Advances in Clinical Medicine
Vol.
13
No.
07
(
2023
), Article ID:
69141
,
5
pages
10.12677/ACM.2023.1371628
替雷利珠单抗免疫相关性Stevens-Johnson 综合征1例
张曼西
西安医学院研工部,陕西 西安
收稿日期:2023年6月21日;录用日期:2023年7月16日;发布日期:2023年7月21日

摘要
目的:探讨Stevens-Johnson综合征的病因、临床特征、诊断、治疗及预后。方法:报道一例使用替雷利珠单抗免疫相关性Stevens-Johnson综合征。查阅文献对Stevens-Johnson综合征的病因、临床特征、诊断、治疗及预后进行探讨分析。结果:71岁的肺腺癌患者,使用卡铂 + 白蛋白紫杉醇联合替雷利珠单抗方案化疗2周期后开始出现周身皮损及皮疹、红斑,头面部为著,眼睑及角膜粘膜可见充血红染,有水泡生成,破溃后有黄色组织液流出,1日前出现发热,且体温波动于38℃至38.5℃间,在停止替雷利珠单抗及对症支持治疗后患者的症状得到改善。结论:替雷利珠单抗已被广泛用于各种癌症的治疗,但需要注意的是,这些药物可能会导致免疫相关不良反应。
关键词
Stevens-Johnson综合征,替雷利珠单抗,免疫相关

A Case of Tislelizumab Immune-Related Stevens-Johnson Syndrome
Manxi Zhang
Research and Engineering Department, Xi’an Medical University, Xi’an Shaanxi
Received: Jun. 21st, 2023; accepted: Jul. 16th, 2023; published: Jul. 21st, 2023

ABSTRACT
Objective: To investigate the etiology, clinical features, diagnosis, treatment and prognosis of Stevens-Johnson syndrome. Methods: To report a case of immune-related Stevens-Johnson syndrome with Tislelizumab. The literature was reviewed to discuss and analyze the etiology, clinical features, diagnosis, treatment, and prognosis of Stevens-Johnson syndrome. Results: A 71-year-old patient with lung adenocarcinoma treated with carboplatin + albumin paclitaxel in combination with Tislelizumab for 2 cycles started to develop peripheral skin lesions and rash and erythema, mainly on the head and face, with congestion and red staining of the eyelids and corneal mucosa, with blister formation and yellow tissue fluid flow after rupture, and a fever that fluctuated between 38˚C and 38.5˚C 1 day ago. The patient’s symptoms improved after discontinuation of Tislelizumab and symptomatic supportive therapy. Conclusion: Tislelizumab has been widely used in the treatment of various cancers, but it is important to note that these drugs may cause immune-related adverse reactions.
Keywords:Stevens-Johnson Syndrome, Tislelizumab, Immune-Related

