Hans Journal of Agricultural Sciences
Vol.
09
No.
04
(
2019
), Article ID:
30006
,
11
pages
10.12677/HJAS.2019.94047
Functional Mechanisms of Greenfeed “Daliwan” Fertilizer
Chew Siok Foon Kelly1*, Ng Zhi Han1*, Muhamad Nizam Amahd Unonis1, Muhamad Izzuddin Khairuddin1, Ng Chang Chai2#
1Greenfeed Agro Sdn Bhd, Kuala Lumpur Malaysia
2Low Input Sustainable Agriculture Practitioner Consortium, University Putra Malaysia, Kuala Lumpur Malaysia

Received: Apr. 11th, 2019; accepted: Apr. 22nd, 2019; published: Apr. 29th, 2019

ABSTRACT
Greenfeed compound fertilizer, commercial name in China “Daliwan”, originated from Malaysia, is a slow release fertilizer based on aluminosilicate as carrier. In China’s application since 2016, “Daliwan” performed excellent in vegetables and fruit trees and gained much positive feedbacks from farmers. Basically, field performances observed included broader and flattened leaves, glossy texture on leaves, early budding, high fruit setting, even on fruit development and longer in shelf-life. In past research, we concluded these performances based on the fertilizer characteristics such as ion gradient activity, pH balancer and also water holding ability. In this report, we further concluded more on these mechanisms based on observation from different parts of crops. The main mechanisms we further summarized here were: root zone effect; ammonium nitrogen being supplied to avoid crops energy consumption on metabolism; induction of dissolution of unavailable phosphate ion. In leave performance, it is concluded to increase the assimilation of ammonium ion and the content of chlorophyll; inhibition of harmful effect of heavy metal to electron transport chain; and lastly, the increase on leaves photosynthesis rate by silica ion.
Keywords:Compound Fertilizer, Greenfeed Agro, Aluminosilicate, Daliwan, Sustainable Development
马来西亚绿丰复合肥“大力丸”产品作用机理
周淑芬1*,黄芷涵1*,Muhamad Nizam Amahd Unonis1, Muhamad Izzuddin Khairuddin1, 吴展才2#
1绿丰农业私人有限公司,马来西亚 吉隆坡
2低投入可持续农业发展联盟,博特拉大学,马来西亚 吉隆坡

收稿日期:2019年4月11日;录用日期:2019年4月22日;发布日期:2019年4月29日

摘 要
马来西亚绿丰公司复合肥“大力丸”,是一款以沸石硅铝酸盐为载体的新型缓释肥料。在各类经作包括蔬菜、果树的使用得到农户的认可。主要田间观察表现为叶片平展、具有光泽度、叶花芽提早、座果率高、果实发育良好、产品贮架期长等效果。本单位曾以肥料本身的特性如具有离子梯度、pH平衡者和持效水分长等因素,总结产品特性。在本篇报告中,将细化产品在根和树体发挥作用的所观察到的效果,结合现有科学文献所总结到的结果,并以本产品在各作物表现的数据,进一步综合归纳产品发挥作用的理论基础。从几个方面可归纳总结:在根系增加根际效益;根部吸收铵离子有助减少作物总体能量耗损;另外可诱导离子交换性磷的再利用。在叶片的表现,可提升铵态氮的同化能力,增加叶绿素含量;减少重金属对电子传递链的破坏;以及硅离子增加叶表光合速率能力等六大方面。
关键词 :复合肥,绿丰农业,硅铝酸盐,大力丸,可持续发展

Copyright © 2019 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
沸石主要由铝和硅等元素组成的硅铝酸盐,此具有正四面立体结构的化合物带有很多孔隙,也赋予了沸石高吸附能力 [1] [2] 。由于沸石空腔内多带有负电荷,因此这些界面,依铝和硅替代程度的不同,具有不同程度的阳离子交换能力。目前报导,其对阳离子的选择性从高到低依次为Cs > Rb > K > NH4 > Ba > Sr > Na > Ca > Fe > Al > Mg > Li [3] ,Pb > NH4 > Cu,Cd > Zn,Co > Ni > Hg [4] 。天然斜发沸石(clinoptilolite)为天然存在量最丰富的沸石矿种,目前其应用的领域包括工业、农业、生态、医药等 [5] [6] 。在农业上,由于其特有的硅铝酸盐结构,它在增添到化肥当中后具有保肥增效的作用。在研究上,它对减少大量元素随水流失的效果极为显著 [7] ,也在促产增收上有大幅的帮助 [8] 。沸石的多孔隙结构,可吸附其自身重量60%的水分,此水分可自由进出其立体三维结构,不影响架构组成 [9] 。因此,在农田水分管理上,沸石资材是一个能抗旱的材料,它的吸附性能让土壤更快速的湿润,并改善水分在土壤的横向分布,有助于根圈对水分的行用效率增加。沸石中的硅元素是作物硅的重要天然来源。作物对于硅的利用可透过主动和被动运输来完成 [10] [11] [12] [13] [14] 。Ma等人认为,硅的吸收靠的是侧根而非根毛。当进入木质部后,硅多被运送至茎部,主要途径是蒸散作用 [15] ,因此,作物体内的硅浓度大小直接反应了作物的蒸散速率 [16] 。一般在老叶累积较新幼叶多,在细胞壁上形成一种植硅体(phytoliths)或称为硅体的结构。
马来西亚绿丰公司产品“大力丸”,为使用沸石硅铝酸盐为载体的新型缓释肥料。在2016年进入中国市场,主要配方为氮磷钾镁20-15-10-2和12-12-20-2,另含有中微量元素钙、硼、硫、铁、锰、锌、硅等。绿丰“大力丸”肥料主要针对春肥、膨果期和采摘后肥三个用肥时期,具体根据果农的施肥习惯使用。按挂果量和树龄大小,每棵大型果树使用16~40粒不等。建议用法为穴施(图1),于根冠滴水线下每株大树取4个穴,将建议用量埋入15~20公分处。在众多田间实践工作当中,其省工省力的效益,尤其是山区果树已为广大老百姓所接受。引进中国当时,为药肥双减政策启动时期,经由2年多在华销售,应用在诸多作物,除了省肥的功效外,对于作物提质增效的效益也十分突出 [17] [18] [19] [20] ,也同时符合了国家减肥增效的需求。在前面的研究当中,本肥料曾就肥料本身的特性如离子梯度、pH平衡者和持效水分长等因素分析过产品机理 [21] 。本篇报告中,将细化产品在作物部位发挥作用的机理,并以本产品在各作物表现的数据,进一步综合总结产品发挥作用的理论基础。

Figure 1. Corn root status with “Daliwan” fertilizer (blue arrow)
图1. 使用“大力丸”玉米在采收后观察其根和“大力丸”(蓝色箭头)分布情况
2. 根际效益
