Hans Journal of Ophthalmology
Vol.07 No.01(2018), Article ID:23976,10
pages
10.12677/HJO.2018.71002
The Effect of Arsenic Trioxide on the Expression of Tnf-α Gene in Adenoid Cystic Carcinoma Acc-2 Cells
Wei Quan1, Tao Jiang1*, Yang Zhou2, Jing Jiang3, Renping Wang4, Xiaojing Pan5
1Department of Ophthalmology, The Affiliated Hospital of Qingdao University, Qingdao Shandong
2Department of Ophthalmology, Qingdao AMASS Ophthalmology Hospital, Qingdao Shandong
3The Infectious Diseases Department, Eastern Branch of the Affiliated Hospital of Qingdao University, Qingdao Shandong
4Health Examination Center, The Affiliated Hospital of Qingdao University, Qingdao Shandong
5Qingdao Ophthalmology Hospital, Qingdao Shandong

Received: Feb. 14th, 2018; accepted: Mar. 1st, 2018; published: Mar. 8th, 2018

ABSTRACT
Objective: The purpose of this study was to investigate the effect of As2O3 on the proliferation and apoptosis of ACC-2 cells cultured in vitro to detect its specific mechanism of ACC-2 cell apoptosis. Methods: ACC-2 cells were cultured in vitro and divided into experimental group and control group with the same amount of cell culturing. In the exponential growth phase the ACC-2 cells cultured in experimental group was added with different concentration of As2O3 (2.0, 4.0, 6.0 and 8.0 μmol/L) and the control group gave equal amount of cell culture medium. After added As2O3, the cells were cultured at different times (12 h, 24 h, 36 h, 48 h). Then the morphological changes of growth inhibition and apoptosis of the ACC-2 cells at each point time in different concentrations of As2O3 were closely observed. Reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemistry test (IHCT) were used to detect the expression changes of TNF-α gene and protein. Results: 1) With the increase of As2O3 concentration and time, it could be seen that the cells became smaller and round, the cell contact was reduced and the cell gap became larger, intracellular particles increased, cell density increased accompanied by vacuolar degeneration, adherent cells decreased, floating necrosis of the cells gradually increased, which shown that As2O3 could inhibit the proliferation of ACC-2 cells, and with the increase of drug concentration and time, the growth inhibition of cells gradually strengthened. 2) Immunohistochemical results showed that TNF-α proteins were expressed in the nucleus and the cytoplasm in TNF-α cells in both the experimental group and the control group. TNF-α proteins were expressed mainly in the nucleus. Positive particles gradually gathered to the nucleus and AIO value was increased gradually as the concentration increased. The difference was statistically significant (P < 0.01). 3) The results of RT-PCR showed that the TNF-α mRNA expressed in ACC-2 cells in both the experimental group and the control group, and its expression decreased slightly with increasing concentrations of As2O3 and prolonging duration of action. However, it was not statistically significant between the concentration and time groups (P > 0.05); it was not statistically significant between the two comparisons at different concentrations (P > 0.05) or different time (P > 0.05), which was different from immunohistochemical results. Conclusion: As2O3 could inhibit the growth and induce the apoptosis of Acc-2 cells cultured in vitro and could increase the expression and secretion of TNF-α protein in Acc-2 cells of adenoid cystic carcinoma, that is, TNF-α is involved in apoptosis of acc-2 cells induced by As2O3 in the protein level.
