Advances in Clinical Medicine
Vol.
13
No.
09
(
2023
), Article ID:
72722
,
8
pages
10.12677/ACM.2023.1392079
基于三维可视化胆道解剖分型与临床意义
杨新涛1,闫军2*
1青海大学附属医院肝胆胰外科,青海 西宁
2北京清华长庚医院肝胆胰中心,北京
收稿日期:2023年8月19日;录用日期:2023年9月14日;发布日期:2023年9月20日

摘要
基于计算机手术辅助规划系统(CASP)将术前胆道CT二维图像进行三维重建,观察了解肝内胆管的分支模式和变异类型。根据右后段胆管(RPSD)右前段胆管(RASD)和左肝管(LHD)的汇合,以及是否存在附属肝管,对胆道进行分类。且操作方便,弥补二维成像不能立体显示肿瘤、胆道间的相互关系。预测术中可能出现的各种因解剖变异而发生的各种情况。现综述其在临床上的进展,希望为临床应用及进一步研究提供新思路。
关键词
三维,成像,胆道解剖分型,综述

Anatomical Classification of Biliary Tract Based on Three-Dimensional Visualization and Its Clinical Significance
Xintao Yang1, Jun Yan2*
1Department of Hepatobiliary and Pancreatic Surgery, Affiliated Hospital of Qinghai University, Xining Qinghai
2Hepatobiliary and Pancreatic Center, Tsinghua Chang Gung Hospital, Beijing
Received: Aug. 19th, 2023; accepted: Sep. 14th, 2023; published: Sep. 20th, 2023

ABSTRACT
Based on the computer aided surgical planning system (CASP), the preoperative CT two-dimensional images of biliary tract were reconstructed, and the branching patterns and variation types of intrahepatic bile duct were observed. According to the confluence of right posterior bile duct (RPSD), right anterior bile duct (RASD) and left hepatic duct (LHD), and whether there is accessory hepatic duct, biliary tract is classified. And the operation is convenient, which makes up for the fact that two-dimensional imaging can’t display the relationship between tumor and biliary tract stereoscopically, and can predict various situations that may occur during operation due to anatomical variation. This paper reviews its clinical progress, hoping to provide new ideas for clinical application and further research.
Keywords:Three Dimensions, Imaging, Anatomical Classification of Biliary Tract, Summary

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
胆道树的解剖结构是众所周知的变化。这种变化是肝胆外科医生的祸根,对他们来说,了解胆道解剖变化是规划和安全进行肝脏手术的关键,从肿瘤切除到分肝移植。了解胆道解剖的常见变体对普通外科医生也很重要,他们在职业生涯中不可避免地会遇到胆囊切除术中胆道结构的广泛变化。胆道解剖 [1] 的变异已被证明具有临床意义,准确了解这些解剖变异对于外科医生避免并发症至关重要。一些报告描述了胆道的解剖学,包括对人类胆道系统的不同方式的研究,如尸检标本、内窥镜逆行胆胰管造影术(ERCP)、磁共振胆胰管造影术(MRCP)、经皮经肝胆道穿刺造影术(PTCD)、导管内超声检查(IDUS)、CT胆道造影等以及对不同分类系统的胆道解剖的详细描述。然而尸检对于活体并不现实,ERCP是胆道造影的黄金标准。然而,这种侵入性技术可能会导致严重的并发症,如急性胰腺炎、穿孔和出血。MRCP虽然为非侵袭性、安全的技术且能提供较详细的胆管分支解剖形态图。但是对于胆管内异常信号无特异性,包括胆石、血块、肿瘤组织及流空效应的气体,不能区别,图像的空间分辨率有待进一步提高。PTCD容易并发胆道感染,胆汁漏。IDUS容易受气体的影响。CT胆道造影的造影剂可能对患者造成严重的过敏性休克。三维可视化则可将二维CT转化为三维图像,通过旋转、放大、对每一个肝段的增加,可以清晰明确的看到胆道引流的肝段,及有无胆道及血管的变异 [2] [3] 。