根际施肥,也称为根圈施肥,最直观的做法是在土面下10~50公分处,以条施、点施,或将肥料放入后覆土的做法。自1970年代开始迄今,一直都有不少研究认为根际施肥优于土表撒施 [22] - [28] 。在田间操作,土表撒施的问题十分显而易见,肥料除了土表随温度和水分流失的问题之外,更有造成根系上浮乃至树体不抗寒冻等问题。Borges和Mallarino等人研究,相较于土表撒施,土面下施肥可增加作物对磷和钾的吸收 [29] ,更有研究表明土面下的施肥方法和提产、增收有很大的关系 [30] [31] [32] 。
3. 沸石增加了根部吸收铵离子,减少作物总体能量耗损
作物偏好吸收的无机盐氮素分别为硝酸态( )和铵态氮( ),而铵态氮是作物较为喜欢被利用的型态。但在通气性较好的土壤中,由于铵态氮的硝化作用和挥发的作用,其浓度往往只有硝态氮的10~1000之1的含量 [33] 。在土壤中,硝酸根离子须经由还原成亚硝根离子(式1),最后还原为铵态离子(式2),才能被作物吸收:
(1)
(2)
相较于直接同化利用铵态离子,此系列的还原作用消耗了作物总能量的15%以上,反之,直接同化铵离子的总耗能只有2%~5% [33] [34] 。前人研究,沸石对于铵态氮的阳离子交换力高达67.51%~79.5% [2] [3] [4] 。在Huang和Petrovic于水稻的研究报告中,应用了含沸石的肥料,作物对氮的利用效率提高了16%~22% [35] ,更少的硝态氮需经由还原过程消耗作物能量,因此作物保有更多能量来进行其他生理活动 [36] 。综上,大力丸在土壤中发挥的效益,即在于吸附了更多的铵态氮素,直接供作物同化作用,减少作物整体能量的消耗。
4. 诱导离子交换性磷的再被利用
在土壤中,磷素易被金属氢氧化物结合形成无效性磷。沸石经由改善土壤的pH (降低酸化)、增加铝和铁元素和其他碱金属和负电离子的土壤离子交换量,来增进磷的有效性。在沸石的存在下,元素诸如钙离子易于被交换给作物利用,此时,为了平衡沸石的电价,土壤中的磷酸盐会被加速溶出,以平衡沸石“耗损”的钙离子 [37] [38] 。在有硅的作用下,磷在土壤的有效性可以提高50% [39] ,但也有报告指出,过度的使用硅,将在根的内表皮细胞产生沉积,导致原生质联丝的障碍,让磷的传送效率降低 [15] 。
5. 铵态氮同化提升,增加叶绿素含量
铵态氮在根部的吸收同化,运输至叶片时,在由谷氨酰胺合成酶的作用下转化为谷氨酰胺,谷氨酰胺进一步转化形成谷氨酸,谷氨酸是叶绿素生合成的主要原料 [34] 。在十字花科的球茎甘蓝当中,提高了铵态氮处理,相较于只供应硝态氮,其叶绿素含量提升了21%,净光合作用的表现也有所上升。在石蒜科的晚香玉上,使用含沸石的肥料也让其叶片的叶绿素含量增加22.7% [40] 。此数据可对应绿丰公司在不同作物的叶绿素增加的百分比为6.69%~18.46% (表1) [40] 。值得一提的是,表1中已发表的作物叶绿素的增加幅度为4.4%~108.3%,数据均为作物在逆境情况下所测。显示如“大力丸”在逆境情况下叶绿素含量可能有类似更高的表现。在表2当中,受到不同逆境的作物的叶绿素a,b都有增加。叶绿素a为光合反应的主色素,而叶绿素b为辅助色素,其中西红柿、小麦的叶绿素a的增幅均高于b。
Table 1. Chlorophyll increase rate of different crops using silica-containing fertilizer
表1. 使用含硅复合肥不同作物总叶绿素增加百分比
Table 2. Chlorophyll a, b increase rate of different crops using silica-containing fertilizer
表2. 使用含硅复合肥不同作物叶绿素a, b增加百分比
6. 硅元素减少电子传递链被重属抑制失活
电子传递链也称为光合磷酸化反应,光合反应系统对于金属离子十分敏感 [42] 。金属离子尤其对光反应II中的受体具有高抑制性 [43] [44] [45] 。在土壤中,单硅酸于土壤常不太活动 [46] ,因此它们通常会与重金属或有机质形成胶体 [47] 。沸石具有可交换的钠、钙和钾等离子,使得它们可和镉、铅、锰、锌等离子作交换 [48] [49] ,直接减少了它们对光合系统的抑制。在作物体内,硅元素形成的多硅酸也可以扮演其在土壤中的角色,免除作物受到重金属离子的胁迫 [50] (图2)。
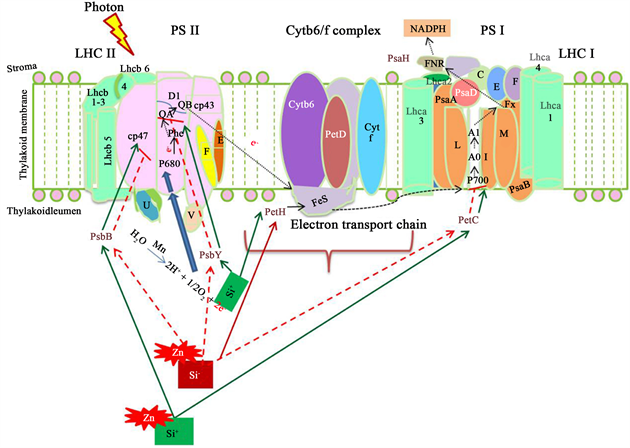
Figure 2. Photosynthesis model indicates silica binding on Zn and its prevention of Zn to bind with photosynthesis receptor. PSII photosynthesis system II; Cytb6f: cytochrome b6/f protein; PSI: photosynthesis system I; LHCI: Light harvest cytochrome I [71] [72] [73]
图2. 光合系统模型示意硅元素可和锌元素结合,减少锌元素和光合系统中的受体结合,影响光合反应。PSII:光合系统II;Cytb6f:色素b6/f蛋白;PSI:光合系统I;LHCI:光接收蛋白I [71] [72] [73]
7. 硅元素增加叶表光合速率能力
植硅体是高等植物吸取可溶性二氧化硅后,沉淀于植物细胞内或细胞外部的含水非晶态二氧化硅颗粒。植硅体在叶片上也产生了更佳的蜡防护层,对叶片形成了更佳的保护 [51] 。此现象可在“大力丸”产品于各个作物的叶片表现上看出(图3)。植硅体的主要存在于叶片表皮细胞,增加了细胞壁的机械性力量,增加纤维素的长度 [52] 。在Liu的研究,植硅体在细胞壁会形成有机硅化物,可和金属如铝、锰、镉等结合 [53] ,防止作物的吸收,减少重金属的危害 [54] [55] 。此外,Quanzhi和Erming的研究中指出,硅对叶片面积的增大有明显的作用(表3、图4),此对截取更多的阳光,提升光合速率有相关性 [56] 。表4中,使用绿丰“大力丸”产品的光合速率提升达12.9%~28.2%。Inanaga也报导使用了含硅肥料,会减少上位叶对下位叶造成的遮荫,因此有更多的叶面积能接收阳光,提高叶片光合作用 [57] 。
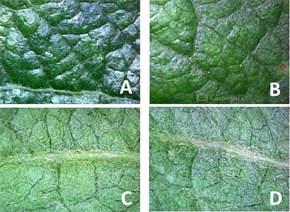
Figure 3. Apple using Greenfeed ((A), (C)) and common fertilizer ((B), (D)) under 300× microscopic observation on leave. (A): Front leaf with glossy-look of cuticle; (B): common fertilizer; (C): Back leaf with coarse surface; (D): common fertilizer
图3. 使用绿丰“大力丸”苹果叶片((A), (C))和常规肥料((B), (D))于300倍显微镜下观察。(A) 叶片正面角质层明显,具有光泽;(B) 常规肥料;(C) 叶背面叶脉较粗;(D) 常规肥料
Table 3. Leaves size (cm) investigation after 77 days applied “Daliwan”
表3. 春施肥使用“大力丸”77天后调查叶片大小(cm)

Figure 4. Apple leaves applied Greenfeed “Daliwan” and common fertilizer after 77 day
图4. 春施肥使用“大力丸”77天后调查苹果叶片大小
Table 4. Photosynthesis rate increase rate of different crops using silica-containing fertilizer
表4. 使用含硅复合肥不同作物光合作用增加百分比
8. 总结
马来西亚绿丰公司复合肥“大力丸”在数千个使用案例的反馈表现,除了其缓释性和省工省肥特性之外,还有复合肥当中的中微量元素,在元素营养配方上提供了一站式的供给。综上归纳点,前人研究和我司于田间走访的反馈,可相互对应,在复合肥方面的节肥、增效上具有高应用推广价值。
致谢
感谢中国农大陈清教授、西北农大周军教授,提出的宝贵意见和对本文不吝提出修改建议。
文章引用
周淑芬,黄芷涵,Muhamad Nizam Amahd Unonis,Muhamad Izzuddin Khairuddin,吴展才. 马来西亚绿丰复合肥“大力丸”产品作用机理
Functional Mechanisms of Greenfeed “Daliwan” Fertilizer[J]. 农业科学, 2019, 09(04): 312-322. https://doi.org/10.12677/HJAS.2019.94047
参考文献
- 1. Mumpton, F.A. (1985) Using Zeolites in Agriculture. In: Innovative Biological Technologies for Lesser Developed Countries, US Congress, Office of Technology Assessment, Washington DC, 127-158.