Keywords:Adenoid Cystic Carcinoma, Arsenic Trioxide, TNF-α, Apoptosis
三氧化二砷对腺样囊性癌ACC-2细胞TNF-α 基因表达的影响
全威1,姜涛1*,周杨2,姜靖3,王仁萍4,潘晓晶5
1青岛大学附属医院眼科,山东 青岛
2青岛阿玛施眼科医院,山东 青岛
3青岛大学附属医院东区感染科,山东 青岛
4青岛大学附属医院健康查体中心,山东 青岛
5青岛眼科医院,山东 青岛

收稿日期:2018年2月14日;录用日期:2018年3月1日;发布日期:2018年3月8日

摘 要
研究目的:本实验旨在通过研究As2O3对体外培养的ACC-2细胞增殖、凋亡的影响,从而探讨其对ACC-2细胞诱导凋亡的具体机制。研究方法:将ACC-2细胞进行体外培养并分为实验组和对照组,于细胞指数生长期向实验组加入不同浓度梯度(2.0、4.0、6.0、8.0 μmol/L)的含As2O3细胞培养液,对照组则给予等量全细胞培养液,加入As2O3后分别培养不同时间(12、24、36、48 h),密切观察各时间点不同浓度As2O3对腺样囊性癌ACC-2细胞的生长抑制作用和细胞凋亡的形态变化,并应用荧光定量RT-PCR和免疫细胞化学技术检测TNF-α基因和蛋白的表达变化。研究结果:1) 随着加入As2O3浓度及时间增加,显微镜下可见胞体缩小变圆,细胞接触减少,细胞间隙变大,胞浆内颗粒样物质增多,胞核固缩,贴壁细胞减少,漂浮坏死的细胞逐渐增多。表示As2O3对ACC-2细胞的生长具有抑制作用,且随着药物浓度的升高,细胞的生长抑制作用逐渐加强。2) 免疫细胞化学检测结果示在细胞核和细胞浆均有TNF-α蛋白表达,以细胞核为主,不同浓度As2O3作用于ACC-2细胞后,阳性颗粒随浓度及作用时间增加逐渐向细胞核聚集并增强,测得的平均光密度值(IOD/area)逐渐增大,不同浓度总体比较TNF-α蛋白表达量差异有统计学意义,但不同处理时间总体比较TNF-α蛋白表达量差异无统计学意义;两两比较,不同浓度除0 μmol/L与2 μmol/L及6 μmol/L与8 μmol/L外其余均有统计学意义(P < 0.01),不同时间除12 h与48 h外其余均无统计学差异(P > 0.05)。3) RT-PCR结果显示在空白对照组和实验组ACC-2细胞中TNF-α mRNA均存在表达,其表达量随着As2O3浓度的增加和作用时间的延长略有下降,但不同浓度及时间点总体比较差异均无统计学意义(P > 0.05),不同浓度两两比较均无统计学差异(P > 0.05),不同时间两两比较均无统计学差异(P > 0.05),这与免疫组化结果不同。结论:As2O3对体外培养的ACC-2细胞具有生长抑制和诱导凋亡作用,能够上调腺样囊性癌ACC-2细胞中TNF-α蛋白的表达及分泌,使TNF-α在蛋白水平参与As2O3诱导ACC-2细胞的凋亡。
关键词 :腺样囊性癌,三氧化二砷,TNF-α,凋亡

Copyright © 2018 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
腺样囊性癌(Adenoid cystic carcinoma, ACC)又被称为圆柱瘤,是一种上皮源性的腺体恶性肿瘤,临床上较为少见,于颜面部以涎腺最常见,其次好发于泪腺 [1] ,属于泪腺上皮源性最常见且恶性程度最高的肿瘤之一,具有极强的浸润性,在泪腺的上皮性肿瘤中其发病率仅次于多形性腺瘤而屈居第二 [2] [3] 。多发于中年,女性多见于男性,平均中位年龄为40岁 [4] [5] 。当肿瘤细胞侵入血管、骨组织及眼外肌时可出现疼痛麻木、眼球突出、眼球运动障碍、上睑下垂和复视等临床症状,视力多不受累;由于浸润性极强,颞上眶缘下相当于泪腺窝部位可触及肿块,触之可有压痛 [1] [6] [7] [8] 。
由于ACC生长较为缓慢,具有极强的浸润性,同时具有嗜神经性生长的特性,早期易发生局部蔓延及血行转移,远处转移以肺转移为主,其次是骨和肝,其治疗较为棘手,目前常用的手术方法是手术切除可见的肿瘤组织连同周围的一部分正常组织,电烙或凿除与肿瘤紧邻的部分骨壁,以尽可能地清除残余肿瘤细胞,术后再辅以放疗或化疗等综合治疗措施。但患者晚期易出现局部复发和远处转移 [2] [9] [10] ,目前常规应用的化疗药物由于其毒副作用影响,应用范围较为局限。因此新的有效的化疗药物的研究对于提高ACC的治疗仍具有重要意义。
三氧化二砷(arsenic trioxide, As2O3)为我国中药砒霜的主要成分,在我国曾用于治疗多种疾病。近年来As2O3已广泛的应用于急性早幼粒细胞白血病的化学药物治疗,特别是对于复发病例,疗效显著、缓解率高 [11] [12] 。目前关于As2O3抗肿瘤作用机制的研究已取得不少新进展,研究最多的机制是诱导肿瘤细胞凋亡 [13] 。
