 19. Chen, X.J., Huang, Q.S., Zhang, F., et al. (2014) ZmCIPK21, a Maize CBL-Interacting Kinase, Enhances Salt Stress Tolerance in Arabidopsis thaliana. International Journal of Molec-ular Sciences, 15, 14819-14834. https://doi.org/10.3390/ijms150814819
- 20. Hu, D.G., Ma, Q.J., Sun, C.H., et al. (2016) Overexpression of MdSOS2L1, a CIPK Protein Kinase, Increases the Antioxidant Metabolites to Enhance Salt Tolerance in Apple and To-mato. Physiologia Plantarum, 156, 201-214. https://doi.org/10.1111/ppl.12354
- 21. Pandey, G.K., Kanwar, P., Singh, A., et al. (2015) Calcineurin B-Like Pro-tein-Interacting Protein Kinase CIPK21 Regulates Osmotic and Salt Stress Responses in Arabidopsis. Plant Physiology, 169, 780-792. https://doi.org/10.1104/pp.15.00623
- 22. Luo, Q., Wei, Q., Wang, R., et al. (2017) BdCIPK31, a Calcineurin B-Like Protein-Interacting Protein Kinase, Regulates Plant Response to Drought and Salt Stress. Frontiers in Plant Sci-ence, 8, 1184. https://doi.org/10.3389/fpls.2017.01184
- 23. Kim, K.N., Lee, J.S., Han, H., et al. (2003) Isolation and Characteri-zation of a Novel Rice Ca2+-Regulated Protein Kinase Gene Involved in Responses to Diverse Signals Including Cold, Light, Cytokinins, Sugars and Salts. Plant Molecular Biology, 52, 1191-1202. https://doi.org/10.1023/B:PLAN.0000004330.62660.a2
- 24. Xiang, Y., Huang, Y. and Xiong, L. (2007) Charac-terization of Stress-Responsive CIPK Genes in Rice for Stress Tolerance Improvement. Plant Physiology, 144, 1416-1428. https://doi.org/10.1104/pp.107.101295
- 25. Huang, C.L., Ding, S., Zhang, H., et al. (2011) CIPK7 Is Involved in Cold Response by Interacting with CBL1 in Arabidopsis thaliana. Plant Science, 181, 57-64. https://doi.org/10.1016/j.plantsci.2011.03.011
- 26. Deng, X.M., Zhou, S.Y., Hu, W., et al. (2013) Ectopic Expres-sion of Wheat TaCIPK14, Encoding a Calcineurin B-Like Protein-Interacting Protein Kinase, Confers Salinity and Cold Tolerance in Tobacco. Physiologia Plantarum, 149, 367-377. https://doi.org/10.1111/ppl.12046
- 27. Yang, T., Shad Ali, G., Yang, L., et al. (2010) Calcium/Calmodulin-Regulated Receptor-Like Kinase CRLK1 Interacts with MEKK1 in Plants. Plant Signal Behavior, 5, 991-994. https://doi.org/10.4161/psb.5.8.12225
- 28. Teige, M., Scheikl, E., Eulgem, T., et al. (2004) The MKK2 Pathway Mediates Cold and Salt Stress Signaling in Arabidopsis. Mo-lecular Cell, 15, 141-152. https://doi.org/10.1016/j.molcel.2004.06.023
- 29. Sangwan, V., Orvar, B.L., Beyerly, J., et al. (2002) Opposite Changes in Membrane Fluidity Mimic Cold and Heat Stress Activation of Distinct Plant MAP Ki-nase Pathways. Plant Journal, 31, 629-638. https://doi.org/10.1046/j.1365-313X.2002.01384.x
- 30. Furuya, T., Matsuoka, D. and Nanmori, T. (2014) Mem-brane Rigidification Functions Upstream of the MEKK1-MKK2-MPK4 Cascade during Cold Acclimation in Arabidopsis thaliana. FEBS Letters, 588, 2025-2030. https://doi.org/10.1016/j.febslet.2014.04.032
- 31. Doherty, C.J., Van Buskirk, H.A., Myers, S.J. and Thomashow, M.F. (2009) Roles for Arabidopsis CAMTA Transcription Factors in Cold-Regulated Gene Expression and Freezing Tolerance. Plant Cell, 21, 972-984. https://doi.org/10.1105/tpc.108.063958
- 32. 丁杨林, 施怡婷, 杨淑华. 植物响应低温胁迫的分子机制研究[J]. 生命科学, 2015, 27(3): 398-405.
- 33. Chinnusamy, V., Zhu, J. and Zhu, J.K. (2007) Cold Stress Regulation of Gene Expression in Plants. Trends Plant Science, 12, 444-451. https://doi.org/10.1016/j.tplants.2007.07.002
- 34. Qin, F., Shinozaki, K. and Yamaguchi-Shinozaki, K. (2011) Achievements and Challenges in Understanding Plant Abiotic Stress Responses and Tolerance. Plant Cell Physiology, 52, 1569-1582. https://doi.org/10.1093/pcp/pcr106
- 35. Shi, Y.T., Ding, Y.L. and Yang, S.H. (2015) Cold Signal Transduction and Its Interplay with Phytohormones during Cold Accli-mation. Plant Cell Physiology, 56, 7-15. https://doi.org/10.1093/pcp/pcu115
- 36. Feng, X.M., Zhao, Q., Zhao, L.L., et al. (2012) The Cold-Induced Basic Helix-Loop-Helix Transcription Factor Gene MdCIbHLHl Encodes an ICE-Like Protein in Apple. BMC Plant Biology, 12, 22. https://doi.org/10.1186/1471-2229-12-22
- 37. Zhao, M.L., Wang, J.N., Shan, W., et al. (2013) Induction of Jasmonate Signalling Regulators MaMYC2s and Their Physical Interactions with MalCEl in Methyl Jasmonate-Induced Chilling Tolerance in Banana Fruit. Plant Cell and Environment, 36, 30-51. https://doi.org/10.1111/j.1365-3040.2012.02551.x
- 38. Shi, Y., Tian, S., Hou, L., Huang, X., Zhang, X., Guo, H. and Yang, S. (2012) Ethylene Signaling Negatively Regulates Freezing Tolerance by Repressing Expression of CBF and Type-A ARR Genes in Arabidopsis. Plant Cell, 24, 2578-2595. https://doi.org/10.1105/tpc.112.098640
- 39. Ding, Y., Li, H., Zhang, X., et al. (2015) OST1 Kinase Modulates Freezing Tolerance by Enhancing ICE1 Stability in Ara-bidopsis. Developmental Cell, 32, 278-289. https://doi.org/10.1016/j.devcel.2014.12.023
- 40. Liu, Z., Jia, Y., Ding, Y., et al. (2017) Plasma Membrane CRPK1-Mediated Phosphorylation of 14-3-3 Proteins Induces Their Nuclear Import to Fine-Tune CBF Signaling during Cold Response. Molecular Cell, 66, 117-128. https://doi.org/10.1016/j.molcel.2017.02.016
- 41. Li, H., Ding, Y., Shi, Y., et al. (2017) MPK3- and MPK6-Mediated ICE1 Phosphorylation Negatively Regulates ICE1 Stability and Freezing Tolerance in Arabidopsis. Developmental Cell, 43, 1-13. https://doi.org/10.1016/j.devcel.2017.09.025