- 2. Bernal, M.P. and Lopez-Real, J.M. (1993) Natural Zeolites and Sepiolite as Ammonium an Ammonia Adsorbent Materials. Bioresource Technology, 43, 27-33. https://doi.org/10.1016/0960-8524(93)90078-P
- 3. Ames, L.L. (1960) The Cation Sieve Properties of Clinoptilolite. American Mineral, 45, 689-700.
- 4. Blanchard, G., Maunaye, M. and Martin, G. (1984) Removal of Heavy Metal from Waters by Means of Natural Zeolites. Water Research, 18, 1501-1507. https://doi.org/10.1016/0043-1354(84)90124-6
- 5. Colella, C. and Mumpton, F.A. (2000) Natural Zeolites for the Third Millennium. De Frede-Editore, Napoli.
- 6. Van Bekkum, H., Flanigen, E.M. and Jansen, J.C. (2001) Introduction to Zeolite Science and Practice. Elsevier Science Publisher, Amsterdam.
- 7. NurAainaa, H., Haruna Ahmed, O. and Ab Majid, N.M. (2018) Effects of Clinoptilolite Zeolite on Phosphorus Dynamics and Yield of Zea mays L. Cultivated on an Acid Soil. PLoS ONE, 13, e0204401. https://doi.org/10.1371/journal.pone.0204401
- 8. Polat, E., Karaca, M., Demir, H. and Onus, A.N. (2004) Use of Natural Zeolite (Clinoptilolite) in Agriculture. Journal of Fruit and Ornamental Plant Research, 12, 183-189.
- 9. Kocakuşak, S., Savaşcı, Ö.T. and Ayok, T. (2001) Doğalzeolitlerveuygulamaalanları. TürkiyeBilimselveTeknikAraştırmaKurumu Marmara AraştırmaMerkezi, MalzemeveKimyaTeknolojileriAraştırmaEnstitüsü, Rapor No: KM, 362.
- 10. Henriet, C., Draye, X., Oppitz, I., Swennen, R. and Delvaux, B. (2006) Effects, Distribution and Uptake of Silicon in Banana (Musa spp.) under Controlled Conditions. Plant and Soil, 287, 359-374. https://doi.org/10.1007/s11104-006-9085-4
- 11. Mitani, N. and Ma, J.F. (2005) Uptake System of Silicon in Different Plant Species. Journal of Experimental Botany, 56, 1255-1261. https://doi.org/10.1093/jxb/eri121
- 12. Ding, T.P., Zhou, J.X., Wan, D.F., Chen, Z.Y., Wang, C.Y. and Zhang, F. (2008) Silicon Isotope Fractionation in Bamboo and Its Significance to the Biogeochemical Cycle of Silicon. Geochimicaet Cosmochimica Acta, 72, 1381-1395. https://doi.org/10.1016/j.gca.2008.01.008
- 13. Liang, Y.C., Sun, W.C., Zhu, Y.G. and Christie, P. (2007) Mechanisms of Silicon-Mediated Alleviation of Abiotic Stresses in Higher Plants: A Review. Environmental Pollution, 147, 422-428. https://doi.org/10.1016/j.envpol.2006.06.008
- 14. Gérard, F., Mayer, K.U., Hodson, M.J. and Ranger, J. (2008) Modelling the Biogeochemical Cycle of Silicon in Soils: Application to a Temperate Forest Ecosystem. Geochimicaet Cosmochimica Acta, 72, 741-758.https://doi.org/10.1016/j.gca.2007.11.010
- 15. Ma, J.F., Goto, S., Tamai, K. and Ichii, M. (2001) Role of Root Hairs and Lateral Roots in Silicon Uptake by Rice. Plant Physiology, 127, 1773-1780. https://doi.org/10.1104/pp.010271
- 16. Raven, J.A. (1983) The Transport and Function of Silicon in Plants. Biological Reviews, 58, 179-207.https://doi.org/10.1111/j.1469-185X.1983.tb00385.x
- 17. 吴展才, 吴思节, Aziz, M.A.A., 陈宥维. 新型缓释肥料对香蕉叶片营养、光合速率、叶绿素含量、产量和果实质量的效益[J]. 农业科学, 2016, 6(3): 49-56.
- 18. 吴展才, 吴思节、Aziz, M.A., Khairuddin, M.I., 陈宥维. 新型含硅型沸石肥料对水稻采收质量和产量的效益[J]. 农业科学, 2016, 6(3): 79-86.
- 19. 吴展才, 刘统棋, 魏金龙, 王发浪. 新型高效缓释肥料绿丰“大力丸”对洛川红富士苹果果实质量的效果[J]. 农业科学, 2018, 8(11): 1307-1311.
- 20. 吴展才, 刘统祺, 魏金龙. 一种新型高效缓释肥在红富士苹果生长期效果研究[J]. 西北园艺(综合), 2018(4): 57-59.
- 21. Tan, Y.W., Unonis, N.A., Khairuddin, I., Shaharuddin, S. and Ng, C.C. (2018) Mechanism in Using Commercial High Efficient Zeolite-Base Greenfeed Slow Release Fertilizers. Journal of Agricultural Chemistry and Environment, 7, 1-9. https://doi.org/10.4236/jacen.2018.71001
- 22. Chaudhary, M.R. and Prihar, S.S. (1974) Comparison of Banded and Broadcast Fertilizer Applications in Relation to Compaction and Irrigation in Maize and Wheat. Agronomy Journal, 66, 560-564. https://doi.org/10.2134/agronj1974.00021962006600040024x
- 23. Wells, K.L. (1982) Band vs. Broadcast Application of Fertilizer. Soil Science News and Views, 3, 147.
- 24. Soon, B.B.F. and Hoong, H.W. (2001) Agronomic Practices to Alleviate Soil and Surface Runoff Losses in an Oil Palm Estate. Malaysia Journal of Soil Science, 6, 53-64.