肿瘤坏死因子(tumor necrosis factor, TNF)最初在小鼠血清中发现,具有杀伤某些肿瘤细胞或使体内肿瘤组织发生出血坏死的作用,是一种多功能因子,在炎症反应、细胞免疫、肿瘤免疫等多种生理和病理过程中发挥关键作用。也有研究发现肿瘤细胞能分泌TNF-α,低剂量、长期TNF-α刺激具有促进血管生成,促进肿瘤进展的作用 [14] [15] [16] 。本实验意在探讨As2O3能否抑制ACC细胞的生长,影响TNF-α的表达,进而探讨As2O3抑制ACC细胞生长的具体机制,为其临床应用提供药理依据。
2. 材料和方法
2.1. 材料
2.1.1. 实验试剂
注射用三氧化二砷(北京双鹭药业股份有限公司);胎牛血清、RPMI-1640培养基(美国Hyclone公司);TNF-α及内参引物序列(上海生工有限公司);TNF-α单克隆抗体(美国Santa Cruz公司);逆转录试剂盒及SYBR荧光染料(北京艾德莱公司);山羊PV-9003试剂盒、浓缩型DAB试剂盒(北京中杉金桥公司);其余试剂均为国产分析纯。
2.1.2. 实验细胞
腺样囊性癌ACC-2细胞(来源于人涎腺ACC)购自青岛大学附属医院中心实验室。
2.1.3. 实验仪器
超净工作台(苏州亿达净化设备有限公司);恒温水浴箱(上海新苗医疗器械有限公司);CO2恒温培养箱(美国VWR公司);微量离心机、低温超速离心机(德国Sigma公司);核酸蛋白分析仪(美国Beckman公司);光学倒置显微镜(日本Olympus公司)。
2.2. 实验方法
2.2.1. 细胞培养及观察
选用人腺样囊性癌ACC-2细胞培养于含10%胎牛血清的RPMI-1640培养基中,在5% CO2,37℃恒温培养箱中培养,每2~3天换液1次。使用复苏传代三次之后处于指数生长期的细胞用于实验。将实验分为实验组和对照组。其中实验组分别加2.0、4.0、6.0、8.0 μmol/L的As2O3 2 mL,分别处理12、24、36、48 h,空白对照组加等量培养液代替As2O3,在同一浓度、时间分别设定3个平行对照。在倒置显微镜下动态观察各组细胞在各时间点不同浓度的形态变化,并照相记录。各组均行苏木素染色以观察细胞核形态变化。
2.2.2. 免疫细胞化学检测TNF-α蛋白表达
取实验组和对照组6孔板细胞,处理12、24、36、48 h,同一时间、浓度设立3个平行对照,行免疫细胞化学操作:1) 吸除6孔板中的旧培养液,取出细胞爬片并用0.01 mol/L PBS洗涤3次;2) 4%多聚甲醛固定20 min,0.01 mol/L PBS冲洗2 min 3次;3) 0.1% TritonX-100作用20 min,0.01 mol/L PBS冲洗2 min × 3 次;4) 3% H2O2去离子水孵育10 min,0.01 mol/L PBS冲洗2 min × 3 次;5) 滴加适量1;200 稀释的山羊抗人TNF-α单克隆抗体工作液,置入湿盒中37℃孵育2 h;6) 滴加适量PV-9003试剂1,湿盒中室温20 min,0.01 mol/L PBS冲洗2 min × 3次;7) 滴加适量PV-9003试剂2,湿盒中室温25 min,0.01 mol/L PBS冲洗2 min × 3次;8) 滴加1:20配制的DAB显色液,室温下显色,显微镜下控制显色时间,见标本细胞核染至棕色时及时蒸馏水充分冲洗终止反应;9) 取需复染的玻片,于玻片滴加1滴苏木素上复染10~30 s,盐酸酒精分化3 s,氨水返蓝5 s,自来水轻轻冲洗1 min,75%、80%、95%、100%酒精梯度各脱水1 min,二甲苯透明1 min,中性树胶封片;10) 显微镜下观察并照相记录。阴性对照用PBS替代一抗,其余步骤相同。光学显微镜下随机选取5个高倍(×400)视野观察并拍照记录,可见TNF-α阳性表达表现为细胞内的棕黄色颗粒,TNF-α蛋白的表达强度以平均光密度值表示,应用Image-pro plus 6.0图像分析软件对染色结果进行分析。
2.2.3. RT-PCR 检测TNF-α基因表达