2. 三维可视化技术
三维可视化技术是基于CT图像数据的重建 [4] 。重建后的三维模型可以提供更直观、更全面的肝实质和肝内管道系统的信息,并且可以调整不同的透明度和旋转角度,使外科医生可以从多个方向和角度观察胆道的解剖结构。可以准确识别胆道的解剖变异和病变位置。三维可视化软件可将肝脏实质、血管系统、胆管系统置于同一空间,立体显示肝脏、肝内血管、胆管与病变的空间位置关系,对肝胆外科手术具有重要的指导意义 [5] 。此外,随着虚拟现实技术和3D打印技术的发展,将重建的3D模型应用于术前模拟操作,使实际操作更安全,创伤更小。胆道阻塞性疾病导致肝内、肝外胆管扩张,CT图像上充分显示胆管系统,与其他肝脏组织的CT值有明显不同。基于这一成像特点,可以使用CAS系统重建一个完整的胆管系统三维模型 [6] 。其隐藏和透明功能可用于立体显示胆道系统。通过旋转和放大,可以从各个角度观察胆道系统的解剖结构,识别胆道的变异情况 [7] 。
技术方法(图1)
① 收集有胆道梗阻疾病患者的三期增强CT,将其影像号输入EDDA (美国)系统后,点击提取按钮,病人的三期影像会自动上传导EDDA系统。
② 进入系统后分别选择显影较好的静脉期、动脉期、和延迟期。厚度一般为1.25 mm~1.5 mm选择完成后点击完成,即可进入三维界面。在系统操作窗口中选择重建模式,选择器官重建,进入器官三维重建的第一步页面。
③ 在CT影像上一层一层将肝脏轮廓画出来,点击完成即可自动生成肝脏三维界面。
④ 肝脏三维生成后,点击胆道三维重建,系统可以自动生成,但大多数比较模糊,需要自己去调整,将每一层扩张的胆道都进行划线,划线完成后即可生成完整的胆道三维重建图像。
⑤ 肝脏和胆道三维重建完成后,在左侧双击病灶,对病灶进行分割,三维描边,即可在肝脏上生成病灶。对重建的图像进行旋转,透明化等一系列处理,即可看到胆道对各个肝段的引流,以及胆道与病灶之间的空间分布关系。
⑥ 确认“完成”选项,结束胆道的三维重建回到系统操作窗口的初始界面。即可获得比较满意的三维胆道图像。
3. 胆道分型
传统上,肝脏手术依赖Couinaud的肝段分类。右前段肝管引流V和VIII段,右后段肝管引流VI和VII段。右肝管是由前、后肝管融合而成。左肝管(B2~B4)引流肝段II、III和IV [8] [9] 。
3.1. 右肝右叶汇合的四种类型(见图1)
1型,最常见的形式,被定义为右后导管排入右前导管形成右肝胆管;2型,其中右后、右前和左肝胆管的三叉在同一点汇合形成共同的肝管;3型,右后胆管引流入左胆管;类型4,其中右后导管直接排入肝总管 [2] [10] 。
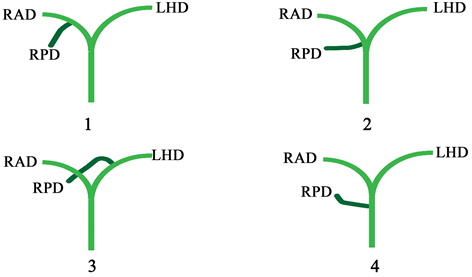
Figure 1. RAD: right anterior bile duct; RPD: right posterior bile duct; LHD: left hepatic duct
图1. RAD:右前段胆管;RPD:右后段胆管;LHD:左肝管
3.2. 根据Nakamura和Varotti等人的分类和对胆管树解剖变异的分类(见图2)
1型,经典型,为右后段肝管汇入右前段肝管形成右肝管,与左肝管汇合成肝总管;2型,其中右后、右前和左肝胆管的三叉在同一点汇合形成共同的肝管;3a型,右前段肝管引流入左胆管;3b型右后段肝管引流入左肝管;4a型:右前段肝管汇入肝总管;4b型右后段肝管汇入肝总管 [11] [12] [13] [14] 。
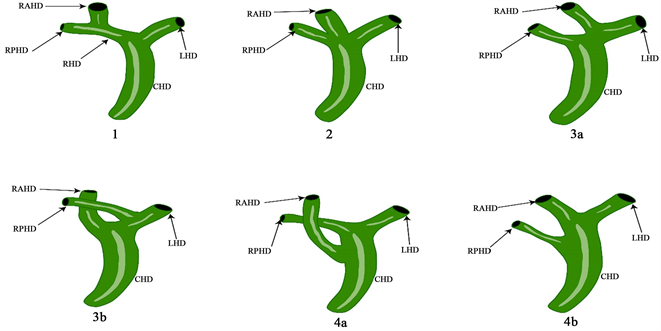
Figure 2. RAHD: right anterior bile duct; RPHD: right posterior bile duct; RHD: right hepatic duct; LHD: left hepatic duct; CHD: common hepatic duct
图2. RAHD:右前段胆管;RPHD:右后段胆管;RHD:右肝管;LHD:左肝管;CHD:肝总管
3.3. 基于韩国连续300例活体肝移植供体术中胆管造影分析(见图3)
类型1是经典型。类型2包括三个汇流,即RASD、RPSD和LHD同时排入CHD。在3型,RPSD异常引流,而在4型,RHD引流到胆囊管。在类型5中,存在副导管。在类型6中,II段和III段分别排入RHD或CHD [15] [16] 。
