QianRen Biology
Vol.02 No.03(2015), Article ID:15852,13
pages
10.12677/QRB.2015.23004
Research Advance in Single-Domain Antibody
Sheng Liu1, Jing Li1, Xingguo Liang1*, Huawei Xin2*
1College of Food Science and Engineering, Ocean University of China, Qingdao Shandong
2Institute of Biology and Medicine, Wuhan University of Science and Technology, Wuhan Hubei
*通讯作者。
Email: Liushengac@163.com, lijouc@ouc.edu.cn, *liangxg@ouc.edu.cn, *xinhuawei@wust.edu.cn
Received: Jul. 24th, 2015; accepted: Aug. 9th, 2015; published: Aug. 12th, 2015
Copyright © 2015 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



ABSTRACT
Single-domain antibody (sdAb) represents a new generation of engineered antibody fragment consisted of a single monomeric variable antibody domain, which is derived from camelid heavy- chain antibodies (HcAbs) or cartilaginous immunoglobin new antigen receptor (IgNAR). These novel antibodies are able to bind selectively to a specific antigen. Due to the advantageous features of sdAbs over conventional antibodies and their derivatives, such as smaller size, high affinity, high stability, high permeability in tissues, low immunogenicity and simple, cost-effective production, etc., sdAbs allow a broad range of applications in biotechnical and therapeutic use. This review provides a detailed overview of the developmental history, structural characteristics, expression and purification, properties and potential applications of the sdAbs. Some exciting thoughts and results of the shark sdAb are especially discussed. The risk assessment and problems with respect to future application of sdAbs have also been highlighted.
Keywords:Single-Domain Antibodies (sdAbs), Nanobodies (Nbs), Heavy-Chain Antibodies (HcAbs), Camel, Shark, Ig New Antigen Receptor (IgNAR)

单域抗体研究进展
刘晟1,李敬1,梁兴国1*,辛化伟2*
1中国海洋大学食品科学与工程学院,山东 青岛
2武汉科技大学生物医学研究院,湖北 武汉
Email: Liushengac@163.com, lijouc@ouc.edu.cn, *liangxg@ouc.edu.cn, *xinhuawei@wust.edu.cn
收稿日期:2015年7月24日;录用日期:2015年8月9日;发布日期:2015年8月12日

摘 要
单域抗体(single-domain antibody, sdAbs)是近年来利用基因工程技术从骆驼科动物和软骨鱼血清中克隆得到的只保留重链可变区的具有抗原结合活性的新型抗体,具有分子量小、特异性高、亲和性好、稳定性高、组织穿透力强、免疫原性低和制备成本低等优点,在诊断、治疗和检测等领域已取得一定成果并获得广泛关注。本文综述了单域抗体的发展历程、结构特征、表达纯化、理化性质及应用方向,特别是对进化上更早出现、抗原结合性能更加多样的鲨鱼sdAbs进行了详尽分析,并对其发展前景和可能面临的问题作了展望。
关键词 :单域抗体,纳米抗体,重链抗体,骆驼,鲨鱼,免疫球蛋白新抗原受体

1. 引言
自上世纪七十年代以来,杂交瘤技术(hybridoma technology),DNA重组(recombinant DNA),蛋白质工程(protein engineering)和噬菌体展示技术(phage display technology)为以抗体为主的生技药品在疾病的治疗和诊断中的研究和发展提供了一个新的平台[1] -[4] ,大大推动了生技药品在临床上的应用和商业上的推广。
1975年,Kohler和Milstein提出了小鼠杂交瘤技术 [3] ,为单克隆抗体(monoclonal antibodies, mAbs)的发展提供了理论基础。相比传统的从血清中提取的多克隆抗体,mAbs具有特异性高、均一性好的优点。只要细胞株不发生基因突变,杂交瘤细胞就可以在体外永久存活并传代 [5] ,产生所需的均一抗体。然而,由于mAbs固有的亲和性的局限性大大限制了其应用范围,其成本高、周期长、工序复杂、体积大、稳定性差、不同批次之间差异较大等不足也对mAbs的临床应用提出了挑战。1988年,Skerra等 [6] 首次运用基因工程技术和分子生物学操作技术在基因水平上设计并改造抗体基因结构,在细菌中同时表达出抗体重链可变区(heavy-chain variable, VH)和轻链可变区(light-chain variable, VL),成功获得了功能性重组抗体,称可变区抗体片段(Fv, Fragment of variable)。以此为基础,研究人员又将重链和轻链可变区进一步连接、获得了功能性单链可变区抗体片段(single-chain variable fragment, scFv)。如图1(a)所示,与之前获得的包含轻链(VL及CL)和重链可变区(VH)及一个重链恒定区(CH1)的抗原结合片段(antigen-binding fragment, Fab)的分子量(约50 kDa)相比,Fv/scFv的相对分子质量更小(约25 kDa),约为γ-免疫球蛋白(immunoglobulin-γ, IgG)全抗的1/6,具有分子量小、组织分布特异性强和免疫原性低等特点。由于缺少重链恒定区片段(Fc),Fv/scFv不能与细胞膜的Fc受体结合,而且分子小,可以穿过血脑屏障,大大扩展了其应用前景。此后,随着分子生物学等基础学科的不断成熟,基因重组技术的飞速发展和对抗体结构更深入的认识,出现了继多克隆抗体、单克隆抗体之后的第三代抗体—基因工程抗体。