取实验组和对照组12孔板细胞,同一时间、浓度设立3个平行对照,处理12、24、36、48 h后,用Trizol提取细胞中的总RNA,紫外分光光度计测定RNA在260 nm和280 nm的吸光度(OD260, OD280)。无蛋白和核酸污染的RNA样本OD260/OD280范围为1.8~2.1。以合格的RNA为模板,加入5 × RT Reaction Mix,Random Primer,TRUE script H-RTase/RI Mix,RNaseFreedH2O配成20 μL反应体系,于25℃孵育10 min,42℃孵育50 min,85℃灭活5 min,4℃永久保存,合成第一链cDNA。扩增反转录所得的cDNA:cDNA模板1 μl,上下游引物各0.5 μl,SYBR12.5 μl,RNase Free dH2O 10.5 μl,总共25 μl。PCR反应过程如下:94℃ 3 min,1个循环;94℃ 10 s,60℃ 34 s,共40个循环;94℃ 15 s,60℃ 30 s,94℃15 s检测溶解曲线。TNF-α及内参引物序列如下:TNF-α引物序列:上游5’-AACCTCCTCTCTGCCATCAA-3’,下游5’-CTGAGTCGGTCACCCTTCTC-3’,产物长度100 bp;内参GAPDH引物序列:上游5’-TCATGGGT GTGAACCATGAGAA-3’,下游5’-GGCATGGACTGTGGTCATGAG-3’,产物长度146 bp。使用荧光定量PCR仪(eppendorf Mastercycler ep realplex)绘制荧光扩增曲线和溶解曲线,得出Ct值,应用2-△△Ct(ΔΔCt = ΔCt待测样本 − ΔCt对照样本,ΔCt = 目的基因Ct值 − 内参Ct值)法分析结果。
2.3. 统计学分析
数据采用SPSS 19.0统计软件进行统计分析,各组数据采用均数±标准差(x ± s)表示,多组之间总体比较采用双因素方差分析,不同浓度和时间各组内两两比较采用LSD-t检验,以P < 0.05为差异有统计学意义。
3. 结果
3.1. ACC-2细胞形态学变化
倒置显微镜下可见正常ACC-2细胞呈现上皮型细胞形态,为不规则多边形,细胞之间紧密相连,内有类圆形核,细胞核分裂相活跃(图1,A)。而细胞经不同浓度As2O3处理后,表现为细胞生长速度减慢,边缘不整齐,细胞间距增大,形态缩小变圆,细胞核固缩,分裂相减少,接触率降低,随着药物浓度增加及作用时间的延长,贴壁细胞不断减少,漂浮坏死的细胞不断增多,当2 μmol/L As2O3刺激24 h时细胞凋亡变化不明显,48 h可见凋亡细胞增多,而4 μmol/L As2O3刺激24 h即观察到细胞凋亡,48 h可见细胞凋亡明显增多,使用6~8 μmol/L As2O3时刺激24 h以内即有明显凋亡,48 h可见大量细胞凋亡(图1,B)。苏木素染色后正常ACC-2细胞可见细胞核呈椭圆形,核大深染,多个核仁,可见核分裂相,散在双核及巨核细胞(图1,C);As2O3刺激后可见细胞皱缩,核固缩,染色加深,胞浆中颗粒增多,呈现明显的凋亡状态(图1,D)。


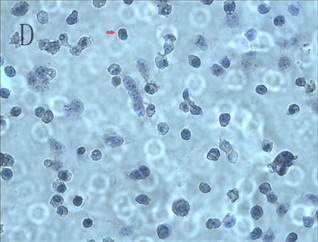

Figure 1. ACC-2 cell morphology observations A: Normal ACC-2 cells were cultured for 48 hours (×200); B: Normal ACC-2 cells were stimulated with 6 μmol/L As2O3 for 48 hours (×200); C: Normal ACC-2 cells were stained with hematoxylin (×400); D: Normal ACC-2 cells were stimulated with 6 μmol/L As2O3 for 48 hours and stained with hematoxylin (×400)
图1. ACC-2细胞形态学观察A:正常ACC-2 细胞传代48 h后(×200);B:6 μmol/L As2O3刺激48 h后,(×200);C:正常ACC-2细胞进行苏木素染色后(×400);D:6 μmol/L As2O3刺激48 h进行苏木素染色后(×400)
3.2. 免疫组化实验结果
各实验组和空白对照组(即0 µmol/L As2O3)均可见TNF-α蛋白阳性表达,表现为胞浆和胞核均可见棕色颗粒,胞核多于胞浆(图2,A);阴性对照表现为胞核及胞浆均无明显着色或仅有极少量棕色颗粒,胞核呈蓝色,表示本次使用的二抗检测系统特异性较好(图2,B)。As2O3刺激后棕色颗粒逐渐向胞核聚集,胞浆减少,随浓度和处理时间增加,聚集程度增强,胞核棕色深度逐渐增强,胞浆逐渐变淡(图3)。
各组平均光密度值(IOD/area)的均数±标准差(x ± s)表示TNF-α蛋白量(见表1),经双因素方差分析,不同浓度总体比较TNF-α蛋白表达量差异有统计学意义(F浓度 = 32.046,P < 0.01);不同处理时间总体比较TNF-α蛋白表达量差异无统计学意义(F时间 = 2.165,P > 0.05);两两比较,不同浓度除0 μmol/L
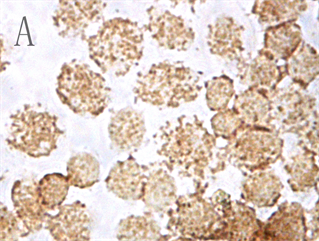
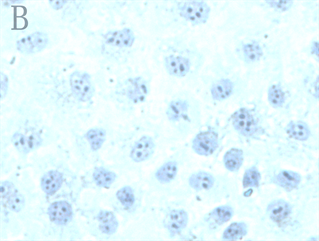