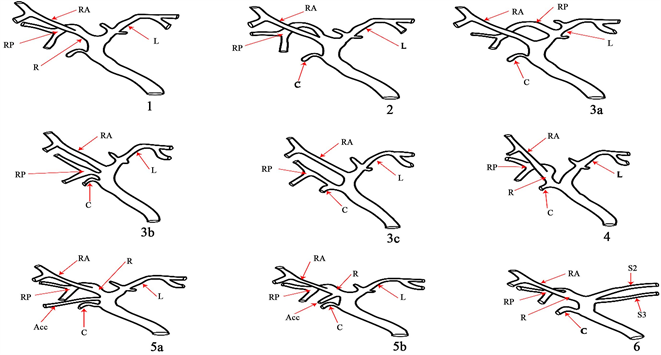
Figure 3. RA: right anterior hepatic duct; RP: right posterior hepatic duct; R: right hepatic duct: L: left hepatic duct: C: cystic duct; ACC: accessory hepatic duct
图3. RA:右前肝管;RP:右后肝管;R:右肝管:L:左肝管:C:胆囊管;ACC:副肝管
3.4. 左肝左叶胆管汇合型可分为三种主要类型(见图4)
A型,其中B2和B3的共同干汇入B4;B型,显示B2、B3和B4的三重汇流;C型,B2与B3和B4的共同干汇合。据报道,97%的病例左肝管位于门静脉上方。Kawarada等人报道,141例肝脏标本中有35.5%的B4在靠近肝门汇合处与左肝管汇合,对于肝门胆管癌患者需要进行扩大肝切除或对于接受右肝叶切除术的患者,术前识别这些分支模式对避免胆管损伤至关重要 [17] [18] [19] 。
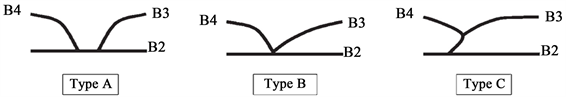
Figure 4. B2: Drainage in the bile duct of the second segment of the left lobe of liver; B3: Drainage of bile duct in the left lobe of liver; B4: Drainage of bile duct in the left lobe of liver
图4. B2:引流肝左叶2段胆管内;B3:引流肝左叶3段胆管;B4:引流肝左叶4段胆管
3.5. Huang等人根据节段性胆汁引流及其频率将左肝导管分为六种类型(见图5)
B1型:引流II段和III段的胆管汇合后分别与IV段和I汇合形成左肝管;B2型:引流II段和III的胆管汇合后与I段形成左肝管,引流IV段的肝管汇入右肝管。B3型:引流II段和III段的胆管汇合后与I段胆管汇合形成左肝管,IV段肝管引流入右前肝管。B4型:IV段和I段肝管汇合后直接引流入肝总管;B5型:I段II段和IV段肝管汇合后直接引流入肝总管;B6型:引流II段和III段的胆管汇合后与IV段形成左肝管,I段单独引流入肝总管 [20] [21] 。
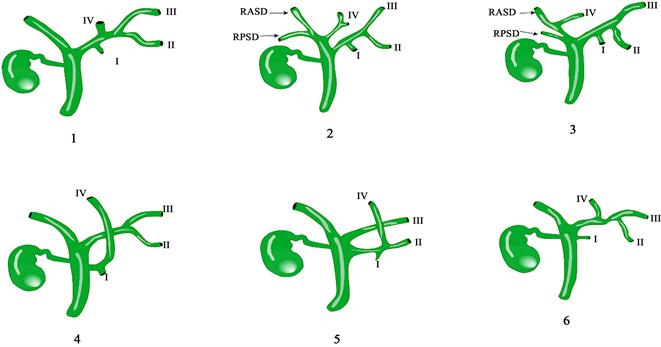
Figure 5. RASD: right anterior bile duct; RPSD: right posterior bile duct; I, II, III and IV drain the bile duct of segment I, II, III and IV of liver respectively
图5. RASD:右前胆管;RPSD:右后胆管;I、II、III、IV分别引流肝I、II、III、IV段胆管
4. 门静脉解剖与胆管变异的相关性
4.1. 根据美国407例活体供肝门静脉解剖与胆管变异的相关性研究
1) 根据RPBD与门静脉的解剖关系,将右侧肝内BD的汇合型分为门静脉上型和门下型(见图6)。
2) 变异型BD的存在与变异型PV的相关性比与正常PV的相关性更高。相比之下,变异HA的存在与变异BD无关。
3) Shimizu等人报道,肝门部胆管癌伴门下RPBD的患者比门静脉上RPBD的患者有更大的负切缘和更安全的重建 [22] [23] 。
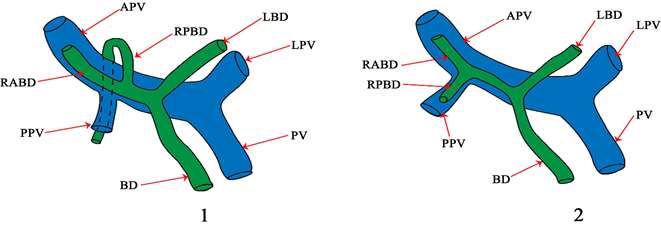
Figure 6. PV: portal vein; LPV: left branch of portal vein; APV: right anterior branch of portal vein; PPV: right posterior branch of portal vein; BD: common bile duct; LBD: left bile duct; RABD: right anterior bile duct; RPBD: right posterior bile duct
图6. PV:门静脉;LPV:门静脉左支;APV:门静脉右前支;PPV:门静脉右后支;BD:胆总管;LBD:左胆管;RABD:右前胆管;RPBD:右后胆管
4.2. 三维可视化技术对于门静脉变异的识别
三维可视化指导下门静脉变异的手术规划正常型门静脉情况下,胆管分离极限点(U点:指门静脉左支水平部与矢状部的转角处;P点:在门静脉右前支、右后支分叉部)是指肝切除术中胆管能从并行的门静脉及肝动脉中剥离出来的极限部位,在这个极限点上游的胆管不能单独分离和切断。通过对极限点(P、U)进行三维可视化分析,可以立体、全方位的观察正常型门静脉、各型变异门静脉P、U点(见图7),从而指导手术方案的制定和精准手术 [24] 。
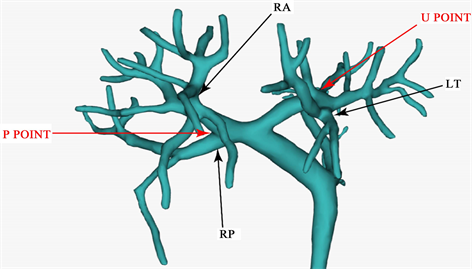