1989年,Hamers-Casterman等 [7] 偶然发现骆驼(单峰驼)血液中有半数的抗体是重链抗体(heavy-chain antibodies, HCAbs),它是一种缺失了轻链的重链二聚体抗体(图1(a))。HCAbs普遍存在于单峰驼(Camelus dromedarius)、亚州双峰驼(Camelus bactrianus)、南美洲的大羊驼(Lama glama)、原驼(Lama guanicoe)、羊
 (a):骆驼IgG1是由两条重链(VH, CH1, Hinge, CH2和CH3)和两条轻链(VL和CL)组成的传统四聚体抗体;两种HCAbs,IgG2和IgG3,缺失了轻链和重链区的CH1,IgG 2的铰链区(hinge)比IgG 3稍长。其VHH在FR2区有4个氨基酸残基与VH有差异(F37, E44, R45, R/L/G47和V37, G44, L45, F/W47),导致重链与轻链分离。(b):软骨鱼IgNAR,同样缺失轻链,每条重链包括5个恒定区(CH1-5)和1个可变区(VNAR),分别在CH1和CH4形成二硫键,聚合成二聚体。
(a):骆驼IgG1是由两条重链(VH, CH1, Hinge, CH2和CH3)和两条轻链(VL和CL)组成的传统四聚体抗体;两种HCAbs,IgG2和IgG3,缺失了轻链和重链区的CH1,IgG 2的铰链区(hinge)比IgG 3稍长。其VHH在FR2区有4个氨基酸残基与VH有差异(F37, E44, R45, R/L/G47和V37, G44, L45, F/W47),导致重链与轻链分离。(b):软骨鱼IgNAR,同样缺失轻链,每条重链包括5个恒定区(CH1-5)和1个可变区(VNAR),分别在CH1和CH4形成二硫键,聚合成二聚体。
Figure 1. Schematic diagram of three types of camel IgG, shark IgNAR and sdAbs
图1. 骆驼的三种抗体、鲨鱼IgNAR和相应的sdAbs示意图
驼(Vicugna pacos)等种类的偶蹄目(Artiodactyla)胼足亚目(Tylopoda)骆驼科(Camelidae)动物血清中 [8] 。1995年之后,又相继在未免疫及免疫的铰口鲨(nurse shark, Ginglymostoma cirratum)、大星鲨(smooth dogfish, Mustelus canis)、白斑角鲨(spiny dogfish, Squalus acanthias)、鰩鱼(skate)和魟鱼(ray)等软骨鱼纲(Chondrichthyes)发现了与HCAbs相似的无轻链的免疫球蛋白新抗原受体(Ig new antigen receptor, IgNAR) [9] - [11] 。鲨鱼血清中含有高达约350 mM尿素(人体中含量低于20 mM),这种恶劣的体内环境极大地增加了IgNAR的稳定性和溶解性。
1997年,Ghahroudi等 [12] 利用噬菌体展示技术获得骆驼重链可变区片段(variable domain of heavy chain of heavy-chain antibody, VHH)基因库,经多轮淘选后得到了只含有一个结构域的最小单元抗原结合片段,被称为单域抗体(single-domain antibodies, sdAbs)。其后,研究人员又从鲨鱼新抗原受体可变区片段(variable NAR, VNAR)基因库 [13] 中获得了结构相似的sdAbs。这种椭球形的小分子抗体的直径仅2.5 nm,长4 nm,相对分子质量仅15 kDa,因此也被称为纳米抗体(nanobodies, Nbs) [14] 。其固有的高特异性、高亲和力和高热稳定性使得sdAbs在其被发现后20多年的研究中取得了瞩目的成果,成为生技药品的一颗新星,在免疫、诊断、医学成像、检测等领域中受到越来越多的关注。
目前已有文献分别综述了骆驼源sdAbs的结构、特性和应用等方面内容,但都不太全面,极少涉及在进化上更早的以鲨鱼为代表的海洋软骨鱼类sdAbs相关研究进展,且近年来sdAbs相关的研究发展较快,涌现出许多新的研究成果和新技术。因此本文综述了近年来单域抗体研究进展,特别是鲨鱼sdAbs的结构及特性,并展望了单域抗体的应用前景和面临的问题。
2. 重链抗体和单域抗体结构特征
单域抗体是重链抗体的可变区结构域,其结构影响抗体VH与VL的结合,决定着抗体的许多性质(图1)。重链抗体广泛存在于骆驼科和软骨鱼纲动物血清中,是趋同进化的典型例证,但由于进化起源上的差异等因素 [10] ,二者的抗体间有许多不同特征。
由于骆驼科动物养殖容易,研究方便,并与人源IgG同源,故早些年的研究对象主要集中于骆驼科动物。如图1(a)所示,骆驼血清中的IgG有三种类型 [15] :由两条重链和两条轻链组成的传统四聚体抗体 IgG1,其相对分子质量和人源IgG接近,为150 kDa;两种缺失轻链和重链的CH1区的HcAbs (IgG2和IgG3),其相对分子质量分别为92 kDa和90 kDa,这是由于IgG2的铰链区(hinge)要长于IgG3型抗体 [16] 。氨基酸序列分析发现 [2] ,骆驼VHH与VH在第二个框架区(framework region 2, FR2)有4个标志性的氨基酸残基不相同 [17] [18] ,使得VHH不能与VL对应的区域结合,进而形成独特的HCAbs重链二聚体抗体结构。
目前所发现的骆驼科动物血清中均同时存在传统IgG和HCAbs,只是二者所占的比例在不同动物中不尽相同 [19] ,在普通的双峰驼中,HCAbs的比例可达50%~80%,而在南美洲大羊驼中可能仅占10%~25%。这对于我们选择何种免疫过的骆驼作为我们的研究对象尤为关键。
随着研究的深入,近年来,sdAbs在以鲨鱼为主的软骨鱼中的研究也越来越受到关注。与骆驼HCAbs相比,软骨鱼IgNAR有5个重链恒定区(constant region of heavy chain, CH),即CH1-5,其晶体结构尚未被探知。与骆驼VHH相同,利用基因工程技术和噬菌体展示技术同样可以将鲨鱼VNAR表达为鲨鱼单域抗体(shark sdAbs),根据VNAR中二硫键的数目与位置 [21] ,研究人员将鲨鱼IgNAR分为三种类型(图2)。每个VNAR均含有两个抗原互补决定区(complementarity-determining region, CDR)和两个超变区(hypervariable region, HV)。其中传统的CDR2缺失,被HV2取代,形成一个柔性接头,连接FR2和FR3。
I型IgNAR只在铰口鲨中被发现过 [22] ,或许只存于这一个物种中,其VNAR的FR2和FR4区分别有一个半胱氨酸(Cys)残基,而其CDR3有两个Cys残基。对I型IgNAR的VNAR晶体结构分析(图3(a)和图3(b) [20] ),研究人员发现除去与其他型VNAR、人类VH、骆驼VHH的FR1和FR3存在的一对维持基本结构的典型的二硫键外,其FR2与CDR3和CDR3与FR4之间还存在两对非典型的二硫键,这使得CDR3折叠成更紧密的结构,并指向HV2。这种结构大大增加了抗体的热稳定性,但同时也限制了其结构的灵活性,因此降低了抗体对抗原的多样性。
II型IgNAR在VNAR的CDR1与CDR3之间形成非典型二硫键,使得CDR3可以像骆驼的VHH (图3(c))的CDR3一样伸展出来,其VNAR的构像与框架也与VHH比较相似。