Figure 2. TNF-α protein staining results in control group and negative control group (ICC, ×400); A: Positive staining of control group showed positive expression of TNF-α protein; B: Negative control staining of PBS instead of primary antibody in control group
图2. 对照组和阴性对照组TNF-α蛋白染色结果(ICC, ×400);A:对照组阳性染色可见TNF-α蛋白表达阳性;B:对照组中PBS代替一抗的阴性对照染色

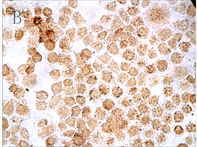

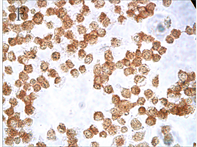
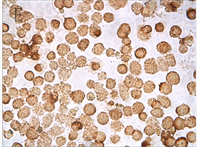
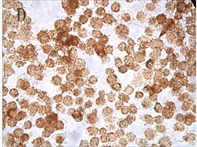
Figure 3. TNF-α protein staining results with different concentration of As2O3 and different times (ICC, ×400); A: Normal ACC-2 cells were stimulated with 2 μmol/L As2O3 for 24 hours; B: Normal ACC-2 cells were stimulated with 2 μmol/L As2O3 for 48 hours; C: Normal ACC-2 cells were stimulated with 4 μmol/L As2O3 for 24 hours; D: Normal ACC-2 cells were stimulated with 4 μmol/L As2O3 for 48 hours; E: Normal ACC-2 cells were stimulated with 6 μmol/L As2O3 for 24 hours; F: Normal ACC-2 cells were stimulated with 6 μmol/L As2O3 for 48 hours
图3. 不同浓度As2O3处理不同时间TNF-α蛋白染色结果(ICC, ×400); A: 2 μmol/L As2O3处理24 h; B: 2 μmol/L As2O3处理48 h; C: 4 μmol/L As2O3处理24 h; D: 4 μmol/L As2O3处理48 h; E: 6 μmol/L As2O3处理24 h; F: 6 μmol/L As2O3处理48 h
与2 μmol/L及6 μmol/L与8 μmol/L外其余均有统计学意义(P < 0.01) (见表2),不同时间除12 h与48 h外其余均无统计学差异(P > 0.05)。
3.3. RT-PCR实验结果
PCR均能检测到各组不同时间点ACC-2细胞中TNF-α mRNA的表达,TNF-α mRNA表达量随着As2O3浓度及作用时间增加略有下降,但不同浓度及时间点总体比较差异均无统计学意义(F浓度 = 0.666,
Table 1. TNF-α protein expression in different time of each group (x ± s, n = 15)
表1. 各组不同作用时间TNF-α蛋白表达量(x ± s, n = 15)
注:0为空白对照组。
Table 2. Comparison results of different two concentrations
表2. 不同浓度两两比较结果
Table 3. The relative expression of TNF-α mRNA in different time of each group (x ± s, n = 6)
表3. 各组不同作用时间TNF-α mRNA相对表达量(x ± s, n = 6)
注:0为空白对照组。
P > 0.05;F时间 = 0.596,P > 0.05);两两比较,不同时间两两比较均无统计学差异(P > 0.05),不同浓度两两比较均无统计学差异(P > 0.05) (见表3)。
4. 讨论