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
越来越多的免疫检查点抑制剂(Immune Checkpoint Inhibitors, ICIs)被批准用于各种肿瘤治疗,然而,随着临床实践中ICIs的广泛应用,但其导致的副作用也不容小觑,即免疫相关不良事件(irAEs) [1] 。与标准化疗或其他生物药物相比,ICIs具有不同的毒性谱,大多数毒性是由于对正常器官的过度免疫 [2] ,超过三分之二的肿瘤免疫治疗相关的irAEs报告病例与ICIs相关。现将1例替雷利珠单抗引起的Stevens-Johnson综合征报道如下。
2. 临床资料
患者,男,71岁,以“确诊肺癌1月余,化疗2周期后,皮疹3日,发热1日”为主诉入院。于2022年7月7日前往陕西省某医院就诊,2022年7月16日患者开始出现周身皮损及皮疹、红斑,头面部为著(见图1,图2),眼睑及角膜粘膜可见充血红染,有水泡生成,破溃后有黄色组织液流出,1日前出现发热,体温波动于38℃至38.5℃间,起病后予以氯雷他定片10 mgpoqd治疗,效果欠佳。既往无过敏史。2022年5月30日在我院确诊为左肺下叶鳞状细胞癌,遂予以卡铂 + 白蛋白紫杉醇联合替雷利珠单抗方案化疗2周期(卡铂500 mg d1ivgtt、白蛋白紫杉醇400 mg d1 ivgtt、替雷利珠单抗200 mgd1 ivgtt),末次使用时间2022年7月12日。化疗及免疫治疗期间复查血常规、肝功能、肾功能、凝血功能等均未见异常。入院后行相关检查:单核细胞计数0.03 (109/L),红细胞2.81 (1012/L),血红蛋白87 (g/L),红细胞压积25.60 (%),白细胞0.43 (109/L),血小板38 (109/L),平均血小板体积13.9 (fl),血小板压积0.053 (%),大型血小板比率56.2 (%),大血小板细胞数22,C反应蛋白194.65 (mg/L),中性粒细胞百分比25.4 (%),超敏C反应蛋白 > 10 (mg/L),淋巴细胞百分比68.2 (%),中性粒细胞计数0.11 (109/L),淋巴细胞计数0.29 (109/L),嗜酸性粒细胞计数0.00 (109/L),电解质,肝功,肾功能:前白蛋白103.55 (mg/dL),总胆固醇1.51 (mmol/L),尿素11.39 (mmol/L),肌酐157 (umol/L),血清5’核苷酸酶测定1 (U/L),胆碱酯酶2829 (U/L),β2-微球蛋白7.19 (mg/L),视黄醇结合蛋白13.84 (mg/L),胱抑素C 2.39 (mg/L),钾2.52 (mmol/L),氯94.9 (mmol/L),钙1.77 (mmol/L),磷0.66 (mmol/L),镁0.54 (mmol/L),甘氨酰脯氨酸二肽氨基肽酶39 (U/L),谷胱甘肽还原酶98.2 (U/L),葡萄糖7.87 (mmol/L),直接胆红素8.21 (umol/L),总蛋白54.20 (g/L),白蛋
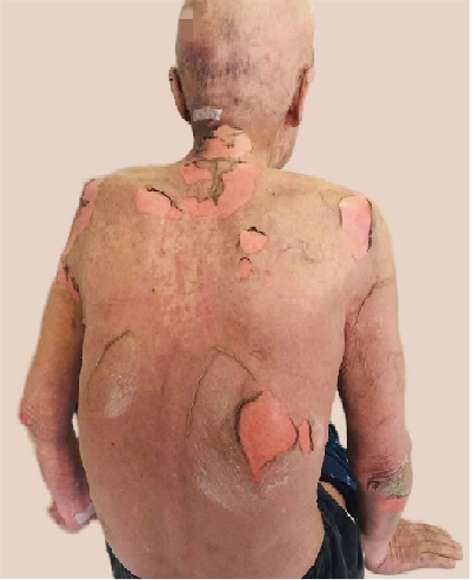
Figure 1. Large erythematous rash and lesions on the back
图1. 背部大片红斑疹及皮损
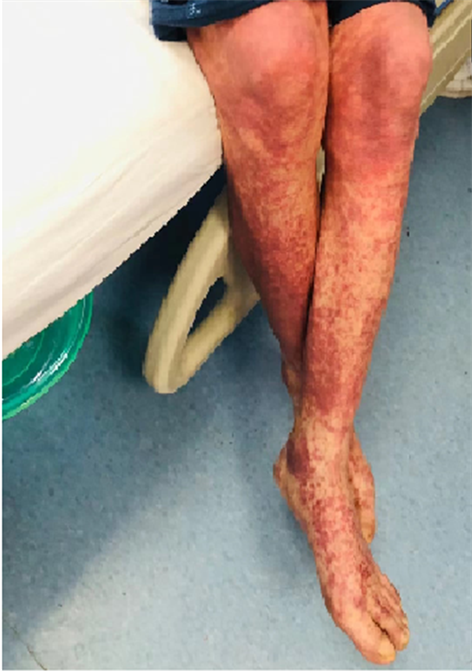
Figure 2. Large dark red rash on both lower extremities
图2. 双下肢出现大片暗红色皮疹
白25.40 (g/L),乳酸脱氢酶598 (U/L)。遂给予沙棘油及激素治疗并给予胃粘膜保护剂,但因沙棘油治疗效果不明且该病有黏膜侵袭可能,遂请皮肤科会诊,考虑为替雷利珠单抗相关免疫副作用,即Stevens-Johnson综合征(SJS),建议立即停用替雷利珠单抗治疗,应足量应用糖皮质激素及注射免疫球蛋白(IVIG)。2天后复查血常规:血常规:白细胞1.51 (109/L),中性粒细胞计数0.83 (109/L),淋巴细胞计数0.56 (109/L),白细胞较前恢复,但患者拒绝后续治疗要求转入专科医院就治。1月后电话随访,皮肤已恢复正常状态,但仍伴有大片色素沉着。
3. 讨论
肺癌每年造成全球209万人死亡 [3] ,发病率高,死亡率高。以含铂类药物为基础的化疗药一直是治疗晚期非小细胞肺癌的一线方案。但具有不可避免的毒副作用。免疫检查点抑制剂(Immune checkpoint inhibitors, ICls)被广泛用于各种癌症的治疗,如转移性黑色素瘤、非小细胞肺癌(NSCLC)、肾细胞癌(RCC)和尿路上皮癌(UC)等。越来越多的PD-1/PD-L1 ICIs已被FDA批准为多种癌症的一线治疗方法。然而,抑制这种相互作用并非是没有代价的,ICIs治疗中的自身炎症毒性发生率逐年增加,据报道,60%的患者中存在肺炎、内分泌、心脏、眼部、肾脏和皮肤的炎症 [4] ,而最常见的免疫相关性毒副反应(immune-related adverse events, irAEs)是皮肤毒性。