- 25. Bakhtiari, M.R. (2014) Selection of Fertilization Method and Fertilizer Application Rate on Corn Yield. Agricultural Engineering International: CIGR Journal, 16, 10-14.
- 26. Grant, C.A. and Bailey, L.D. (1997) Effects of Phosphorus and Zinc Fertiliser Management on Cadmium Accumulation in Flaxseed. Journal of the Science of Food and Agriculture, 73, 307-314. https://doi.org/10.1002/(SICI)1097-0010(199703)73:3<307::AID-JSFA732>3.0.CO;2-3
- 27. Sepahvand, P., Sajedi, N., Mousavi, S.K. and Ghiasvand, M. (2014) Effects of Nitrogen Application Method and Weed Control on Corn Yield and Yield Components. Pakistan Journal of Biological Sciences, 17, 497-503.https://doi.org/10.3923/pjbs.2014.497.503
- 28. Sims, A.L. and Smith, L.J. (2002) Use of Starter Fertilizer to Reduce Broadcast Applications of Phosphorus. Sugarbeet Research and Extention Board. Sugarbeet Research Extention Reports, 33, 94-99.
- 29. Borges, R. and Mallarino, A.P. (2001) Deep Banding Phosphorus and Potassium Fertilizers for Corn Managed with Ridge Tillage. Soil Science Society of America Journal, 65, 376-384. https://doi.org/10.2136/sssaj2001.652376x
- 30. Mallarino, A.P., Bordoli, J.M. and Borges, R. (1999) Phosphorus and Potassium Placement Effects on Early Growth and Nutrient Uptake of No-Till Corn and Relationships with Grain Yield. Agronomy Journal, 91, 37-45.https://doi.org/10.2134/agronj1999.00021962009100010007x
- 31. Malhi, S.S., Zentner, R.P. and Heier, K. (2001) Banding Increases Effectiveness of Fertilizer P for Alfalfa Production. Nutrient Cycling in Agroecosystems, 59, 1-11. https://doi.org/10.1023/A:1009889218829
- 32. Singh, D.K., Sale, P.W.G. and Routley, R.R. (2005) Increasing Phosphorus Supply in Subsurface Soil in Northern Australia: Rationale for Deep Placement and the Effects with Various Crops. Plant and Soil, 269, 35-44.https://doi.org/10.1007/s11104-004-2475-6
- 33. von Wirén, N., Gojon, A., Chaillou, S. and Raper Jr., D. (2001) Mechanisms and Regulation of Ammonium Uptake in Higher Plants. In: Lea, P.J. and Morot-Gaudry, J.F., Eds., Plant Nitrogen, Springer, Berlin, Heidelberg, 61-77. https://doi.org/10.1007/978-3-662-04064-5_3
- 34. Hopkins, W.G. and Huner, N.P. (2009) Introduction to Plant Physiology. John Wiley & Sons, New York.
- 35. Huang, T.Z. and Petrovic, A. (1994) Clinoptilolite Zeolite Influence on Nitrate Leaching and Nitrogen Use Efficiency in Simulated Sand-Based Golf Greens. Journal of Environmental Quality, 23, 1190-1194. https://doi.org/10.2134/jeq1994.00472425002300060009x
- 36. Jakkula, V.S. and Wani, S.P. (2018) Zeolites: Potential Soil Amendments for Improving Nutrient and Water Use Efficiency and Agriculture Productivity. Scientific Reviews & Chemical Communications, 8, 1-15.
- 37. Allen, E.R., Hossner, L.R., Ming, D.W. and Henninger, D.L. (1993) Solubility and Cation Exchange in Phosphate Rock and Saturated Clinoptilolite Mixtures. Soil Science Society of America Journal, 57, 1368-1374.https://doi.org/10.2136/sssaj1993.03615995005700050034x
- 38. Pickering, H.W., Menzies, N.W. and Hunter, M.N. (2002) Zeolite/Rock Phosphate—A Novel Slow Release Phosphorus Fertiliser for Potted Plant Production. Scientia Horticulturae, 94, 333-343.https://doi.org/10.1016/S0304-4238(02)00006-7
- 39. Greger, M., Landberg, T. and Vaculík, M. (2018) Silicon Influences Soil Availability and Accumulation of Mineral Nutrients in Various Plant Species. Plants, 7, 41. https://doi.org/10.3390/plants7020041
- 40. Majid, B., Hassan, S. and Saeid, E. (2012) Growth and Flowering of Tuberose as Affected by Adding Natural Zeolite to the Culture Medium. Journal of Plant Nutrition, 35, 1491-1496. https://doi.org/10.1080/01904167.2012.689909
- 41. Greenfeed Bulletin Issue 9, 13, 15, 16, 17 (Not Published).https://issuu.com/greenfeed/docs/greenfeed_bulletin_volume_9 http://www.greenfeed.com.my/bulletin/greenfeed-bulletin-issue-13/ http://www.greenfeed.com.my/bulletin/greenfeed-bulletin-issue-15/ http://www.greenfeed.com.my/bulletin/greenfeed-bulletin-issue-16/ http://www.greenfeed.com.my/bulletin/greenfeed-bulletin-issue-17/
- 42. Prasad, M.N.V. and Strzałka, K. (1999) Impact of Heavy Metals on Photosynthesis. In: Heavy Metal Stress in Plants, Springer, Berlin, Heidelberg, 117-138. https://doi.org/10.1007/978-3-662-07745-0_6
- 43. Cedeno-Maldonado, A., Swader, J.A. and Heath, R.L. (1972) The Cupric Ion as an Inhibitor of Photosynthetic Electron Transport in Isolated Chloroplasts. Plant Physiology, 50, 698-701. https://doi.org/10.1104/pp.50.6.698
- 44. Vierke, G. and Struckmeier, P. (1977) Binding of Copper (II) to Proteins of the Photosynthetic Membrane and Its Correlation with Inhibition of Electron Transport in Class II Chloroplasts of Spinach. Zeitschrift für Naturforschung C, 32, 605-610. https://doi.org/10.1515/znc-1977-7-820
- 45. Yruela, I., Pueyo, J.J., Alonso, P.J. and Picorel, R. (1996) Photoinhibition of Photosystem II from Higher Plants Effect of Copper Inhibition. Journal of Biological Chemistry, 271, 27408-27415. https://doi.org/10.1074/jbc.271.44.27408
- 46. Khalid, R.A. and Silva, J.A. (1980) Residual Effect of Calcium Silicate on pH, Phosphorus, and Aluminium in a Tropical Soil Profile. Soil Science and Plant Nutrition, 26, 87-98. https://doi.org/10.1080/00380768.1980.10433215
- 47. Datnoff, L.E., Snyder, G.H. and Korndörfer, G.H. (2001) Silicon in Agriculture (Vol. 8). Elsevier, Amsterdam, Netherlands.