Figure 7. U point: refers to the corner between the horizontal part and the sagittal part of the left branch of portal vein; Point P: at the bifurcation of right anterior branch and right posterior branch of portal vein; RA: right anterior branch of portal vein; RP: right posterior branch of portal vein
图7. U点:指门静脉左支水平部与矢状部的转角处;P点:在门静脉右前支、右后支分叉部;RA:门静脉右前支;RP:门静脉右后支
5. 讨论
肝脏有四组导管系统,每组都有复杂的结构,其中胆道的解剖变异最多。术前了解这些变化的模式可能有助于预防和处理手术过程中的胆道损伤。可以使用多种用于评估体内胆道的成像方式。ERCP和PTC作为此目的的金标准,可以准确显示胆道的走行;然而,它们很少在术前使用,因为它们是侵入性方法并且容易出现急性胰腺炎和胆管炎等并发症。作为一种非侵入性方法,CT被用作胆道系统术前评估的有用方式,数据采集相对简单方便。此外,胆道解剖学的术前评估在指导手术中起着关键作用。本研究基于CT图像数据,利用CAS系统建立胆道三维模型,识别变异类型,有助于进行术前模拟程序,制定最佳手术方案,提高手术效果,实现手术的准确性和安全性。
文章引用
杨新涛,闫 军. 基于三维可视化胆道解剖分型与临床意义
Anatomical Classification of Biliary Tract Based on Three-Dimensional Visualization and Its Clinical Significance[J]. 临床医学进展, 2023, 13(09): 14855-14862. https://doi.org/10.12677/ACM.2023.1392079
参考文献
- 1. Li, X., Duan, R., He, Y., et al. (2022) Application of Three-Dimensional Visualization Technology in the Anatomical Variations of Hilar Bile Ducts in Chinese Population. Frontiers in Surgery, 9, Article 934183. https://doi.org/10.3389/fsurg.2022.934183
- 2. Cucchetti, A., Peri, E., Cescon, M., et al. (2011) Anatomic Varia-tions of Intrahepatic Bile Ducts in a European Series and Meta-Analysis of the Literature. Journal of Gastrointestinal Surgery, 15, 623-630. https://doi.org/10.1007/s11605-011-1447-4
- 3. Nakamura, T., Tanaka, K., Kiuchi, T., et al. (2002) Anatomical Variations and Surgical Strategies in Right Lobe Living Donor Liver Transplantation: Lessons from 120 Cases. Trans-plantation, 73, 1896-1903. https://doi.org/10.1097/00007890-200206270-00008
- 4. 曾宁. 肝门部胆管癌三维可视化诊治平台的构建及临床应用研究[D]: [博士学位论文]. 广州: 南方医科大学, 2017.
- 5. Fang, C., An, J., Bruno, A., et al. (2020) Consensus Recommendations of Three-Dimensional Visualization for Diagnosis and Management of Liver Diseases. Hepatology International, 14, 437-453. https://doi.org/10.1007/s12072-020-10052-y
- 6. Chen, H., He, Y. and Jia, W. (2020) Precise Hepatectomy in the Intelligent Digital Era. International Journal of Biological Sciences, 16, 365-373. https://doi.org/10.7150/ijbs.39387