III型IgNAR一般只在软骨鱼年幼时(小于1岁)出现 [23] ,其结构与II型IgNAR比较相似,只是在VNAR的CDR1有一个保守的色氨酸(Trp/W)残基。其CDR3仅有约15个氨基酸残基,缺乏多样性,却形成保护新生鱼的第一道防线 [11] ,在成年软骨鱼免疫系统成熟的过程中发挥了重要作用。
另外,Liu等 [24] 还发现了没有形成二硫键的IIb型以及同IIb型结构相似但和III型一样在CDR1有一个保守色氨酸残基的IIIb型IgNAR。但制备出的缺少二硫键的sdAbs稳定性差,亲和力低,其应用前景有限。
 黑色为与人源VH和驼源VHH相同的典型二硫键,绿色和红色为非典型二硫键。
黑色为与人源VH和驼源VHH相同的典型二硫键,绿色和红色为非典型二硫键。
Figure 2. Three IgNAR isotypes from chondrichthyes divided by the numble and site of VNAR
图2. 软骨鱼IgNAR按VNAR的二硫键的数目和位置划分出的三种类型
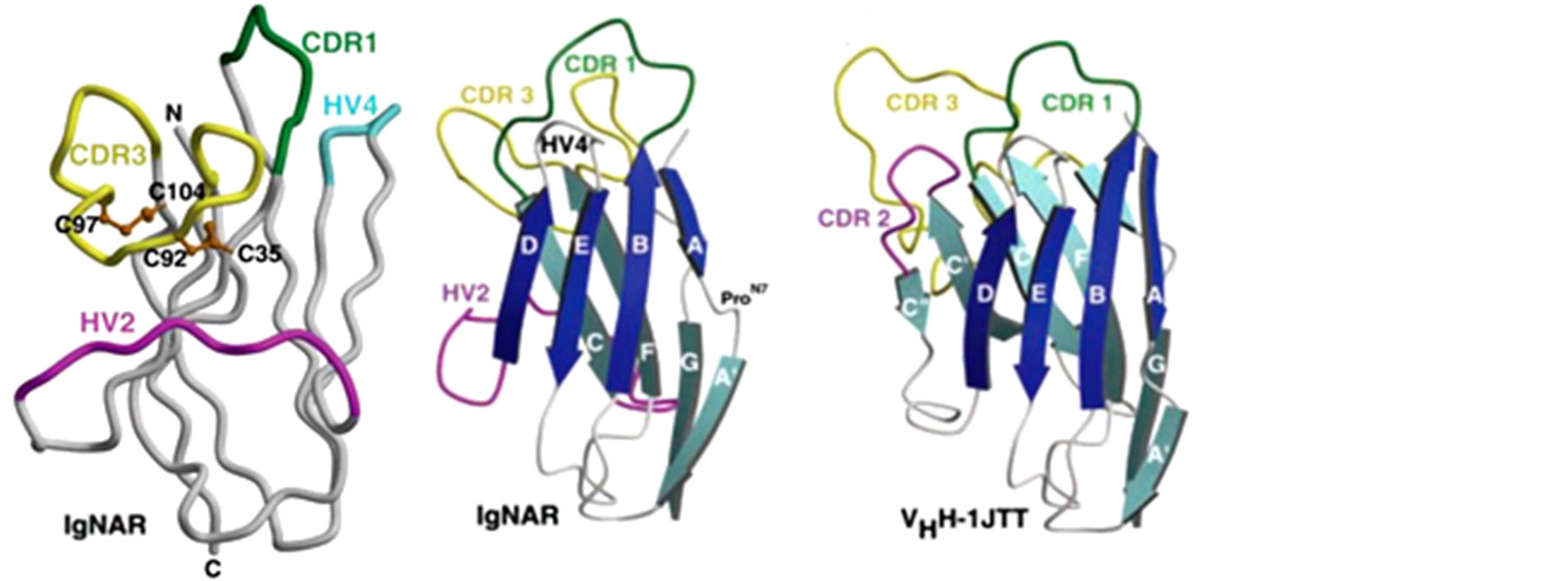 (a)(b)(c)(a):铰口鲨Ⅰ型IgNAR的VNAR区晶体结构图,有两对二硫键分别连接FR2与CDR3和CDR3与FR4,使得CDR3的螺旋折叠区指向HV2。(b):Ⅰ型VNAR区晶体结构图,其结构相比驼源VHH更紧凑,比VHH约小20%。(c):骆驼VHH区晶体结构图,只有一对二硫键连接CDR1和CDR3,使得CDR3螺旋伸展出来。
(a)(b)(c)(a):铰口鲨Ⅰ型IgNAR的VNAR区晶体结构图,有两对二硫键分别连接FR2与CDR3和CDR3与FR4,使得CDR3的螺旋折叠区指向HV2。(b):Ⅰ型VNAR区晶体结构图,其结构相比驼源VHH更紧凑,比VHH约小20%。(c):骆驼VHH区晶体结构图,只有一对二硫键连接CDR1和CDR3,使得CDR3螺旋伸展出来。
Figure 3. Crystal structures of VNAR and VHH [20]
图3. VNAR与VHH晶体结构图 [20]
3. 单域抗体的表达与纯化
目前,sdAbs已经可以成熟地采用基因工程的方法进行大量表达,sdAbs制备的核心是建立一个容量较大的基因库,并从中淘选出目的基因片段。
3.1. 单域抗体基因文库的建立
驼源与鲨鱼源sdAbs在抗体的表达纯化及特性分析的操作方法相仿,一般从新鲜的经免疫的或未经免疫的动物外周血液淋巴细胞中提取RNA,逆转录后建立抗体cDNA文库,之后可借助噬菌体展示技术 [25] 将抗体cDNA连接到噬菌粒载体转化到大肠杆菌(图4),再进行基因库的保藏与目标基因的淘选工作,以大大提高目标产物的筛选效率,也有的采用酵母展示技术 [26] [27] 和核糖体展示技术 [28] [29] 等。
根据抗体基因的来源,我们将噬菌体抗体文库分为两类 [30] [31] :天然库(naïve library)和合成库
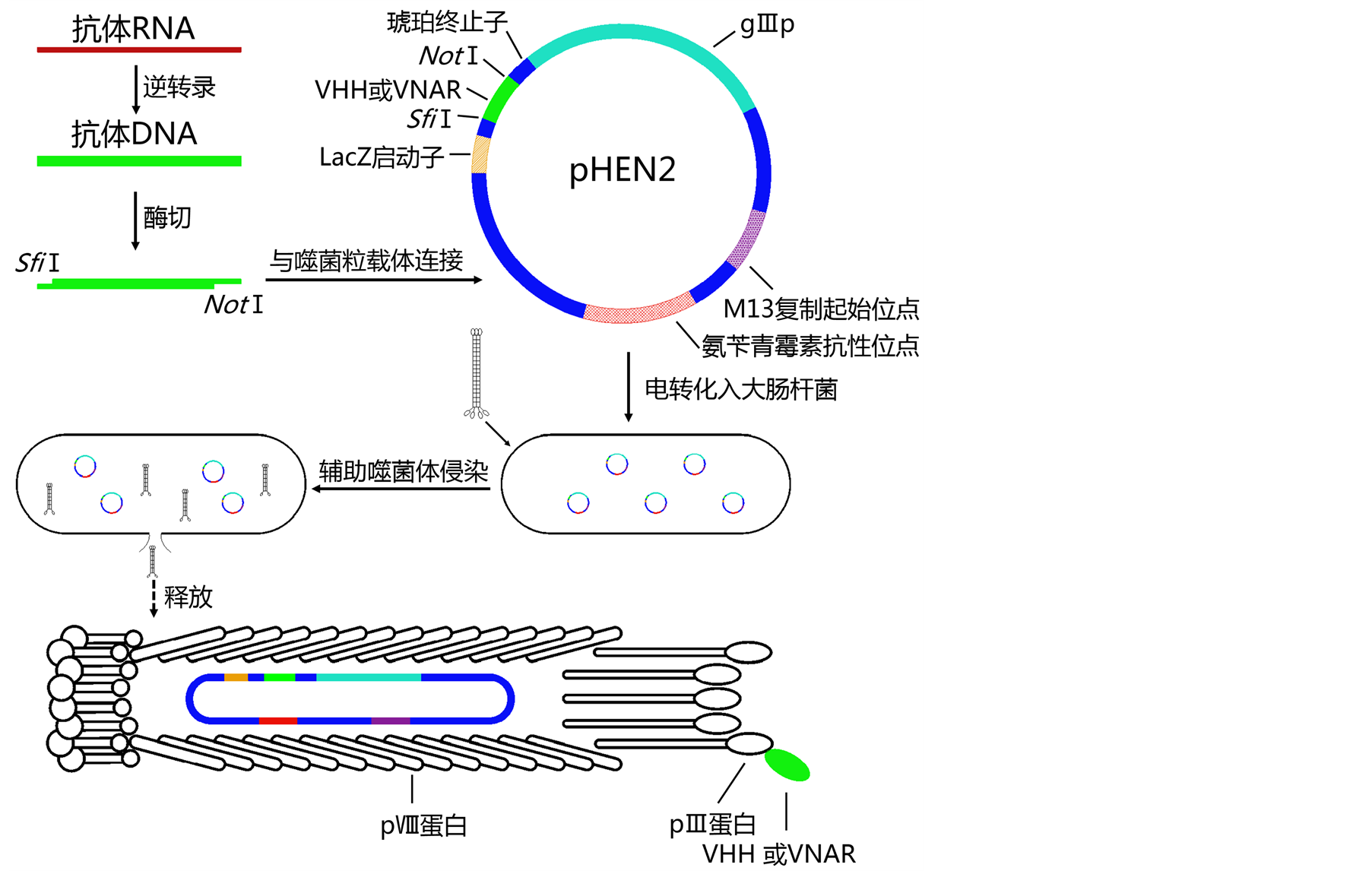 从外周血淋巴细胞中提取的抗体RNA经逆转录后形成cDNA第一条链,PCR扩增后形成双链的cDNA文库。抗体DNA经双酶切后与具有两个对应的粘性末端(SfiI和NotI)的pHEN2噬菌粒载体连接,电转化至电感受态大肠杆菌,形成噬菌体文库。淘选前,需用烈性的辅助噬菌体侵染大肠杆菌,激活重组噬菌粒的表达,使大肠杆菌裂解。表达出的pIII-VHH(VNAR)融合蛋白可随机地装载到噬菌体衣壳蛋白上。
从外周血淋巴细胞中提取的抗体RNA经逆转录后形成cDNA第一条链,PCR扩增后形成双链的cDNA文库。抗体DNA经双酶切后与具有两个对应的粘性末端(SfiI和NotI)的pHEN2噬菌粒载体连接,电转化至电感受态大肠杆菌,形成噬菌体文库。淘选前,需用烈性的辅助噬菌体侵染大肠杆菌,激活重组噬菌粒的表达,使大肠杆菌裂解。表达出的pIII-VHH(VNAR)融合蛋白可随机地装载到噬菌体衣壳蛋白上。
Figure 4. Schematic diagram of sdAbs phage display
图4. SdAbs的噬菌体展示示意图
(synthetic library)。天然库是未加修饰的完全拷贝动物的抗体基因建立的基因库;合成库包括全合成库和半合成库(semi-synthetic library)。因抗体的框架区基因序列具有一定保守性,而CDR的基因序列则多变并决定抗体的抗原结合性质。因此,半合成库是将天然库与全合成库相结合 [32] ,只合成相对高变的序列,在大大减少工作量的同时保证噬菌体抗体文库的多样性。目前相关研究主要采用半合成库,其成功度取决于文库的大小和质量。一个能让人满意的噬菌体抗体文库至少要达到109个单位的拷贝数 [33] ,这将直接影响最终筛选出的单域抗体的特异性、亲和性以及稳定性。从头开始建立一个全新的基因库需要约4~6个月,市面上已经有相关的sdAbs噬菌体基因库出售 [11] 。
噬菌体展示技术一般选用pIII蛋白和pVIII蛋白展示,前者是噬菌体尾部蛋白的组成部分,可以允许大片段的插入,后者是噬菌体衣壳蛋白,只能展示一段非常短的肽链(6~8个残基),如图4所示。而编码驼源VHH的基因约为360 nt (IgNAR与之相仿),表达的sdAbs约由120个氨基酸残基组成,因此研究者通常采用PHEN2,MS2等 [34] 噬菌体进行基于pⅢ蛋白的单价展示。这些噬菌体一般是溶源性温和噬菌体,在短时间内不能快速增殖,因此在进行扩增淘选时还需要在辅助噬菌体 [35] 帮助下裂解宿主细胞。
3.2. 目标抗体的淘选
淘选(panning)是从海量的噬菌体抗体文库中采用固相化的抗原富集特异性目标抗体基因的过程。由于噬菌体展示技术可以使融合蛋白表达在噬菌体衣壳表面,利用抗原富集目标抗体的同时使携带了目标抗体基因的噬菌体富集。将这些目标抗体基因分离出来可以很容易地进行表达纯化与分析。
要得到特异性较高的目标抗体基因一般需三轮淘选,每轮淘选可以纯化约4~10倍 [36] 。由于文库中每种噬菌体抗体的拷贝数很少,而库容量巨大,因此第一轮淘选条件要尽量地非严谨,否则会适得其反,增加偶然因素造成的风险。第一轮淘选后,每种噬菌体的拷贝数大幅提高,随后其操作严谨性也应大幅提高。
3.3. 重组抗体的表达纯化
SdAbs可在细菌、真菌和植物中大量表达,即便是在细菌中也能够形成二硫键,可保证目标sdAbs结构的正确性和功能的完整性。在表达前,通常给目标抗体基因加上组蛋白标签(6XHis tag),这样就可以通过固相金属镍亲和柱高效地纯化目标抗体。如有必要,还可以经凝胶过滤等蛋白质分离技术对目标抗体进一步纯化。如Liu等 [37] 获得的羊驼和鲨鱼sdAbs产物的得率分别为10~20 mg/L和0.5~1.0 mg/L。
4. 单域抗体的理化性质
4.1. 单域抗体对抗原的特异性
单域抗体CDR区是主要的抗原结合区域,其基因库的多样性决定了淘选得到的目标抗体对抗原的特异识别能力。Liu等 [38] 淘选出的鲨鱼源抗葡萄球菌肠毒素B(SEB)和抗蓖麻毒素(ricin) sdAbs对结构相似的非特异性抗原的结合率分别低于1%和0.1%,表现出高度抗原结合选择性。目前,研究者们已经制备出多种具有高度特异性的基于VHH或IgNAR的sdAbs,可靶向于噬菌体、病毒、细菌和真菌等微生物及其分泌的毒素等大分子蛋白质。如Walper、Graef和Goldman等从驼源和鲨鱼单域抗体库中淘选出高特异性抗炭疽芽孢杆菌(Bacillus anthracis)的孢子和营养细胞 [39] ,抗金黄色葡萄球菌肠毒素B (Staphylococcus aureus Enterotoxin B, SEB) [40] ,抗相思子凝集素(abrus agglutinin) [41] 的sdAbs,可用于免疫和检测等领域。