- 48. Erdem, E., Karapinar, N. and Donat, R. (2004) The Removal of Heavy Metal Cations by Natural Zeolites. Journal of Colloid and Interface Science, 280, 309-314. https://doi.org/10.1016/j.jcis.2004.08.028
- 49. Shaheen, S.M., Derbalah, A.S. and Moghanm, F.S. (2012) Removal of Heavy Metals from Aqueous Solution by Zeolite in Competitive Sorption System. International Journal of Environmental Science and Development, 3, 362-367.https://doi.org/10.7763/IJESD.2012.V3.248
- 50. Emamverdian, A., Ding, Y., Xie, Y. and Sangari, S. (2018) Silicon Mechanisms to Ameliorate Heavy Metal Stress in Plants. BioMedical Research International, 2018, Article ID: 8492898. https://doi.org/10.1155/2018/8492898
- 51. Blanke, M.M., Bacher, W., Pring, R.J., and Baker, E.A. (1996) Ammonium Nutrition Enhance Chlorophyll and Glaucousness in Kohlrabi. Annals of Botany, 78, 559-604. https://doi.org/10.1006/anbo.1996.0166
- 52. He, C., Ma, J. and Wang, L. (2015) A Hemicelluloses-Bound Form of Silicon with Potential to Improve the Mechanical Properties and Regeneration of the Cell Wall of Rice. New Phytologist, 206, 1051-1062. https://doi.org/10.1111/nph.13282
- 53. Liu, J., Ma, J., He, C., Li, X., Zhang, W., Xu, F. and Wang, L. (2013) Inhibition of Cadmium Ion Uptake in Rice (Oryza sativa) Cells by a Wall-Bound Form of Silicon. New Pytologist, 200, 691-699. https://doi.org/10.1111/nph.12494
- 54. Richmond, K.E. and Sussman, M. (2003) Got Silicon? The Non-Essential Beneficial Plant Nutrient. Current Opinion in Plant Biology, 6, 268-272. https://doi.org/10.1016/S1369-5266(03)00041-4
- 55. Ma, J.F., Mitani, N., Nagao, S., Konishi, S., Tamai, K., Iwashita, T. and Yano, M. (2004) Characterization of the Silicon Uptake System and Molecular Mapping of the Silicon Transporter Gene in Rice. Plant Physiology, 136, 3284-3289. https://doi.org/10.1104/pp.104.047365
- 56. Quanzhi, Z. and Erming, G. (1998) Effect of Silicon Application on Rice in a Rice Area along the Yellow River. Department of Agronomy, 32, 308-313.
- 57. Inanaga, S., Okasaka, A. and Tanaka, S. (1995) Does Silicon Exist in Association with Organic Compounds in Rice Plant? Soil Science and Plant Nutrition, 41, 111-117. https://doi.org/10.1080/00380768.1995.10419564
- 58. Xie, Z., Song, F., Xu, H., Shao, H. and Song, R. (2014) Effects of Silicon on Photosynthetic Characteristics of Maize (Zea mays L.) on Alluvial Soil. The Scientific World Journal, 2014, Article ID: 718716. https://doi.org/10.1155/2014/718716
- 59. Chen, W., Yao, X., Cai, K. and Chen, J. (2011) Silicon Alleviates Drought Stress of Rice Plants by Improving Plant Water Status, Photosynthesis and Mineral Nutrient Absorption. Biological Trace Element Research, 142, 67-76.https://doi.org/10.1007/s12011-010-8742-x
- 60. Silva, O.N., Lobato, A.K.S., Ávila, F.W., Costa, R.C.L., Neto, C.O., Santos Filho, B.G. and Cardoso, M.S. (2012) Silicon-Induced Increase in Chlorophyll Is Modulated by the Leaf Water Potential in Two Water-Deficient Tomato Cultivars. Plant, Soil and Environment, 58, 481-486. https://doi.org/10.17221/213/2012-PSE
- 61. Ali, S., Farooq, M.A., Yasmeen, T., Hussain, S., Arif, M.S., Abbas, F. and Zhang, G. (2013) The Influence of Silicon on Barley Growth, Photosynthesis and Ultra-Structure under Chromium Stress. Ecotoxicology and Environmental Safety, 89, 66-72. https://doi.org/10.1016/j.ecoenv.2012.11.015
- 62. Farooq, M.A., Ali, S., Hameed, A., Ishaque, W., Mahmood, K. and Iqbal, Z. (2013) Alleviation of Cadmium Toxicity by Silicon Is Related to Elevated Photosynthesis, Antioxidant Enzymes; Suppressed Cadmium Uptake and Oxidative Stress in Cotton. Ecotoxicology and Environmental Safety, 96, 242-249. https://doi.org/10.1016/j.ecoenv.2013.07.006
- 63. Feng, J., Shi, Q., Wang, X., Wei, M., Yang, F. and Xu, H. (2010) Silicon Supplementation Ameliorated the Inhibition of Photosynthesis and Nitrate Metabolism by Cadmium (Cd) Toxicity in Cucumis sativus L. Scientia Horticulturae, 123, 521-530. https://doi.org/10.1016/j.scienta.2009.10.013
- 64. Yang, Y.H., Chen, S.M., Chen, Z., Zhang, H.Y., Shen, H.G., Hua, Z.C. and Li, N. (1999) Silicon Effects on Aluminum Toxicity to Mungbean Seedling Growth. Journal of Plant Nutrition, 22, 693-700. https://doi.org/10.1080/01904169909365664
- 65. Hussain, I., Ashraf, M.A., Rasheed, R., Asghar, A., Sajid, M.A. and Iqbal, M. (2015) Exogenous Application of Silicon at the Boot Stage Decreases Accumulation of Cadmium in Wheat (Triticum aestivum L.) Grains. Brazilian Journal of Botany, 38, 223-234. https://doi.org/10.1007/s40415-014-0126-6
- 66. Tripathi, D.K., Singh, V.P., Prasad, S.M., Chauhan, D.K., Dubey, N.K. and Rai, A.K. (2014) Silicon-Mediated Alleviation of Cr(VI) Toxicity in Wheat Seedlings as Evidenced by Chlorophyll Fluorescence, Laser Induced Breakdown Spectroscopy and Anatomical Changes. Journal Ecotoxicology and Environmental Safety, 113, 133-144.https://doi.org/10.1016/j.ecoenv.2014.09.029
- 67. Bae, E.J., Lee, K.S., Huh, M.R. and Lim, C.S. (2012) Silicon Significantly Alleviates the Growth Inhibitory Effects of NaCl in Salt-Sensitive ‘Perfection’ and ‘Midnight’ Kentucky Bluegrass (Poa pratensis L). Horticulture, Environment, and Biotechnology, 53, 477-483. https://doi.org/10.1007/s13580-012-0094-3
- 68. Tuna, A.L., Kaya. C., Higgs, D., Murillo-Amador, B., Aydemir, S. and Girgin, A.R. (2008) Silicon Improves Salinity Tolerance in Wheat Plants. Environmental and Experimental Botany, 62, 10-16. https://doi.org/10.1016/j.envexpbot.2007.06.006
- 69. Al-Aghabary, K., Zhu, Z. and Shi, Q.H. (2005) Influence of Silicon Supply on Chlorophyll Content, Chlorophyll Fluorescence, and Antioxidative Enzyme Activities in Tomato Plants under Salt Stress. Journal of Plant Nutrition, 27, 2101-2115. https://doi.org/10.1081/PLN-200034641
- 70. Hattori, T., Inanaga, S., Araki, H., An, P., Morita, S., Luxová, M. and Lux, A. (2005) Application of Silicon Enhanced Drought Tolerance in Sorghum bicolor. Physiologia Plantarum, 123, 459-466.https://doi.org/10.1111/j.1399-3054.2005.00481.x
- 71. Kawakami, K., Iwai, M., Ikeuchi, M., Kamiya, N. and Shen, J.R. (2007) Location of PsbY in Oxygen-Evolving Photosystem II Revealed by Mutagenesis and X-Ray Crystallography. FEBS Letters, 581, 4983-4987.https://doi.org/10.1016/j.febslet.2007.09.036
- 72. Lunde, C., Jensen, P.E., Haldrup, A., Knoetzel, J. and Scheller, H.V. (2000) The PSI-H Subunit of Photosystem I Is Essential for State Transitions in Plant Photosynthesis. Nature, 408, 613-615. https://doi.org/10.1038/35046121
- 73. Song, A., Li, P., Fan, F., Li, Z. and Liang, Y. (2014) The Effect of Silicon on Photosynthesis and Expression of Its Relevant Genes in Rice (Oryza sativa L.) under High-Zinc Stress. PLoS ONE, 9, e113782. https://doi.org/10.1371/journal.pone.0113782
- 74. Mumpton, F.A. (1985) Using Zeolites in Agriculture. In: Innovative Biological Technologies for Lesser Developed Countries, US Congress, Office of Technology Assessment, Washington DC, 127-158.