Stevens-Johnson (SJS)综合征是一种皮肤黏膜相关的不良反应,被认为是一种以表皮脱离为特征的复杂的迟发性超敏反应,虽然很罕见,但死亡率接近30% [5] 。其特征是流感样症状,包括发烧、厌食、不适,随后出现红斑疹,表现为暗红色、紫癜性斑点,对称地分散在颈部、躯干、四肢和近端四肢。随后扩散到整个体表区域,发展为松弛性水泡,其特征是表皮脱离,并与口腔、眼部和生殖器粘膜的炎症和疼痛有关 [6] 。SJS/TEN的发病机制尚不清楚,已知ICIs通过抑制PD-1/PD-L1和细胞毒性T淋巴细胞相关蛋白CTLA-4通路来触发CD4+/CD8+ T细胞的激活。免疫检查点分子功能的中断导致免疫耐受不稳定,并最终导致不良事件 [7] 。该患者确诊肺腺癌1月余,确诊后予以卡铂和白蛋白紫杉醇联合替雷利珠单抗2周期后,第3天出现全身皮肤反应,并在停药及系统使用类固醇后症状得到缓解。国外相关报道纳武利尤单抗所致皮疹在服药的第4个月出现,最近一次使用在就诊前1周,体格检查发现红斑斑块覆盖了较大范围的体表区域,手臂、躯干、腿部和面部均有破溃,与该病例相似 [8] 。虽然美国食品药物管理局(U.S. Food and Drug Administration, FDA)发布的白蛋白紫杉醇不良反应中有SJS/TEN,但国内外大量报道证实免疫抑制剂与该病的发生更为密切。例如姚宇雄等人 [9] 报道一例替雷利珠单抗治疗上尿路上皮癌的病例,此患者出现腹部、臀部等多处皮肤对称泛发性分布红斑及丘疹、瘀点及瘀斑,与该患者相似。虽然不能完全证实替雷利珠单抗导致SJS,但根据国内外不良反应因果判断原则及评价方法即诺氏(Naranjo’s)评估量表法分析,在该不良反应出现前使用过可疑药物,且停用后不良反应有所改善,不良反应的轻重程度与可疑药物剂量变化有关等,替雷利珠单抗与SJS的相关性为5分,评定为很可能,即具有客观证据或定量检测结果支持。
结合国外相关文献,对SIS/TEN的治疗首先应尽早停用免疫抑制剂并使用含银敷料以减少感染的风险,并提高环境温度来减少由于表皮受损而导致的低热症的风险,建议立即转诊到烧伤中心,也可预防性使用抗生素治疗,一旦用免疫疗法建立了再上皮化,就应开始减少类固醇的使用。对于类固醇难治性糜烂、静脉注射免疫球蛋白(IVIG) [10] 、血浆透析和口服环孢素都能有效阻止疾病的进展 [11] 。且在高度怀疑SJS/TEN时,必须使用1%利多卡因溶液 [12] 、制霉菌素、0.1%醋酸软膏 [13] ,皮肤裸露的患者应及时入住重症监护病房。但该病的治疗原则并无统一标准,根据中国Stevens-Johnson综合征/中毒性表皮坏死松解症诊疗专家共识 [14] ,早期足量系统应用糖皮质激素控制病情进展,对于中重度SJS/TEN患者,可给予1.5~2 mg∙kg−1∙d−1起始量(泼尼松当量),一般7~10 d,控制病情后可逐渐减量。推荐IVIG的剂量为400 mg∙kg−1∙d−1,连用3~5 d;环孢素可单独用于SJS/TEN的治疗,推荐剂量3~5 mg∙kg−1∙d−1。同时TNF-α拮抗剂也可单用或与糖皮质激素联用,并尽早使用 [15] 。但该患者入院时,肝肾功、血常规、凝血各项指标都存在异常,此时使用激素治疗是否会增加发生败血症的几率,Kojiro Morita等人 [16] 发现SJS/TEN患者早期全身使用皮质类固醇与住院死亡率的改善无关,但K. Kridin等人 [17] 认为全身使用糖皮质激素能够降低感染风险,使患者受益。
综上所述,对于此类患者应警惕ICIs所带来的不良反应,并且及时诊断及治疗显得至关重要。多学科诊疗也应及时在各个学科基础上为病人制定出最佳治疗模式,使病人获得最大受益。
文章引用
张曼西. 替雷利珠单抗免疫相关性Stevens-Johnson综合征1例
A Case of Tislelizumab Immune-Related Stevens-Johnson Syndrome[J]. 临床医学进展, 2023, 13(07): 11633-11637. https://doi.org/10.12677/ACM.2023.1371628
参考文献