4.2. 单域抗体的亲和性
目前,关于人源scFv抗体的研究也很热门。scFv是由一条VL和一条VH随机地连接而成,这使得抗体的多样性提高,但同时也会因其不确定的组合而降低抗体的亲和性。而sdAbs则没有这方面的后顾之忧,如驼源VHH的热动力学结合与解离常数为105~106 M−1∙s−1 (kon)和10−2~10−4 s−1 (kd),表现出良好的亲和性 [33] 。Liu等 [38] 淘选出的亲和力更高的铰口鲨源sdAbs,因其HV4的特殊序列,与抗原间的范德华力更强,其解离常数仅10−7 s−1,与传统IgG相当。另外,由于sdAbs的CDR3区较长,可以深入到抗原内部与之更好地结合。
4.3. 单域抗体的稳定性
热稳定性是sdAbs相对于传统IgG、Fab、scFv抗体最明显的优势。一方面由于sdAbs分子尺寸小,结构紧凑;另一方面,VNAR非典型二硫键也赋予了sdAbs优良的热稳定性 [40] [42] 。SdAbs的变性温度在70℃~80℃之间 [37] [43] ,远高于scFv的57℃ [44] 。即便在95℃下加热一小时,来源于星鲨的sdAbs仍然能够保持一定的活性 [24] ,这一性质大大拓展了sdAbs的应用领域。有趣的是,研究人员还发现,有些sdAbs的活性会在复性后获得更好的活性,可能是由于复性过程使蛋白质更好地折叠 [38] 。
额外的非典型二硫键不仅影响sdAbs分子的热稳定性,同时还影响其化学稳定性。这使得它可以在约60℃~80℃、2.3~3.3 M的盐酸胍溶液中保持活性 [45] ,抵抗变性剂引起的蛋白质失活,这一特性使得sdAbs可以加入到洗发水中治疗因头皮屑中的糠秕马拉色氏霉菌引起的皮下感染 [46] 。同时这些二硫键还可以保护sdAbs避免被胰凝乳蛋白酶降解 [47] ,因此这类sdAbs还可以用于口服。然而,为增加sdAbs的亲和力和稳定性而人为地增加二硫键数目或改变二硫键位置的同时也会影响CDR3对抗原的特异性,故需要综合考虑。
5. 单域抗体在科研中的应用
作为一类相对分子质量小、特异性高、亲和性好、稳定性高的新型纳米抗体,sdAbs同时还具有周期短(在有完整抗体库下,筛选周期仅1~2个月)、成本低、货架期长等优点。其可开发成诊断和治疗疾病的试剂,作为科学研究的新工具,或者作为检测工具的新型材料,已经在医疗、科研及检测等领域中崭露头角。至2014年 [11] [48] ,研究人员基于骆驼和鲨鱼已开发出约80种sdAbs,用于免疫、治疗和传感器领域,其中有7种处于I期临床试验阶段,3种处于I/II期,4种处于II期。目前,尚无相关的sdAbs产品上市。
5.1. 单域抗体在医学中的应用
SdAbs最主要的应用是代替传统IgG抗体或mAbs抗体等在诊断、治疗和免疫检测中的应用。由于sdAbs可以很容易地在细菌或真菌中大量表达,并且其稳定性高、药效好、货架期长,可降低诊断病菌和中和毒素的成本。
由于sdAbs相对分子质量小、尺寸小,并且缺失了Fc结构域,从而避免了与其它免疫蛋白分子如补体蛋白相结合,不会聚合形成大分子抗体衍生物,因此免疫原性低 [49] ,并且易于携带靶向药物进入细胞内部 [50] 或穿越血脑屏障 [51] ,对传统药物很难到达的部位进行治疗。另外,也可筛选出特定的sdAbs直接作用于许多可进入并定位于细胞内或大脑区域的毒素,如蓖麻毒素(ricin)、肉毒梭菌神经毒素(Clostridium botulinum neurotoxin, BoNT)等,这样的sdAbs不仅可以用于检测还可以用于中和毒素 [52] [53] 。
SdAbs还可以用于医学显影方面 [54] ,如通过识别表皮生长因子受体(epidermal growth factor receptor, EGFR)等靶标来诊断肿瘤 [55] [56] 。应用传统单克隆抗体进行放射性元素(99mTc或68Ga)标记 [57] 和体内显影的缺点是,由于标记抗体分子量较大,组织穿透性差,显像剂滞留于血液中,而且抗体的半衰期长(可达数天),影响图像质量 [58] [59] ,并导致检测间隔长,限制了单克隆抗体在人体中的应用。而sdAbs相对分子质量小,半衰期仅1~2小时 [54] ,存在着在体内时间过短、放射性元素剂量过大等问题。因此可以将抗体N端和C端融合形成多价聚合体 [60] [61] ,适当延长其半衰期,降低辐射量,降低信噪比,增强显影效果。这样一方面可减少剂量,减轻肾脏负担,另一方面增加抗体亲和力。
5.2. 单域抗体在科研中的应用
SdAbs还因其对靶标物质的高亲和性和识别位点的多样性等特点被用来作为科学研究的辅助工具。如将sdAbs结合到亲和色谱柱的固定相上来捕获特定组分,在蛋白质纯化中获得良好的效果 [62] 。通过将绿色荧光蛋白(green fluorescent protein, GFP)等荧光蛋白基因与sdAbs基因融合 [63] [64] ,构建标记的重组抗体,在体内表达,可跟踪抗原在体内的活动。并且由于许多细菌病毒等抗原一般含有多抗原表位,而sdAbs分子尺寸小,其空间位阻小,可以更多地结合到抗原表面 [65] ,提高观测的灵敏度。
基于抗体可与特异性蛋白质深入结合、稳定蛋白质构象的特点,很早就有应用抗体辅助不稳定蛋白质结晶来分析其结构和功能特性的提议和尝试;然而传统抗体都或多或少地存在分子量大、不稳定等因素 [66] [67] ,导致结晶效果一直不尽人意。因此,sdAbs的快速发展为这一设想提供了新的突破口,目前已经可以制备重组抗体亲和试剂作为结晶伴侣试剂 [68] 。这些亲和试剂的小尺寸和灵活的CDR3片段可以插入高度动态下的蛋白质空隙中,或与蛋白质表面位点紧密结合,保护疏水位点,稳定蛋白质构象,与蛋白质形成共结晶 [69] ,通过X射线衍射技术来探知蛋白质的空间结构。
5.3. 单域抗体在检测中的应用
从骆驼和鲨鱼抗体基因库中筛选出的抗霍乱毒素和葡萄球菌肠毒素的高亲和性sdAbs可与金纳米颗粒(gold nanoparticles, AuNPs)共轭结合后组成复合材料用于生物传感器上 [70] [71] ,实现对靶标物的快速检测。另一个应用是用于酶联免疫吸附反应(enzyme-linked immunosorbent assay, ELISA)的双抗夹心法中替代二抗 [24] [72] ,降低检测成本,获得更准确的结果。Anderson等 [73] 还探索了用sdAbs制作量子点的方法,成功制作出粒径仅12 nm的用二氢硫辛酸(dihydrolipoic acid, DHLA)配基包裹的靶向蓖麻毒素的sdAbs量子点,用于表面等离子体谐振(surface plasmon resonance, SPR)检测蓖麻毒素,检测限仅为mAbs同类的1/2。
SdAbs的另一个特点是可识别小分子的半抗原位点,这是传统抗体通常所不能做到的。利用这一特性可将sdAbs组装在试纸条中用于海关机场等场所,用免疫层析法检测除草剂、TNT和治疗药物等敏感物质 [74] - [76] ,这种方法可简化检测步骤,降低检测时间和费用,并且在设备缺乏的地区也可准确快速检测。
6. 前景展望与存在的问题
单域抗体因其小尺寸、高特异性、高亲和性、高稳定性、高组织穿透性、低免疫原性和生产周期短等优点,在其被发现的二十多年时间里,在免疫、诊断、疾病治疗、材料、晶体学和食品科学等领域均取得丰硕的成果,其更是为各类学科创建了一个新的研究平台。