- 75. Bernal, M.P. and Lopez-Real, J.M. (1993) Natural Zeolites and Sepiolite as Ammonium an Ammonia Adsorbent Materials. Bioresource Technology, 43, 27-33. https://doi.org/10.1016/0960-8524(93)90078-P
- 76. Ames, L.L. (1960) The Cation Sieve Properties of Clinoptilolite. American Mineral, 45, 689-700.
- 77. Blanchard, G., Maunaye, M. and Martin, G. (1984) Removal of Heavy Metal from Waters by Means of Natural Zeolites. Water Research, 18, 1501-1507. https://doi.org/10.1016/0043-1354(84)90124-6
- 78. Colella, C. and Mumpton, F.A. (2000) Natural Zeolites for the Third Millennium. De Frede-Editore, Napoli.
- 79. Van Bekkum, H., Flanigen, E.M. and Jansen, J.C. (2001) Introduction to Zeolite Science and Practice. Elsevier Science Publisher, Amsterdam.
- 80. NurAainaa, H., Haruna Ahmed, O. and Ab Majid, N.M. (2018) Effects of Clinoptilolite Zeolite on Phosphorus Dynamics and Yield of Zea mays L. Cultivated on an Acid Soil. PLoS ONE, 13, e0204401. https://doi.org/10.1371/journal.pone.0204401
- 81. Polat, E., Karaca, M., Demir, H. and Onus, A.N. (2004) Use of Natural Zeolite (Clinoptilolite) in Agriculture. Journal of Fruit and Ornamental Plant Research, 12, 183-189.
- 82. Kocakuşak, S., Savaşcı, Ö.T. and Ayok, T. (2001) Doğalzeolitlerveuygulamaalanları. TürkiyeBilimselveTeknikAraştırmaKurumu Marmara AraştırmaMerkezi, MalzemeveKimyaTeknolojileriAraştırmaEnstitüsü, Rapor No: KM, 362.
- 83. Henriet, C., Draye, X., Oppitz, I., Swennen, R. and Delvaux, B. (2006) Effects, Distribution and Uptake of Silicon in Banana (Musa spp.) under Controlled Conditions. Plant and Soil, 287, 359-374. https://doi.org/10.1007/s11104-006-9085-4
- 84. Mitani, N. and Ma, J.F. (2005) Uptake System of Silicon in Different Plant Species. Journal of Experimental Botany, 56, 1255-1261. https://doi.org/10.1093/jxb/eri121
- 85. Ding, T.P., Zhou, J.X., Wan, D.F., Chen, Z.Y., Wang, C.Y. and Zhang, F. (2008) Silicon Isotope Fractionation in Bamboo and Its Significance to the Biogeochemical Cycle of Silicon. Geochimicaet Cosmochimica Acta, 72, 1381-1395. https://doi.org/10.1016/j.gca.2008.01.008
- 86. Liang, Y.C., Sun, W.C., Zhu, Y.G. and Christie, P. (2007) Mechanisms of Silicon-Mediated Alleviation of Abiotic Stresses in Higher Plants: A Review. Environmental Pollution, 147, 422-428. https://doi.org/10.1016/j.envpol.2006.06.008
- 87. Gérard, F., Mayer, K.U., Hodson, M.J. and Ranger, J. (2008) Modelling the Biogeochemical Cycle of Silicon in Soils: Application to a Temperate Forest Ecosystem. Geochimicaet Cosmochimica Acta, 72, 741-758.https://doi.org/10.1016/j.gca.2007.11.010
- 88. Ma, J.F., Goto, S., Tamai, K. and Ichii, M. (2001) Role of Root Hairs and Lateral Roots in Silicon Uptake by Rice. Plant Physiology, 127, 1773-1780. https://doi.org/10.1104/pp.010271
- 89. Raven, J.A. (1983) The Transport and Function of Silicon in Plants. Biological Reviews, 58, 179-207.https://doi.org/10.1111/j.1469-185X.1983.tb00385.x
- 90. 吴展才, 吴思节, Aziz, M.A.A., 陈宥维. 新型缓释肥料对香蕉叶片营养、光合速率、叶绿素含量、产量和果实质量的效益[J]. 农业科学, 2016, 6(3): 49-56.
- 91. 吴展才, 吴思节、Aziz, M.A., Khairuddin, M.I., 陈宥维. 新型含硅型沸石肥料对水稻采收质量和产量的效益[J]. 农业科学, 2016, 6(3): 79-86.
- 92. 吴展才, 刘统棋, 魏金龙, 王发浪. 新型高效缓释肥料绿丰“大力丸”对洛川红富士苹果果实质量的效果[J]. 农业科学, 2018, 8(11): 1307-1311.
- 93. 吴展才, 刘统祺, 魏金龙. 一种新型高效缓释肥在红富士苹果生长期效果研究[J]. 西北园艺(综合), 2018(4): 57-59.
- 94. Tan, Y.W., Unonis, N.A., Khairuddin, I., Shaharuddin, S. and Ng, C.C. (2018) Mechanism in Using Commercial High Efficient Zeolite-Base Greenfeed Slow Release Fertilizers. Journal of Agricultural Chemistry and Environment, 7, 1-9. https://doi.org/10.4236/jacen.2018.71001
- 95. Chaudhary, M.R. and Prihar, S.S. (1974) Comparison of Banded and Broadcast Fertilizer Applications in Relation to Compaction and Irrigation in Maize and Wheat. Agronomy Journal, 66, 560-564. https://doi.org/10.2134/agronj1974.00021962006600040024x
- 96. Wells, K.L. (1982) Band vs. Broadcast Application of Fertilizer. Soil Science News and Views, 3, 147.
- 97. Soon, B.B.F. and Hoong, H.W. (2001) Agronomic Practices to Alleviate Soil and Surface Runoff Losses in an Oil Palm Estate. Malaysia Journal of Soil Science, 6, 53-64.
- 98. Bakhtiari, M.R. (2014) Selection of Fertilization Method and Fertilizer Application Rate on Corn Yield. Agricultural Engineering International: CIGR Journal, 16, 10-14.
- 99. Grant, C.A. and Bailey, L.D. (1997) Effects of Phosphorus and Zinc Fertiliser Management on Cadmium Accumulation in Flaxseed. Journal of the Science of Food and Agriculture, 73, 307-314. 3.0.CO;2-3>https://doi.org/10.1002/(SICI)1097-0010(199703)73:3<307::AID-JSFA732>3.0.CO;2-3
- 100. Sepahvand, P., Sajedi, N., Mousavi, S.K. and Ghiasvand, M. (2014) Effects of Nitrogen Application Method and Weed Control on Corn Yield and Yield Components. Pakistan Journal of Biological Sciences, 17, 497-503.https://doi.org/10.3923/pjbs.2014.497.503
- 101. Sims, A.L. and Smith, L.J. (2002) Use of Starter Fertilizer to Reduce Broadcast Applications of Phosphorus. Sugarbeet Research and Extention Board. Sugarbeet Research Extention Reports, 33, 94-99.