泪腺腺样囊性癌(lacrimal gland adenoid cystic carcinoma, LGACC)具有浸润转移性极强且预后差的特性,由于其极易复发和转移的特性,且肿瘤细胞易沿神经生长,手术往往很难完全切除癌组织,术后有较高的局部复发率和远处转移率,预后并不理想。对该病的治疗是一直是临床上亟待解决的重要问题,虽然化疗是重要的辅助治疗手段,但目前临床上常规应用的化疗药物效果并不理想,寻求新的有效的化疗药物便是研究该病的目的之一。而As2O3对急性早幼粒细胞白血病的有效治疗作用为其他恶性肿瘤的化疗药物研究提供了新思路。本实验采用的腺样囊性癌ACC-2细胞属于与泪腺ACC组织同源性极高的人类涎腺ACC,其与泪腺ACC组织具有极为相似的生理生化特性。
三氧化二砷As2O3作为砒霜的主要成分,作为我国一味古老的中药具有多种治疗功效和明确的毒性,上世纪中后期,我国学者使用其治疗急性早幼粒细胞白血病(acute promyelocytic leukemia, APL)取得显著疗效,未出现严重不良反应,证实其完全缓解率可达85% [11] [12] ,也有研究发现,As2O3也对多种实体肿瘤如肺癌、肝癌、胃癌等生长存在抑制作用 [17] [18] [19] [20] 。本实验中我们观察到,细胞生长抑制效果随As2O3浓度和作用时间增加逐渐增强,出现细胞生长速度减慢、细胞形态缩小变圆、细胞核固缩、分裂相减少、接触率降低、凋亡数量明显增加等。
Carswell等 [21] 于1975年首次通过实验证实并报道,用卡介苗注射或用细菌内毒素感染小鼠后,在小鼠血清中可检测到一种蛋白类物质,该物质可对多种体外培养的肿瘤细胞株产生细胞抑制或杀伤作用,进而引起肿瘤组织出血坏死,因此将其命名为肿瘤坏死因子(tumor necrosis factor, TNF)。后来把TNF按结构分为两种:TNF-α和TNF-β。TNF-α是一种单核因子,主要由活化的单核细胞和巨噬细胞产生,TNF-β是一种淋巴因子,由活化的T细胞和NK细胞产生,目前研究多为TNF-α。研究表明,TNF-α对肿瘤有双向生物学特性,不仅有抗肿瘤的作用,还存在直接促进肿瘤生长的作用。但是,在总体情况下,主要表现为前者的趋势。
本实验用As2O3刺激腺样囊性癌ACC-2细胞,发现TNF-α蛋白表达明显升高,且具有随药物浓度变化呈现依赖性。因此,As2O3使TNF-α蛋白表达和分泌升高,诱导细胞凋亡,从而抑制腺样囊性癌ACC-2细胞生长,可能的分子机制为 [22] :1) 肿瘤组织细胞周期的各个时期均可受TNF-α的影响,主要有以下2个机制:A诱导其他加速细胞周期的蛋白,间接促进肿瘤生长;B直接诱导活化肿瘤细胞内有丝分裂调控基因,其可促进DNA合成并加快S期过程,使细胞周期缩短。但高剂量的TNF-α反而延长细胞周期。2) 低剂量TNF-α激活核因子-κB (nuclear factor-kappa B, NF-κB)致肿瘤细胞周期调节紊乱而发生凋亡逃逸,NF-κB是一种重要的调控肿瘤凋亡的核转录因子,而TNF-α能激活经典的NF-κB信号通路。当细胞受到TNF-α等因子的刺激,激活的IKKb磷酸化NF-κB的抑制剂I-κB,使I-κB快速降解,并释放了NF-κB。结果,NF-κB的异源性的聚合物进入细胞核内与它同源性的DNA结合,诱导一系列与肿瘤免疫、细胞增殖、血管形成及转移相关基因的表达 [23] [24] [25] [26] 。高剂量的TNF-α则抑制NF-κB途径,间接起到了抑制肿瘤细胞生长的作用。Zheng等 [27] 发现,高剂量的TNF-α在体外能显著抑制口腔鳞癌细胞株的迁移、表达。与本研究的腺样囊性癌细胞株有相似的生物学效应。
综上所述,As2O3诱导细胞凋亡可以作为当前一种新的肿瘤治疗策略。本实验发现TNF-α在蛋白水平参与As2O3诱导ACC-2细胞的凋亡。As2O3能够上调腺样囊性癌ACC-2细胞中TNF-α蛋白的表达及分泌,高浓度TNF-α加速了肿瘤细胞的凋亡,在抑制肿瘤生长中发挥了重要的作用。同时本次实验对As2O3作用于ACC的分子机制进行了更深一步的研究,进一步证实了对ACC的体外抑瘤作用,为其应用于临床提供了一定的理论依据。随着细胞凋亡与肿瘤发生发展关系的进一步阐明以及细胞凋亡分子调控机制研究的不断深入,相信As2O3在ACC实验与临床治疗中发挥重要作用的时刻即将到来。
基金项目
山东省自然科学基金资助项目(No. ZR2012HM062)。
参考文献
[1] Friedrich, R.E. and Bleckmann, V. (2003) Adenoid Cystic Carcinoma of Salivary and Lacrimal Gland Origin: Localization, Classification, Clinical Pathological Correlation, Treatment Results and Long-Term Follow-Up Control in 84 Patients. Anticancer Research, 23, 93l-940.