无可否认,单域抗体将在未来的研究与应用中发挥越来越重要的作用,但同时也要正视其在目前研究中的困境。虽然一些筛选出的抗体药物已经获得批准,进入II期临床试验阶段 [77] ,但有研究指出,大剂量重复使用融合多价单域抗体也将使机体对单域抗体产生免疫原性,最终结果只有通过一定数目病人的临床试验研究才会得以展现 [11] 。另外,虽然目前筛选特异性抗体的时间已经大大缩短,但要获得具有完整功能的理想产品仍然是一件费时费力的事。与传统抗体的应用规模,系统成熟度和市场认可度相比,单域抗体的研究和应用还处于探索阶段,其大规模的应用还需克服相当的困难,解决一些现实的问题。当然,这些问题将随着研究的深入,分析手段和分析工具的革新而逐步解决。
我国目前关于单域抗体的研究还处于起步阶段。一方面是由于单域抗体发展时间较短,研究还处于基础阶段;另一方面受限于骆驼和鲨鱼等研究材料的高价格。贺生芳 [78] 和丁志凌 [79] 分别对Intein介导的单域抗体在大肠杆菌中的表达纯化和99mTc标记的单域抗体在肿瘤放射免疫显影中作了初步探究,取得了不错的进展。未来单域抗体将成为一个强大的工具箱来辅助尖端科学的研究,在高特异性药剂的研发、胞内成像、结晶、快速检测及食品解毒等领域具有不可估量的发展潜力。
基金项目
本成果获得国家自然科学基金面上项目(31371385)和山东省自然科学基金杰出青年基金(JQ201204)资助。
文章引用
刘 晟,李敬,梁兴国,辛化伟. 单域抗体研究进展
Research Advance in Single-Domain Antibody[J]. 千人·生物, 2015, 02(03): 26-38. http://dx.doi.org/10.12677/QRB.2015.23004
参考文献 (References)
- 1. Cohen, S.N., Chang, A.C., Boyer, H.W. and Helling, R.B. (1973) Construction of biologically functional bacterial plasmids in vitro. Proceedings of the National Academy of Sciences of the United States of America, 70, 3240-3244. http://dx.doi.org/10.1073/pnas.70.11.3240
- 2. De, M.A. (2011) Biotechnological applications of recombinant sin-gle-domain antibody fragments. Microbial Cell Factories, 10, 44-58. http://dx.doi.org/10.1186/1475-2859-10-44
- 3. Kohler, G. and Milstein, C. (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 256, 495-497. http://dx.doi.org/10.1038/256495a0
- 4. Smith, G.P. (1985) Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science (New York, NY), 228, 1315-1317. http://dx.doi.org/10.1126/science.4001944
- 5. Weiner, L.M. (2006) Fully human therapeutic monoclonal antibodies. Journal of Immunotherapy, 29, 1-9. http://dx.doi.org/10.1097/01.cji.0000192105.24583.83
- 6. Skerra, A. and Pluckthun, A. (1988) Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science (New York, NY), 240, 1038-1041. http://dx.doi.org/10.1126/science.3285470
- 7. Hamers-Casterman, C., Atarhouch, T., Muyldermans, S., Robinson, G., Hamers, C., Songa, E.B., Bendahman, N. and Hamers, R. (1993) Naturally occurring antibodies devoid of light chains. Nature, 363, 446-448. http://dx.doi.org/10.1038/363446a0
- 8. Muyldermans, S. and Lauwereys, M. (1999) Unique single-domain antigen binding fragments derived from naturally occurring camel heavy-chain antibodies. Journal of Molecular Recognition, 12, 131-140. http://dx.doi.org/10.1002/(SICI)1099-1352(199903/04)12:2<131::AID-JMR454>3.0.CO;2-M
- 9. Greenberg, A.S., Avila, D., Hughes, M., Hughes, A., Mckinney, E.C. and Flajnik, M.F. (1995) A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature, 374, 168-173. http://dx.doi.org/10.1038/374168a0
- 10. Flajnik, M.F., Deschacht, N. and Muyldermans, S. (2011) A case of convergence: Why did a simple alternative to canonical antibodies arise in sharks and camels? Plos Biology, 9, 1-5. http://dx.doi.org/10.1371/journal.pbio.1001120
- 11. Kovaleva, M., Ferguson, L., Steven, J., Porter, A. and Barelle, C. (2014) Shark variable new antigen receptor biologics— A novel technology platform for therapeutic drug development. Expert Opinion on Biological Therapy, 14, 1527-1539. http://dx.doi.org/10.1517/14712598.2014.937701
- 12. Goyvaerts, C., Robays, L., De Groeve, K., Raes, G., De Baetselier, P., Thielemans, K. and Breckpot, K. (2010) Targeting lentiviral vectors to dendritic cells by the nanobody display technology. Human Gene Therapy, 21, 1440-1441.