- 7. 方驰华, 刘允怡, 曾宁, 等. 肝门部胆管癌三维可视化精准诊治中国专家共识(2019版) [J]. 中国实用外科杂志, 2020, 40(03): 260-266. https://doi.org/10.19538/j.cjps.issn1005-2208.2020.03.06
- 8. Hyodo, T., Kumano, S., Kushihata, F., et al. (2012) CT and MR Cholangiography: Advantages and Pitfalls in Perioperative Evaluation of Biliary Tree. The British Journal of Radiology, 85, 887-896. https://doi.org/10.1259/bjr/21209407
- 9. Limanond, P., Raman, S., Ghobrial, R., et al. (2004) The Utility of MRCP in Preoperative Mapping of Biliary Anatomy in Adult-to-Adult Living Related Liver Transplant Donors. Journal of Magnetic Resonance Imaging, 19, 209-215. https://doi.org/10.1002/jmri.10446
- 10. Kim, H., Kim, M., Lee, S., et al. (2002) Normal Structure, Variations, and Anomalies of the Pancreaticobiliary Ducts of Koreans: A Nationwide Cooperative Prospective Study. Gastrointestinal Endoscopy, 55, 889-896. https://doi.org/10.1067/mge.2002.124635
- 11. Chaib, E., Kanas, A.F., Galvão, F.H.F. and D’Albuquerque, L.A.C. (2014) Bile Duct Confluence: Anatomic Variations and Its Classification. Surgical and Radiologic Anatomy, 36, 105-109. https://doi.org/10.1007/s00276-013-1157-6
- 12. Deka, P., Islam, M., Jindal, D., et al. (2014) Analysis of Biliary Anatomy according to Different Classification Systems. Indian Journal of Gastroenterology, 33, 23-30. https://doi.org/10.1007/s12664-013-0371-9
- 13. Gazelle, G., Lee, M. and Mueller, P. (1994) Cholangiographic Segmental Anatomy of the Liver. RadioGraphics, 14, 1005-1013. https://doi.org/10.1148/radiographics.14.5.7991810
- 14. Kashyap, R., Bozorgzadeh, A., Abt, P., et al. (2008) Stratifying Risk of Biliary Complications in Adult Living Donor Liver Transplantation by Magnetic Resonance Cholan-giography. Transplantation, 85, 1569-1572. https://doi.org/10.1097/TP.0b013e31816ff21f
- 15. Choi, J., Kim, T., Kim, K., et al. (2003) Anatomic Variation in Intrahepatic Bile Ducts: An Analysis of Intraoperative Cholangiograms in 300 Consecutive Donors for Living Donor Liver Transplantation. Korean Journal of Radiology, 4, 85-90. https://doi.org/10.3348/kjr.2003.4.2.85