- 102. Borges, R. and Mallarino, A.P. (2001) Deep Banding Phosphorus and Potassium Fertilizers for Corn Managed with Ridge Tillage. Soil Science Society of America Journal, 65, 376-384. https://doi.org/10.2136/sssaj2001.652376x
- 103. Mallarino, A.P., Bordoli, J.M. and Borges, R. (1999) Phosphorus and Potassium Placement Effects on Early Growth and Nutrient Uptake of No-Till Corn and Relationships with Grain Yield. Agronomy Journal, 91, 37-45.https://doi.org/10.2134/agronj1999.00021962009100010007x
- 104. Malhi, S.S., Zentner, R.P. and Heier, K. (2001) Banding Increases Effectiveness of Fertilizer P for Alfalfa Production. Nutrient Cycling in Agroecosystems, 59, 1-11. https://doi.org/10.1023/A:1009889218829
- 105. Singh, D.K., Sale, P.W.G. and Routley, R.R. (2005) Increasing Phosphorus Supply in Subsurface Soil in Northern Australia: Rationale for Deep Placement and the Effects with Various Crops. Plant and Soil, 269, 35-44.https://doi.org/10.1007/s11104-004-2475-6
- 106. von Wirén, N., Gojon, A., Chaillou, S. and Raper Jr., D. (2001) Mechanisms and Regulation of Ammonium Uptake in Higher Plants. In: Lea, P.J. and Morot-Gaudry, J.F., Eds., Plant Nitrogen, Springer, Berlin, Heidelberg, 61-77. https://doi.org/10.1007/978-3-662-04064-5_3
- 107. Hopkins, W.G. and Huner, N.P. (2009) Introduction to Plant Physiology. John Wiley & Sons, New York.
- 108. Huang, T.Z. and Petrovic, A. (1994) Clinoptilolite Zeolite Influence on Nitrate Leaching and Nitrogen Use Efficiency in Simulated Sand-Based Golf Greens. Journal of Environmental Quality, 23, 1190-1194. https://doi.org/10.2134/jeq1994.00472425002300060009x
- 109. Jakkula, V.S. and Wani, S.P. (2018) Zeolites: Potential Soil Amendments for Improving Nutrient and Water Use Efficiency and Agriculture Productivity. Scientific Reviews & Chemical Communications, 8, 1-15.
- 110. Allen, E.R., Hossner, L.R., Ming, D.W. and Henninger, D.L. (1993) Solubility and Cation Exchange in Phosphate Rock and Saturated Clinoptilolite Mixtures. Soil Science Society of America Journal, 57, 1368-1374.https://doi.org/10.2136/sssaj1993.03615995005700050034x
- 111. Pickering, H.W., Menzies, N.W. and Hunter, M.N. (2002) Zeolite/Rock Phosphate—A Novel Slow Release Phosphorus Fertiliser for Potted Plant Production. Scientia Horticulturae, 94, 333-343.https://doi.org/10.1016/S0304-4238(02)00006-7
- 112. Greger, M., Landberg, T. and Vaculík, M. (2018) Silicon Influences Soil Availability and Accumulation of Mineral Nutrients in Various Plant Species. Plants, 7, 41. https://doi.org/10.3390/plants7020041
- 113. Majid, B., Hassan, S. and Saeid, E. (2012) Growth and Flowering of Tuberose as Affected by Adding Natural Zeolite to the Culture Medium. Journal of Plant Nutrition, 35, 1491-1496. https://doi.org/10.1080/01904167.2012.689909
- 114. Greenfeed Bulletin Issue 9, 13, 15, 16, 17 (Not Published).https://issuu.com/greenfeed/docs/greenfeed_bulletin_volume_9 http://www.greenfeed.com.my/bulletin/greenfeed-bulletin-issue-13/ http://www.greenfeed.com.my/bulletin/greenfeed-bulletin-issue-15/ http://www.greenfeed.com.my/bulletin/greenfeed-bulletin-issue-16/ http://www.greenfeed.com.my/bulletin/greenfeed-bulletin-issue-17/
- 115. Prasad, M.N.V. and Strzałka, K. (1999) Impact of Heavy Metals on Photosynthesis. In: Heavy Metal Stress in Plants, Springer, Berlin, Heidelberg, 117-138. https://doi.org/10.1007/978-3-662-07745-0_6
- 116. Cedeno-Maldonado, A., Swader, J.A. and Heath, R.L. (1972) The Cupric Ion as an Inhibitor of Photosynthetic Electron Transport in Isolated Chloroplasts. Plant Physiology, 50, 698-701. https://doi.org/10.1104/pp.50.6.698
- 117. Vierke, G. and Struckmeier, P. (1977) Binding of Copper (II) to Proteins of the Photosynthetic Membrane and Its Correlation with Inhibition of Electron Transport in Class II Chloroplasts of Spinach. Zeitschrift für Naturforschung C, 32, 605-610. https://doi.org/10.1515/znc-1977-7-820
- 118. Yruela, I., Pueyo, J.J., Alonso, P.J. and Picorel, R. (1996) Photoinhibition of Photosystem II from Higher Plants Effect of Copper Inhibition. Journal of Biological Chemistry, 271, 27408-27415. https://doi.org/10.1074/jbc.271.44.27408
- 119. Khalid, R.A. and Silva, J.A. (1980) Residual Effect of Calcium Silicate on pH, Phosphorus, and Aluminium in a Tropical Soil Profile. Soil Science and Plant Nutrition, 26, 87-98. https://doi.org/10.1080/00380768.1980.10433215
- 120. Datnoff, L.E., Snyder, G.H. and Korndörfer, G.H. (2001) Silicon in Agriculture (Vol. 8). Elsevier, Amsterdam, Netherlands.
- 121. Erdem, E., Karapinar, N. and Donat, R. (2004) The Removal of Heavy Metal Cations by Natural Zeolites. Journal of Colloid and Interface Science, 280, 309-314. https://doi.org/10.1016/j.jcis.2004.08.028
- 122. Shaheen, S.M., Derbalah, A.S. and Moghanm, F.S. (2012) Removal of Heavy Metals from Aqueous Solution by Zeolite in Competitive Sorption System. International Journal of Environmental Science and Development, 3, 362-367.https://doi.org/10.7763/IJESD.2012.V3.248
- 123. Emamverdian, A., Ding, Y., Xie, Y. and Sangari, S. (2018) Silicon Mechanisms to Ameliorate Heavy Metal Stress in Plants. BioMedical Research International, 2018, Article ID: 8492898. https://doi.org/10.1155/2018/8492898
- 124. Blanke, M.M., Bacher, W., Pring, R.J., and Baker, E.A. (1996) Ammonium Nutrition Enhance Chlorophyll and Glaucousness in Kohlrabi. Annals of Botany, 78, 559-604. https://doi.org/10.1006/anbo.1996.0166
- 125. He, C., Ma, J. and Wang, L. (2015) A Hemicelluloses-Bound Form of Silicon with Potential to Improve the Mechanical Properties and Regeneration of the Cell Wall of Rice. New Phytologist, 206, 1051-1062. https://doi.org/10.1111/nph.13282
- 126. Liu, J., Ma, J., He, C., Li, X., Zhang, W., Xu, F. and Wang, L. (2013) Inhibition of Cadmium Ion Uptake in Rice (Oryza sativa) Cells by a Wall-Bound Form of Silicon. New Pytologist, 200, 691-699. https://doi.org/10.1111/nph.12494
- 127. Richmond, K.E. and Sussman, M. (2003) Got Silicon? The Non-Essential Beneficial Plant Nutrient. Current Opinion in Plant Biology, 6, 268-272. https://doi.org/10.1016/S1369-5266(03)00041-4
- 128. Ma, J.F., Mitani, N., Nagao, S., Konishi, S., Tamai, K., Iwashita, T. and Yano, M. (2004) Characterization of the Silicon Uptake System and Molecular Mapping of the Silicon Transporter Gene in Rice. Plant Physiology, 136, 3284-3289. https://doi.org/10.1104/pp.104.047365
- 129. Quanzhi, Z. and Erming, G. (1998) Effect of Silicon Application on Rice in a Rice Area along the Yellow River. Department of Agronomy, 32, 308-313.