- 1. Ramos-Casals, M., Brahmer, J.R., Callahan, M.K., et al. (2020) Immune-Related Adverse Events of Checkpoint Inhibi-tors. Nature Reviews Disease Primers, 6, Article No. 38. https://doi.org/10.1038/s41572-020-0160-6
- 2. Bhardwaj, M., Chiu, M.N. and Pilkhwal, S.A.H.S. (2022) Ad-verse Cutaneous Toxicities by PD-1/PD-L1 Immune Checkpoint Inhibitors: Pathogenesis, Treatment, and Surveillance. Cutaneous and Ocular Toxicology, 41, 73-90. https://doi.org/10.1080/15569527.2022.2034842
- 3. Xia, C., Dong, X., Li, H., et al. (2022) Cancer Statistics in China and United States, 2022: Profiles, Trends, and Determinants. Chinese Medical Journal (England), 135, 584-590. https://doi.org/10.1097/CM9.0000000000002108
- 4. Baxi, S., Yang, A., Gennarelli, R.L., et al. (2018) Im-mune-Related Adverse Events for Anti-PD-1 and Anti-PD-L1 Drugs: Systematic Review and Meta-Analysis. BMJ, 360, k793. https://doi.org/10.1136/bmj.k793
- 5. Dodiuk-Gad, R.P., Chung, W.H., Valeyrie-Allanore, L., et al. (2015) Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: An Update. American Journal of Clinical Dermatology, 16, 475-493. https://doi.org/10.1007/s40257-015-0158-0
- 6. Riano, I., Cristancho, C. and Treadwell, T. (2020) Ste-vens-Johnson Syndrome-Like Reaction after Exposure to Pembrolizumab and Recombinant Zoster Vaccine in a Patient with Metastatic Lung Cancer. Journal of Investigative Medicine High Impact Case Reports, 8. https://doi.org/10.1177/2324709620914796
- 7. Fessas, P., Possamai, L.A., Clark, J., et al. (2020) Immunotoxici-ty from Checkpoint Inhibitor Therapy: Clinical Features and Underlying Mechanisms. Immunology, 159, 167-177. https://doi.org/10.1111/imm.13141
- 8. Lu, J., Thuraisingam, T., Chergui, M., et al. (2019) Nivolumab-Associated DRESS Syndrome: A Case Report. JAAD Case Reports, 5, 216-218. https://doi.org/10.1016/j.jdcr.2018.11.017
- 9. 姚雄宇, 董秀哲, 侯国军, 等. 替雷利珠单抗免疫相关性Stevens-Johnson综合征1例[J]. 中国医院药学杂志, 2023, 43(2): 237-238.