- 13. Nuttall, S.D., Krishnan, U.V., Hattarki, M., De Gori, R., Irving, R.A. and Hudson, P.J. (2001) Isolation of the new antigen receptor from wobbegong sharks, and use as a scaffold for the display of protein loop libraries. Molecular Immunology, 38, 313-326. http://dx.doi.org/10.1016/S0161-5890(01)00057-8
- 14. Rahbarizadeh, F., Ahmadvand, D. and Sharifzadeh, Z. (2011) Nanobody: An old concept and new vehicle for immunotargeting. Immunological Investigations, 40, 299-338. http://dx.doi.org/10.3109/08820139.2010.542228
- 15. Swain, M.D., Anderson, G.P., Zabetakis, D., Bernstein, R.D., Liu, J.L., Sherwood, L.J., Hayhurst, A. and Goldman, E.R. (2010) Llama-derived single-domain antibodies for the detection of botulinum A neurotoxin. Analytical and Bioanalytical Chemistry, 398, 339-348. http://dx.doi.org/10.1007/s00216-010-3905-3
- 16. Smolarek, D., Hattab, C., Hassanzadeh-Ghassabeh, G., Cochet, S., Gutiérrez, C., de Brevern, A.G., et al. (2010) A recombinant dromedary antibody fragment (VHH or nanobody) directed against human Duffy antigen receptor for chemokines. Cellular and Molecular Life Sciences, 67, 3371-3387. http://dx.doi.org/10.1007/s00018-010-0387-6
- 17. Bond, C.J., Wiesmann, C., Marsters, J.C. and Sidhu, S.S. (2005) A structure-based database of antibody variable domain diversity. Journal of Molecular Biology, 348, 699-709. http://dx.doi.org/10.1016/j.jmb.2005.02.063
- 18. Araste, F., Ebrahimizadeh, W., Rasooli, I., Rajabibazl, M. and Gargari, S.L.M. (2014) A novel VHH nanobody against the active site (the CA domain) of tumor-associated, carbonic anhydrase isoform IX and its usefulness for cancer diagnosis. Biotechnology Letters, 36, 21-28. http://dx.doi.org/10.1007/s10529-013-1340-1
- 19. Blanc, M.R., Anouassi, A., Abed, M.A., Tsikis, G., Canepa, S., Labas, V., Belghazi, M. and Bruneau, G. (2009) A one-step exclusion-binding procedure for the purification of functional heavy-chain and mammalian-type gamma- globulins from camelid sera. Biotechnology and Applied Biochemistry, 54, 207-212. http://dx.doi.org/10.1042/BA20090208
- 20. Stanfield, R.L., Dooley, H., Flajnik, M.F. and Wilson, I.A. (2004) Crystal structure of a shark single-domain antibody V region in complex with lysozyme. Science, 305, 1770-1773. http://dx.doi.org/10.1126/science.1101148
- 21. Dooley, H. and Flajnik, M.F. (2006) Antibody repertoire development in cartilaginous fish. Developmental and Comparative Immunology, 30, 43-56. http://dx.doi.org/10.1016/j.dci.2005.06.022
- 22. Stanfield, R.L., Dooley, H., Flajnik, M.F. and Wilson, I.A. (2004) Crystal structure of a shark single-domain antibody V region in complex with lysozyme. Science, 305, 1770-1773. http://dx.doi.org/10.1126/science.1101148
- 23. Diaz, M., Stanfield, R.L., Greenberg, A.S. and Flajnik, M.F. (2002) Structural analysis, selection, and ontogeny of the shark new antigen receptor (IgNAR): Identification of a new locus preferentially expressed in early development. Immunogenetics, 54, 501-512. http://dx.doi.org/10.1007/s00251-002-0479-z
- 24. Liu, J.L., Anderson, G.P., Delehanty, J.B., Baumann, R., Hayhurst, A. and Goldman, E.R. (2007) Selection of cholera toxin specific IgNAR single-domain antibodies from a naive shark library. Molecular Immunology, 44, 1775-1783. http://dx.doi.org/10.1016/j.molimm.2006.07.299
- 25. Shoae-Hassani, A., Mortazavi-Tabatabaei, S.A., Sharif, S., Madadi, S., Rezaei-Khaligh, H. and Verdi, J. (2013) Recombinant lambda bacteriophage displaying nanobody towards third domain of HER-2 epitope inhibits proliferation of breast carcinoma SKBR-3 cell line. Archivum Immunologiae et Therapiae Experimentalis, 61, 75-83. http://dx.doi.org/10.1007/s00005-012-0206-x
- 26. Schroter, C., Gunther, R., Rhiel, L., Becker, S., Toleikis, L., Doerner, A., Becker, J., Schonemann, A., Nasu, D., Neuteboom, B., Kolmar, H. and Hock, B. (2015) A generic approach to engineer antibody pH-switches using combinatorial histidine scanning libraries and yeast display. mAbs, 7, 138-151. http://dx.doi.org/10.4161/19420862.2014.985993
- 27. Boder, E.T., Raeeszadeh-Sarmazdeh, M. and Price, J.V. (2012) Engineering antibodies by yeast display. Archives of Biochemistry and Biophysics, 526, 99-106. http://dx.doi.org/10.1016/j.abb.2012.03.009
- 28. Heyduk, E. and Heyduk, T. (2014) Ribosome display enhanced by next generation sequencing: A tool to identify antibody-specific peptide ligands. Analytical Biochemistry, 464, 73-82. http://dx.doi.org/10.1016/j.ab.2014.07.014
- 29. Pan, Y.B., Mao, W.P., Liu, X.X., Xu, C., He, Z.J., Wang, W.Q. and Yan, H. (2012) Selection of single chain variable fragments specific for the human-inducible costimulator using ribosome display. Applied Biochemistry and Biotechnology, 168, 967-979. http://dx.doi.org/10.1007/s12010-012-9800-y
- 30. Jiang, W.Z., Rosenberg, J.N., Wauchope, A.D., Tremblay, J.M., Shoemaker, C.B., Weeks, D.P. and Oyler, G.A. (2013) Generation of a phage-display library of single-domain camelid VHH antibodies directed against Chlamydomonas reinhardtii antigens, and characterization of V(H)Hs binding cell-surface antigens. Plant Journal, 76, 709-717. http://dx.doi.org/10.1111/tpj.12316
- 31. Abbady, A.Q., Al-Mariri, A., Zarkawi, M., Al-Assad, A. and Muyldermans, S. (2011) Evaluation of a nanobody phage display library constructed from a Brucella-immunised camel. Veterinary Immunology and Immunopathology, 142, 49- 56. http://dx.doi.org/10.1016/j.vetimm.2011.04.004
- 32. Goldman, E.R., Anderson, G.P., Liu, J.L., Delehanty, J.B., Sherwood, L.J., Osborn, L.E., Cummins, L.B. and Hayhurst, A. (2006) Facile generation of heat-stable antiviral and antitoxin single domain antibodies from a semisynthetic llama library. Analytical Chemistry, 78, 8245-8255. http://dx.doi.org/10.1021/ac0610053
- 33. Muyldermans, S. (2013) Nanobodies: Natural single-domain antibodies. Annual Review of Biochemistry, 82, 775-797. http://dx.doi.org/10.1146/annurev-biochem-063011-092449
- 34. Liu, J.L., Zabetakis, D., Goldman, E.R. and Anderson, G.P. (2013) Selection and evaluation of single domain antibodies toward MS2 phage and coat protein. Molecular Immunology, 53, 118-125. http://dx.doi.org/10.1016/j.molimm.2012.07.010
- 35. Goldman, E.R., Anderson, G.P., Bernstein, R.D. and Swain, M.D. (2010) Amplification of immunoassays using phage- displayed single domain antibodies. Journal of Immunological Methods, 352, 182-185. http://dx.doi.org/10.1016/j.jim.2009.10.014
- 36. Behdani, M., Zeinali, S., Khanahmad, H., Karimipour, M., Asadzadeh, N., Azadmanesh, K., Khabiri, A., Schoonooghe, S., Anbouhi, M.H., Hassanzadeh-Ghassabeh, G. and Muyldermans, S. (2012) Generation and characterization of a functional nanobody against the vascular endothelial growth factor receptor-2; angiogenesis cell receptor. Molecular Immunology, 50, 35-41. http://dx.doi.org/10.1016/j.molimm.2011.11.013
- 37. Liu, J.L., Zabetakis, D., Brown, J.C., Anderson, G.P. and Goldman, E.R. (2014) Thermal stability and refolding capability of shark derived single domain antibodies. Molecular Immunology, 59, 194-199. http://dx.doi.org/10.1016/j.molimm.2014.02.014
- 38. Liu, J.L., Anderson, G.P. and Goldman, E.R. (2007) Isolation of anti-toxin single domain antibodies from a semi-synthetic spiny dogfish shark display library. BMC Biotechnology, 7, 78-88. http://dx.doi.org/10.1186/1472-6750-7-78
- 39. Walper, S.A., Anderson, G.P., Lee, P.A.B., Glaven, R.H., Liu, J.L., Bernstein, R.D., Zabetakis, D., Johnson, L., Czarnecki, J.M. and Czarnecki, J.M. (2012) Rugged single domain antibody detection elements for Bacillus anthracis spores and vegetative cells. Plos One, 3, 1-10.