- 130. Inanaga, S., Okasaka, A. and Tanaka, S. (1995) Does Silicon Exist in Association with Organic Compounds in Rice Plant? Soil Science and Plant Nutrition, 41, 111-117. https://doi.org/10.1080/00380768.1995.10419564
- 131. Xie, Z., Song, F., Xu, H., Shao, H. and Song, R. (2014) Effects of Silicon on Photosynthetic Characteristics of Maize (Zea mays L.) on Alluvial Soil. The Scientific World Journal, 2014, Article ID: 718716. https://doi.org/10.1155/2014/718716
- 132. Chen, W., Yao, X., Cai, K. and Chen, J. (2011) Silicon Alleviates Drought Stress of Rice Plants by Improving Plant Water Status, Photosynthesis and Mineral Nutrient Absorption. Biological Trace Element Research, 142, 67-76.https://doi.org/10.1007/s12011-010-8742-x
- 133. Silva, O.N., Lobato, A.K.S., Ávila, F.W., Costa, R.C.L., Neto, C.O., Santos Filho, B.G. and Cardoso, M.S. (2012) Silicon-Induced Increase in Chlorophyll Is Modulated by the Leaf Water Potential in Two Water-Deficient Tomato Cultivars. Plant, Soil and Environment, 58, 481-486. https://doi.org/10.17221/213/2012-PSE
- 134. Ali, S., Farooq, M.A., Yasmeen, T., Hussain, S., Arif, M.S., Abbas, F. and Zhang, G. (2013) The Influence of Silicon on Barley Growth, Photosynthesis and Ultra-Structure under Chromium Stress. Ecotoxicology and Environmental Safety, 89, 66-72. https://doi.org/10.1016/j.ecoenv.2012.11.015
- 135. Farooq, M.A., Ali, S., Hameed, A., Ishaque, W., Mahmood, K. and Iqbal, Z. (2013) Alleviation of Cadmium Toxicity by Silicon Is Related to Elevated Photosynthesis, Antioxidant Enzymes; Suppressed Cadmium Uptake and Oxidative Stress in Cotton. Ecotoxicology and Environmental Safety, 96, 242-249. https://doi.org/10.1016/j.ecoenv.2013.07.006
- 136. Feng, J., Shi, Q., Wang, X., Wei, M., Yang, F. and Xu, H. (2010) Silicon Supplementation Ameliorated the Inhibition of Photosynthesis and Nitrate Metabolism by Cadmium (Cd) Toxicity in Cucumis sativus L. Scientia Horticulturae, 123, 521-530. https://doi.org/10.1016/j.scienta.2009.10.013
- 137. Yang, Y.H., Chen, S.M., Chen, Z., Zhang, H.Y., Shen, H.G., Hua, Z.C. and Li, N. (1999) Silicon Effects on Aluminum Toxicity to Mungbean Seedling Growth. Journal of Plant Nutrition, 22, 693-700. https://doi.org/10.1080/01904169909365664
- 138. Hussain, I., Ashraf, M.A., Rasheed, R., Asghar, A., Sajid, M.A. and Iqbal, M. (2015) Exogenous Application of Silicon at the Boot Stage Decreases Accumulation of Cadmium in Wheat (Triticum aestivum L.) Grains. Brazilian Journal of Botany, 38, 223-234. https://doi.org/10.1007/s40415-014-0126-6
- 139. Tripathi, D.K., Singh, V.P., Prasad, S.M., Chauhan, D.K., Dubey, N.K. and Rai, A.K. (2014) Silicon-Mediated Alleviation of Cr(VI) Toxicity in Wheat Seedlings as Evidenced by Chlorophyll Fluorescence, Laser Induced Breakdown Spectroscopy and Anatomical Changes. Journal Ecotoxicology and Environmental Safety, 113, 133-144.https://doi.org/10.1016/j.ecoenv.2014.09.029
- 140. Bae, E.J., Lee, K.S., Huh, M.R. and Lim, C.S. (2012) Silicon Significantly Alleviates the Growth Inhibitory Effects of NaCl in Salt-Sensitive ‘Perfection’ and ‘Midnight’ Kentucky Bluegrass (Poa pratensis L). Horticulture, Environment, and Biotechnology, 53, 477-483. https://doi.org/10.1007/s13580-012-0094-3
- 141. Tuna, A.L., Kaya. C., Higgs, D., Murillo-Amador, B., Aydemir, S. and Girgin, A.R. (2008) Silicon Improves Salinity Tolerance in Wheat Plants. Environmental and Experimental Botany, 62, 10-16. https://doi.org/10.1016/j.envexpbot.2007.06.006
- 142. Al-Aghabary, K., Zhu, Z. and Shi, Q.H. (2005) Influence of Silicon Supply on Chlorophyll Content, Chlorophyll Fluorescence, and Antioxidative Enzyme Activities in Tomato Plants under Salt Stress. Journal of Plant Nutrition, 27, 2101-2115. https://doi.org/10.1081/PLN-200034641
- 143. Hattori, T., Inanaga, S., Araki, H., An, P., Morita, S., Luxová, M. and Lux, A. (2005) Application of Silicon Enhanced Drought Tolerance in Sorghum bicolor. Physiologia Plantarum, 123, 459-466.https://doi.org/10.1111/j.1399-3054.2005.00481.x
- 144. Kawakami, K., Iwai, M., Ikeuchi, M., Kamiya, N. and Shen, J.R. (2007) Location of PsbY in Oxygen-Evolving Photosystem II Revealed by Mutagenesis and X-Ray Crystallography. FEBS Letters, 581, 4983-4987.https://doi.org/10.1016/j.febslet.2007.09.036
- 145. Lunde, C., Jensen, P.E., Haldrup, A., Knoetzel, J. and Scheller, H.V. (2000) The PSI-H Subunit of Photosystem I Is Essential for State Transitions in Plant Photosynthesis. Nature, 408, 613-615. https://doi.org/10.1038/35046121
- 146. Song, A., Li, P., Fan, F., Li, Z. and Liang, Y. (2014) The Effect of Silicon on Photosynthesis and Expression of Its Relevant Genes in Rice (Oryza sativa L.) under High-Zinc Stress. PLoS ONE, 9, e113782. https://doi.org/10.1371/journal.pone.0113782
NOTES
*本文共同第一作者为周淑芬和黄芷涵。
#通讯作者。