[2] Ueda, S., Goto, H., Matsubayashi, J. and Nagao, T. (2014) Adenoid Cystic Carcinoma of the Lacrimal Gland: Clinicopathological Study. Nippon Ganka Gakkai Zasshi, 118, 963-967.
[3] Ducrey, N., Villemure, J.G., Jaques, B., et al. (2002) Cystic Adenocarcinomas of the Lacrimal Gland. Klin Monatsbl Augenheilkd, 219, 23l-234. https://doi.org/10.1055/s-2002-30672
[4] Lawal, A.-O., Adisa, A.-O., Kolude, B., Adeyemi, B.-F. and Olajide, M.-A. (2013) A Review of 413 Salivary Gland Tumours in the Head and Neck Region. Journal of Clinical and Experimental Dentistry, 5, e218-e222. https://doi.org/10.4317/jced.51143
[5] Martinez-Rodriguez, N., Leco-Berrocal, I., Rubio-Alonso, L., Arias-Irimia, O. and Martinez-Gonzalez, J.M. (2011) Epidemiology and Treatment of Adenoid Cystic Carcinoma of the Minor Salivary Glands: A Meta-Analytic Study. Medicina Oral, Patologia Oral y Cirugia Bucal, 16, e884-e889. https://doi.org/10.4317/medoral.17200
[6] Luksic, I., Suton, P., Macan, D. and Dinjar, K. (2014) Intraoral Adenoid Cystic Carcinoma: Is the Presence of Perineural Invasion Associated with the Size of the Primary Tumour, Local Extension, Surgical Margins, Distant Metastases, and Outcome? The British Journal of Oral & Maxillofacial Surgery, 52, 214-218. https://doi.org/10.1016/j.bjoms.2013.11.009
[7] Terasaki, M., Tokutomi, T., Maruiwa, H., Sugita, Y., Harada, H. and Shigemori, M. (2000) High-Grade Adenoid Cystic Carcinoma Originating from the Lacrimal Gland. Brain Tumor Pathology, 17, 159-163. https://doi.org/10.1007/BF02484288
[8] Wang, F., Sun, F.Y., Guo, X.X., Xia, S. and Tang, D.R. (2013) Imaging Feature and Clinical Histological Analysis of 12 Adenoid Cystic Carcinoma of the Lacrimal Gland. Chinese Journal of Ophthalmology, 49, 47-51.
[9] Singh, F.M., Mak, S.Y. and Bonington, S.C. (2015) Patterns of Spread of Head and Neck Adenoid Cystic Carcinoma. Clinical Radiology, 70, 644-653. https://doi.org/10.1016/j.crad.2015.01.013
[10] Soignet, S.L., Frankel, S.R., Douer, D., Tallman, M.S., Kantarjian, H., Calleja, E., et al. (2001) United States Multicenter Study of Arsenic Trioxide in Relapsed Acute Promyelocytic Leukemia. Journal of Clinical Oncology, 19, 3852-3860. https://doi.org/10.1200/JCO.2001.19.18.3852
[11] Lengfelder, E., Hofrnann, W.K. and Nowak, D. (2012) Impact of Arsenic Trioxide in the Treatment of Acute Promyelocytic Leukemia. Leukemia, 26, 433-442. https://doi.org/10.1038/leu.2011.245
[12] Keyhani, M. (2012) Use of Arsenic Trioxide as a First-Time Single Agent in the Treatment of Acute Promyelocytic Leukemia. Journal of Clinical Oncology, 30, 217-222. https://doi.org/10.1200/JCO.2011.38.7654
[13] Gupta, S., Yel, L., Kim, D., et al. (2003) Arsenic Trioxide Induces Apoptosis in Peripheral Blood T Lymphocyte Subsets by Inducing Oxidative Stress: A Role of Bcl-2. Molecular Cancer Therapeutics, 2, 711-719.
[14] Malik, S.T., Griffin, D.B., Fiers, W. and Balkwill, F.R. (1989) Paradoxical Effects of Tumour Necrosis Factor in Experimental Ovarian Cancer. International Journal of Cancer, 44, 918-925.