- 10. Storandt, M.H. and Seth, R. (2021) A Case of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis in a Patient Receiving Chemo-Immunotherapy with Pemetrexed and Pembrolizumab. Current Problems in Cancer: Case Reports, 3, Article ID: 100048. https://doi.org/10.1016/j.cpccr.2020.100048
- 11. Saw, S., Lee, H.Y. and Ng, Q.S. (2017) Pembrolizumab-Induced Stevens-Johnson Syndrome in Non-Melanoma Patients. European Jour-nal of Cancer, 81, 237-239. https://doi.org/10.1016/j.ejca.2017.03.026
- 12. Dasanu, C.A. (2019) Late-Onset Ste-vens-Johnson Syndrome Due to Nivolumab Use for Hepatocellular Carcinoma. Journal of Oncology Pharmacy Practice, 25, 2052-2055. https://doi.org/10.1177/1078155219830166
- 13. Gracia-Cazaña, T., Padgett, E., Calderero, V., et al. (2021) Nivolumab-Associated Stevens-Johnson Syndrome in a Patient with Lung Cancer. Dermatology Online Journal, 27, Article No. 13. https://doi.org/10.5070/D3273052777
- 14. Adverse Drug Reaction Research Center of Chinese Society of Dermatology (2021) Expert Consensus on the Diagnosis and Treatment of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Chinese Journal of Dermatology, 54, 376-381.
- 15. Zhang, S., Tang, S., Li, S., et al. (2020) Bi-ologic TNF-alpha Inhibitors in the Treatment of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Sys-temic Review. Journal of Dermatological Treatment, 31, 66-73. https://doi.org/10.1080/09546634.2019.1577548
- 16. Morita, K., Matsui, H., Michihata, N., et al. (2019) Associa-tion of Early Systemic Corticosteroid Therapy with Mortality in Patients with Stevens-Johnson Syndrome or Toxic Epi-dermal Necrolysis: A Retrospective Cohort Study Using a Nationwide Claims Database. American Journal of Clinical Dermatology, 20, 579-592. https://doi.org/10.1007/s40257-019-00443-9
- 17. Kridin, K., Bruggen, M.C., Chua, S.L., et al. (2021) Assessment of Treatment Approaches and Outcomes in Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Insights from a Pan-European Multicenter Study. JAMA Dermatology, 157, 1182-1190. https://doi.org/10.1001/jamadermatol.2021.3154