- 40. Graef, R.R., Anderson, G.P., Doyle, K.A., Zabetakis, D., Sutton, F.N., Liu, J.L., Serrano-Gonzalez, J., Goldman, E.R. and Cooper, L.A. (2011) Isolation of a highly thermal stable lama single domain antibody specific for Staphylococcus aureus enterotoxin B. BMC Biotechnology, 11, 86-96. http://dx.doi.org/10.1186/1472-6750-11-86
- 41. Goldman, E.R., Anderson, G.P., Zabetakis, D., Walper, S., Liu, J.L., Bernstein, R., Calm, A., Carney, J.P., O’Brien, T.W., Walker, J.L. and Garber, E.A.E. (2011) Llama-derived single domain antibodies specific for Abrus agglutinin. Toxins, 3, 1405-1419. http://dx.doi.org/10.3390/toxins3111405
- 42. Hagihara, Y. and Saerens, D. (2014) Engineering disulfide bonds within an antibody. Biochimica et Biophysica Acta— Proteins and Proteomics, 1844, 2016-2023. http://dx.doi.org/10.1016/j.bbapap.2014.07.005
- 43. Wolper, S.A., Battle, S.R., Lee, P.A.B., Zabetakis, D., Turner, K.B., Buckley, P.E., Calm, A.M., Welsh, H.S., Warner, C.R., Zacharko, M.A., Goldman, E.R. and Anderson, G.P. (2014) Thermostable single domain antibody-maltose binding protein fusion for Bacillus anthracis spore protein BclA detection. Analytical Biochemistry, 447, 64-73. http://dx.doi.org/10.1016/j.ab.2013.10.031
- 44. Sircar, A., Sanni, K.A., Shi, J.Y. and Gray, J.J. (2011) Analysis and modeling of the variable region of camelid single- domain antibodies. Journal of Immunology, 186, 6357-6367. http://dx.doi.org/10.4049/jimmunol.1100116
- 45. Dumoulin, M., Conrath, K., Van Meirhaeghe, A., Meersman, F., Heremans, K., Frenken, L.G.J., Muyldermans, S., Wyns, L. and Matagne, A. (2002) Single-domain antibody fragments with high conformational stability. Protein Science, 11, 500-515. http://dx.doi.org/10.1110/ps.34602
- 46. Dolk, E., van der Vaart, M., Hulsik, D.L., Vriend, G., de Haard, H., Spinelli, S., Cambillau, C., Frenken, L. and Verrips, T. (2005) Isolation of llama antibody fragments for prevention of dandruff by phage display in shampoo. Applied and Envi-ronmental Microbiology, 71, 442-450. http://dx.doi.org/10.1128/AEM.71.1.442-450.2005
- 47. Hussack, G., Hirama, T., Ding, W., MacKenzie, R. and Tanha, J. (2011) Engineered single-domain antibodies with high protease resistance and thermal stability. PloS ONE, 6, 1-6. http://dx.doi.org/10.1371/journal.pone.0028218
- 48. Wesolowski, J., Alzogaray, V., Reyelt, J., Unger, M., Juarez, K., Urrutia, M., Cauerhff, A., Danquah, W., Rissiek, B., Scheuplein, F., Schwarz, N., Adriouch, S., Boyer, O., Seman, M., Licea, A., Serreze, D.V., Goldbaum, F.A., Haag, F. and Koch-Nolte, F. (2009) Single domain antibodies: Promising experimental and therapeutic tools in infection and immunity. Medical Microbiology and Immunology, 198, 157-174. http://dx.doi.org/10.1007/s00430-009-0116-7
- 49. Coppieters, K., Dreier, T., Silence, K., de Haard, H., Lauwereys, M., Casteels, P., Beirnaert, E., Jonckheere, H., de Wiele, C.V., Staelens, L., Hostens, J., Revets, H., Remaut, E., Elewaut, D. and Rottiers, P. (2006) Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of col-lagen-induced arthritis. Arthritis and Rheumatism, 54, 1856-1866. http://dx.doi.org/10.1002/art.21827
- 50. Alzogaray, V., Danquah, W., Aguirre, A., Urrutia, M., Berguer, P., Vescovi, E.G., Haag, F., Koch-Nolte, F. and Goldbaum, F.A. (2011) Single-domain llama antibodies as specific intracellular inhibitors of SpvB, the actin ADP-ribosylating toxin of Salmonella typhimurium. Faseb Journal, 25, 526-534. http://dx.doi.org/10.1096/fj.10-162958
- 51. Li, T.F., Bourgeois, J.P., Celli, S., Glacial, F., Le Sourd, A.M., Mecheri, S., Weksler, B., Romero, I., Couraud, P.O., Rougeon, F. and Lafaye, P. (2012) Cell-penetrating anti-GFAP VHH and corresponding fluorescent fusion protein VHH-GFP spontaneously cross the blood-brain barrier and specifically recognize astrocytes: Application to brain imaging. Faseb Journal, 26, 3969-3979. http://dx.doi.org/10.1096/fj.11-201384
- 52. Hmila, I., Abdallah, B.A.B., Saerens, D., Benlasfar, Z., Conrath, K., El Ayeb, M., Muyldermans, S. and Bouhaouala- Zahar, B. (2008) VHH, bivalent domains and chimeric heavy chain-only antibodies with high neutralizing efficacy for scorpion toxin AahI’. Molecular Immunology, 45, 3847-3856. http://dx.doi.org/10.1016/j.molimm.2008.04.011
- 53. Anderson, G.P., Matney, R., Liu, J.L., Hayhurst, A. and Goldman, E.R. (2007) Multiplexed fluid array screening of phage displayed anti-ricin single domain antibodies for rapid assessment of specificity. Biotechniques, 43, 806-811. http://dx.doi.org/10.2144/000112600
- 54. Chakravarty, R., Goel, S. and Cai, W.B. (2014) Nanobody: The “magic bullet” for molecular imaging? Theranostics, 4, 386-398. http://dx.doi.org/10.7150/thno.8006
- 55. Vosjan, M.J.W.D., Vercammen, J., Kolkman, J.A., Stigter-van Walsum, M., Revets, H. and van Dongen, G.A.M.S. (2012) Nanobodies targeting the hepatocyte growth factor: Potential new drugs for molecular cancer therapy. Molecular Cancer Therapeutics, 11, 1017-1025. http://dx.doi.org/10.1158/1535-7163.MCT-11-0891
- 56. Hernot, S., Unnikrishnan, S., Du, Z., Cosyns, B., Broisat, A., Muyldermans, S., Lahoutte, T., Klibanov, A.L. and Devoogdt, N. (2012) Nanobody-coupled microbubbles as novel molecular tracer. European Heart Journal, 33, 403-404. http://dx.doi.org/10.1016/j.jconrel.2011.12.007
- 57. Huang, L., Gainkam, L.O.T., Caveliers, V., Vanhove, C., Keyaerts, M., De Baetselier, P., Bossuyt, A., Revets, H. and Lahoutte, T. (2008) SPECT imaging with 99mTc-labeled EGFR-specific nanobody for in vivo monitoring of EGFR expression. Molecular Imaging and Biology, 10, 167-175. http://dx.doi.org/10.1007/s11307-008-0133-8
- 58. Oliveira, S., van Dongen, G.A.M.S., Stigter-van Walsum, M., Roovers, R.C., Stam, J.C., Mali, W., van Diest, P.J. and van Bergen en Henegouwen, P.M.P. (2012) Rapid visualization of human tumor xenografts through optical imaging with a near-infrared fluorescent anti-epidermal growth factor receptor nanobody. Molecular Imaging, 11, 33-46.