[15] Malik, S.T., Naylor, M.S., East, N., Oliff, A. and Balkwill, F.R. (1990) Cells Secreting Tumour Necrosis Factor Show Enhanced Metastasis in Nude Mice. European Journal of Cancer, 26, 1031-1034. https://doi.org/10.1016/0277-5379(90)90044-T
[16] Balkwill, F. (2002) Tumor Necrosis Factor or Tumor Promoting Factor? Cytokine & Growth Factor Reviews, 13, 135-141.
[17] Chen, G.Q., Zhu, J., Shi, X.G., Ni, J.H., Zhong, H.J., Si, G.Y., et al. (1996) In Vitro Studies on Cellular and Molecular Mechanisms of Arsenic Trioxide (As2O3) in the Treatment of Acute Promyelocytic Leukemia: As2O3 Induces NB4 Cell Apoptosis with Downregulation of Bcl-2 Expression and Modulation of PML-RAR Alpha/PML Proteins. Blood, 88, 1052-1061.
[18] Lin, C.C., Hsu, C., Hsu, C.H., Hsu, W.L., Cheng, A.L. and Yang, C.H. (2007) Arsenic Trioxide in Patients with Hepatocellular Carcinoma: A Phase II Trial. Investigational New Drugs, 25, 77-84. https://doi.org/10.1007/s10637-006-9004-9
[19] Zheng, C.Y., Lam, S.K., Li, Y.Y., Fong, B.M., Mak, J.C. and Ho, J.C. (2013) Combination of Arsenic Trioxide and Chemotherapy in Small Cell Lung Cancer. Lung Cancer, 82, 222-230. https://doi.org/10.1016/j.lungcan.2013.08.022
[20] Hu, X.M., Yuan, B., Tanaka, S., Song, M.M., Onda, K., Tohyama, K., et al. (2014) Arsenic Disulfide-Triggered Apoptosis and Erythroid Differentiation in Myelodysplastic Syndrome and Acute Myeloid Leukemia Cell Lines. Hematology, 19, 352-360. https://doi.org/10.1179/1607845413Y.0000000138
[21] Carswell, E.A., Old, L.J., Kassel, R.L., et al. (1975) An Endotoxin-Induced Serum Factor That Causes Necrosis of Tumors. Proceedings of the National Academy of Sciences of the United States of America, 72, 3666-3670. https://doi.org/10.1073/pnas.72.9.3666
[22] Volland, S., Amtmann, E. and Sauer, G. (1994) TNF Accelerates the S-Phase of the Cell Cycle in Tumor Cells. International Journal of Cancer, 56, 698-705. https://doi.org/10.1002/ijc.2910560515
[23] Mantovani, A. (2009) Cancer: Inflaming Metastasis. Nature, 457, 36-37. https://doi.org/10.1038/457036b
[24] Sethi, G., Sung, B. and Aggarwal, B.B. (2008) TNF: A Master Switch for Inflammation to Cancer. Frontiers in Bioscience, 13, 5094-5107.
[25] Thompson, E.W., Newgreen, D.F. and Tarin, D. (2005) Carcinoma Invasion and Metastasis: A Role for Epithelial— Mesenchymal Transition? Cancer Research, 65, 5991-5995; discussion 5995. https://doi.org/10.1158/0008-5472.CAN-05-0616
[26] Luo, J.L., Kamata, H. and Karin, M. (2005) IKK/NF-κB Signaling: Balancing Life and Death—A New Approach to Cancer Therapy. The Journal of Clinical Investigation, 115, 2625-2632. https://doi.org/10.1172/JCI26322
[27] Zheng, Z., Luan, X., Zha, J., Li, Z., Wu, L., Yan, Y., Wang, H., Hou, D., Huang, L., Huang, F., Zheng, H., Ge, L. and Guan, H. (2017) TNF-α Inhibits the Migration of Oral Squamous Cancer Cells Mediated by miR-765-EMP3-p66Shc Axis. Cell Signalling, 34, 102-109. https://doi.org/10.1016/j.cellsig.2017.03.009
知网检索的两种方式:
1. 打开知网页面http://kns.cnki.net/kns/brief/result.aspx?dbPrefix=WWJD
下拉列表框选择:[ISSN],输入期刊ISSN:2333-5394,即可查询
2. 打开知网首页http://cnki.net/
左侧“国际文献总库”进入,输入文章标题,即可查询
投稿请点击:http://www.hanspub.org/Submission.aspx
期刊邮箱:hjo@hanspub.org