- 16. Sharma, V., Saraswat, V.A., Baijal, S.S. and Choudhuri, G. (2008) Anatomic Variations in Intrahepatic Bile Ducts in a North In-dian Population. Journal of Gastroenterology and Hepatology, 23, e58-e62. https://doi.org/10.1111/j.1440-1746.2008.05418.x
- 17. Abou-Khalil, J. and Bertens, K. (2019) Embryology, Anatomy, and Imaging of the Biliary Tree. The Surgical Clinics of North America, 99, 163-174. https://doi.org/10.1016/j.suc.2018.12.005
- 18. Sasaki, R., Kondo, T., Oda, T., et al. (2011) Impact of Three-Dimensional Analysis of Multidetector Row Computed Tomography Cholangioportography in Operative Planning for Hilar Cholangiocarcinoma. American Journal of Surgery, 202, 441-448. https://doi.org/10.1016/j.amjsurg.2010.06.034
- 19. Kim, R., Sakamoto, S., Haider, M., et al. (2005) Role of Mag-netic Resonance Cholangiography in Assessing Biliary Anatomy in Right Lobe Living Donors. Transplantation, 79, 1417-1421. https://doi.org/10.1097/01.TP.0000159793.02863.D2
- 20. Kishi, Y., Imamura, H., Sugawara, Y., et al. (2010) Evaluation of Donor Vasculobiliary Anatomic Variations in Liver Graft Procurements. Surgery, 147, 30-39. https://doi.org/10.1016/j.surg.2009.06.017
- 21. Reichert, P., Renz, J., D’albuquerque, L., et al. (2000) Surgical Anatomy of the Left Lateral Segment as Applied to Living-Donor and Split-Liver Transplantation: A Clinicopathologic Study. Annals of Surgery, 232, 658-664. https://doi.org/10.1097/00000658-200011000-00007
- 22. Takeishi, K., Shirabe, K., Yoshida, Y., et al. (2015) Correlation between Portal Vein Anatomy and Bile Duct Variation in 407 Living Liver Donors. American Journal of Transplantation, 15, 155-160. https://doi.org/10.1111/ajt.12965
- 23. Kitami, M., Takase, K., Murakami, G., et al. (2006) Types and Frequencies of Biliary Tract Variations Associated with a Major Portal Venous Anomaly: Analysis with Multi-Detector Row CT Cholangiography. Radiology, 238, 156-166. https://doi.org/10.1148/radiol.2381041783
- 24. Endo, I., Shimada, H., Sugita, M., et al. (2007) Role of Three-Dimensional Imaging in Operative Planning for Hilar Cholangiocarcinoma. Surgery, 142, 666-675. https://doi.org/10.1016/j.surg.2007.05.018
NOTES
*通讯作者。