- 59. Broisat, A., Hernot, S., Toczek, J., De Vos, J., Riou, L.M., Martin, S., Ahmadi, M., Thielens, N., Wernery, U., Caveliers, V., Muyldermans, S., Lahoutte, T., Fagret, D., Ghezzi, C. and Devoogdt, N. (2012) Nanobodies targeting mouse/ human VCAM1 for the nuclear imaging of atherosclerotic lesions. Circulation Research, 110, 927-937. http://dx.doi.org/10.1161/CIRCRESAHA.112.265140
- 60. Muller, M.R., Saunders, K., Grace, C., Jin, M., Piche-Nicholas, N., Steven, J., O’Dwyer, R., Wu, L.Y., Khetemenee, L., Vugmeyster, Y., Hickling, T.P., Tchistiakova, L., Olland, S., Gill, D., Jensen, A. and Barelle, C.J. (2012) Improving the pharmacokinetic properties of biologics by fusion to an anti-HSA shark VNAR domain. mAbs, 4, 673-685. http://dx.doi.org/10.4161/mabs.22242
- 61. Tang, J.C.Y., Szikra, T., Kozorovitskiy, Y., Teixiera, M., Sabatini, B.L., Roska, B. and Cepko, C.L. (2013) A nanobody-based system using fluorescent proteins as scaffolds for cell-specific gene manipulation. Cell, 154, 928-939. http://dx.doi.org/10.1016/j.cell.2013.07.021
- 62. Franco, E.J., Sonneson, G.J., DeLegge, T.J., Hofstetter, H., Horn, J.R. and Hofstetter, O. (2010) Production and characterization of a genetically engineered anti-caffeine camelid antibody and its use in immunoaffinity chromatography. Journal of Chromatography B—Analytical Technologies in the Biomedical and Life Sciences, 878, 177-186. http://dx.doi.org/10.1016/j.jchromb.2009.06.017
- 63. Rothbauer, U., Zolghadr, K., Tillib, S., Nowak, D., Schermelleh, L., Gahl, A., Backmann, N., Conrath, K., Muyldermans, S., Cardoso, M.C. and Leonhardt, H. (2006) Targeting and tracing antigens in live cells with fluorescent nanobodies. Nature Methods, 3, 887-889. http://dx.doi.org/10.1038/nmeth953
- 64. Hernot, S., Unnikrishnan, S., Du, Z.M., Shevchenko, T., Cosyns, B., Broisat, A., Toczek, J., Caveliers, V., Muyldermans, S., Lahoutte, T., Klibanov, A.L. and Devoogdt, N. (2012) Nanobody-coupled microbubbles as novel molecular tracer. Journal of Controlled Release, 158, 346-353. http://dx.doi.org/10.1016/j.jconrel.2011.12.007
- 65. Caussinus, E., Kanca, O. and Affolter, M. (2012) Fluorescent fusion protein knockout mediated by anti-GFP nanobody. Nature Structural & Molecular Biology, 19, 117-121. http://dx.doi.org/10.1038/nsmb.2180
- 66. Sennhauser, G. and Grutter, M.G. (2008) Chaperone-assisted crystallography with DARPins. Structure, 16, 1443-1453. http://dx.doi.org/10.1016/j.str.2008.08.010
- 67. Ermolenko, D.N., Zherdev, A.V. and Dzantiev, B.B. (2004) Antibodies as specific chaperones. Biochemistry—Moscow, 69, 1233-1238. http://dx.doi.org/10.1007/s10541-005-0069-4
- 68. Koide, S. (2009) Engineering of recombinant crystallization chaperones. Current Opinion in Structural Biology, 19, 449-457. http://dx.doi.org/10.1016/j.sbi.2009.04.008
- 69. Abskharon, R., Soror, S., Giachin, G., Pardon, E., El Hassan, H., Le, N., Legname, G., Wohlkonig, A. and Steyaert, J. (2012) Nanobody-stabilize the crystal structure of full-length human PrP. Prion, 6, 115-115.
- 70. Van de Broek, B., Devoogdt, N., D’Hollander, A., Gijs, H.L., Jans, K., Lagae, L., Muyldermans, S., Maes, G. and Borghs, G. (2011) Specific cell targeting with nanobody conjugated branched gold nanoparticles for photothermal therapy. Acs Nano, 5, 4319-4328. http://dx.doi.org/10.1021/nn1023363
- 71. Haselberg, R., Oliveira, S., van der Meel, R., Somsen, G.W. and de Jong, G.J. (2014) Capillary electrophoresis-based assessment of nanobody affinity and purity. Analytica Chimica Acta, 818, 1-6. http://dx.doi.org/10.1016/j.aca.2014.01.048
- 72. He, T., Wang, Y.R., Li, P.E., Zhang, Q., Lei, J.E., Zhang, Z.E., Ding, X.X., Zhou, H.Y. and Zhang, W. (2014) Nanobody-based enzyme immunoassay for aflatoxin in agro-products with high tolerance to cosolvent methanol. Analytical Chemistry, 86, 8873-8880. http://dx.doi.org/10.1021/ac502390c
- 73. Anderson, G.P., Glaven, R.H., Algar, W.R., Susumu, K., Stewart, M.H., Medintz, I.L. and Goldman, E.R. (2013) Single domain antibody-quantum dot conjugates for ricin detection by both fluoroimmunoassay and surface plasmon resonance. Analytica Chimica Acta, 786, 132-138. http://dx.doi.org/10.1016/j.aca.2013.05.010
- 74. Alvarez-Rueda, N., Behar, G., Ferre, V., Pugniere, M., Roquet, F., Gastinel, L., Jacquot, C., Aubry, J., Baty, D., Barbet, J. and Birkle, S. (2007) Generation of llama single-domain antibodies against methotrexate, a prototypical hapten. Molecular Immunology, 44, 1680-1690. http://dx.doi.org/10.1016/j.molimm.2006.08.007
- 75. Doyle, P.J., Arbabi-Ghahroudi, M., Gaudette, N., Furzer, G., Savard, M.E., Gleddie, S., McLean, M.D., Mackenzie, C.R. and Hall, J.C. (2008) Cloning, expression, and characterization of a single-domain antibody fragment with affinity for 15-acetyl-deoxynivalenol. Molecular Immunology, 45, 3703-3713. http://dx.doi.org/10.1016/j.molimm.2008.06.005
- 76. Anderson, G.P. and Goldman, E.R. (2008) TNT detection using llama antibodies and a two-step competitive fluid array immunoassay. Journal of Immunological Methods, 339, 47-54. http://dx.doi.org/10.1016/j.jim.2008.08.001
- 77. Peyvandi, F., Breems, D.A., Knoebl, P., De Man, C., Wu, K.L., Lyssens, C. and Holz, J.B. (2011) First results of the phase II TITAN trial: Anti-von Willebrand factor nanobody as adjunctive treatment for patients with acquired thrombotic thrombocytopenic purpura. Journal of Thrombosis and Haemostasis, 9, 720-721.
- 78. 贺生芳 (2013) Intein介导的纳米抗体在大肠杆菌中的表达和纯化. 硕士论文, 西北农林科技大学, 杨凌.
- 79. 丁志凌 (2013) 99mTc-EGFR Nanobodies用于肿瘤放射免疫显像的初步研究. 博士论文, 华中科技大学, 武汉.