Journal of Advances in Physical Chemistry
Vol.
12
No.
01
(
2023
), Article ID:
61906
,
8
pages
10.12677/JAPC.2023.121002
Design Rules of Ordered Structure Formed by AB Diblock Copolymers and Nanoparticles in Dilute Solution
Yang Li1*, Kefei Wang1, Haiyan Hu1, Zhichao Wang1, Yanchun Li2
李洋1*,王科飞1,胡海燕1,王志超1,李延春2
1吉林工商学院工学院,吉林 长春
2吉林大学化学学院理论化学研究所,吉林 长春
收稿日期:2023年1月4日;录用日期:2023年2月17日;发布日期:2023年2月27日
摘要
利用耗散粒子动力学方法,我们系统研究了纳米粒子接枝AB两嵌段共聚物在稀溶液中的自组装过程,不仅得到了胶束和囊泡等有序结构,还探究了该类体系的设计规则。我们发现嵌段共聚物的亲疏水比例对有序结构起到决定性的影响,纳米粒子复合体系的浓度对最终的结构也有一定程度的影响。无论是形成胶束还是形成囊泡,都会先形成纳米簇的聚集体,进而聚集甚至弯曲形成更加有序的结构。结合实验现象,我们通过调控初始结构中纳米粒子的排列方式,发现囊泡有两种截然不同的形成机制。最后,我们总结了这类自组装的设计规则,即亲疏水比例大于2:1时,很容易形成胶束,亲疏水比例小于1:2时,则很容易形成囊泡。
关键词
AB两嵌段共聚物,纳米粒子,耗散粒子动力学,胶束,囊泡
1Technology School, Jilin Business and Technology College, Changchun Jilin
2Institute of Theoretical Chemistry, College of Chemistry, Jilin University, Changchun Jilin
Received: Jan. 4th, 2023; accepted: Feb. 17th, 2023; published: Feb. 27th, 2023

AB共聚物和纳米粒子组装形成有序结构的设计规则



ABSTRACT
We use Dissipative Particle Dynamics (DPD) simulation method to study the self-assembly behavior of AB diblock copolymers and nanoparticles composite system in dilute solution, certain ordered structures such as micelles and vesicles has been obtained. The results show that the hydrophobicity ratio of block copolymer play a decisive influence on ordered structures, the concentration of the nanoparticles also have a certain degree of impact on the final structures. Either to form micelles or to form vesicles, they will first form aggregates composed of nanoclusters, and then more ordered structure are formed. Combined with experimental methods, through the regulation of the initial structure of the arrangement of nanoparticles, vesicles have two different formation mechanisms. Further summarizes the design rules of this type of material, i.e. when the hydrophobicity ratio is greater than 2:1, it is easy to form micelles, however, when the hydrophobicity ratio is less than 1:2, it is very easy to form vesicles.
Keywords:AB Diblock Copolyme, Nanoparticle, Dissipative Particle Dynamics, Micelle, Vesicle

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
在具有不同选择性的稀溶液中,嵌段共聚物可以自组装形成胶束和囊泡等有序结构。通过加入具有特殊性能的纳米粒子,则可以使得这些有序结构具有更加优异的光学、电学、力学和磁学性质。一方面,纳米粒子因具有一定的硬度,组装过程中往往成为有序结构的骨架,从而大大增加了材料的各项性能和稳定性;另一方面,纳米粒子因具有丰富的功能,能够对嵌段共聚物起到修饰和调控作用等。最近几十年,嵌段共聚物和纳米粒子复合体系的结构和性能的研究成为热点,已经广泛应用于催化、太阳能电池和药物运输等领域 [1] - [7] 。
Mai等人 [8] 重点关注了特定浓度下聚苯乙烯-b-聚氧化乙烯(PS-b-PEO)、聚氧化乙烯-b-聚苯乙烯-b-聚丙烯酸(PEO-b-PS-b-PAA)、铅交联的聚氧化乙烯-b-聚苯乙烯-b-聚丙烯酸(PEO-b-PS-b-P(APb))、硫辛酸酯-b-聚苯乙烯-b-聚丙烯酸(TE-PS270-b-PAA15)、硫辛酸酯-b-聚苯乙烯-b-聚丙烯酸接枝金纳米粒子等复合体系,给出了其在水溶液中自组装形成囊泡的可能机理。Guo等人 [9] 研究了硫化镉交联的聚苯乙烯-b-聚丙烯酸-b-聚甲基丙烯酸甲酯(PS-b-PAA-b-PMMA)和硫化镉交联的聚苯乙烯-b-聚丙烯酸-b-聚甲基丙烯酸(PS-b-PAA-b-PMAA)在水溶液中自组装形成胶束、蠕虫状胶束和囊泡的过程。Song等人 [10] 研究了由金@聚乙二醇/聚甲基丙烯酸甲酯(Au@PEG/PMMA)组装形成的囊泡的pH响应特性。Hu等人 [11] 将聚氧化乙烯-b-聚硫辛酸-b-聚苯乙烯(PEO-b-P(LAMP-co-GMA)-b-PS)中的聚硫辛酸与金纳米粒子结合,该复合物进一步自组装,可以形成胶束、纳米棒和囊泡等有序结构。He等人 [4] [12] 系统研究了金纳米粒子接枝聚苯乙烯和聚氧化乙烯后,自组装形成纳米簇、胶束、囊泡和管状结构,并发现具有很好的生物应用。
以上的实验表明,纳米粒子和聚合物复合体系在选择性溶剂条件下,可以形成胶束和囊泡等结构,然而这些有序结构的形成机理和过程并不清晰。因此,我们结合实验的结果,设计了如图1所示的纳米粒子和聚合物复合体系的粗粒化模型,进而研究这类材料组装的设计规则。在粗粒化模拟方法方面,耗散粒子动力学(Dissipative Particle Dynamics, DPD)已经广泛应用于研究软物质的性质 [13] - [20] ,特别是嵌段共聚物与纳米粒子的自组装 [21] [22] [23] [24] 。本文采用DPD方法对嵌段共聚物接枝的纳米粒子在水溶液中的自组装结构、机理和设计规则进行了探索,所得到的结果对实验将起到一定的促进作用。
 A representative architecture of AuNP tethered with 8 short linear block copolymer chains C30 (AxBy)8.
A representative architecture of AuNP tethered with 8 short linear block copolymer chains C30 (AxBy)8.
Figure 1. The coarse-grained model
图1. 粗粒化模型
2. 研究方法及模型构
在DPD方法中,所有相互作用的粒子的运动遵循牛顿运动方程 [25] [26] [27] [28] :
, .
作用到粒子上的力包括保守力、耗散力、随机力以及弹簧力,它们都是对相互作用力:
,
,
,
,
.
其中,保守力 是一个软作用力, 为粒子i和j之间的最大排斥力, , , , 。耗散力 和随机力 分别起着消耗和补偿能量的作用。 和 是依赖于r的权重函数,它们分别描述了两个力随着粒子间距离增加而衰减的情况。 是具有高斯分布且具有单位方差的随机数。Español和Warren [26] 认为耗散力和随机力必须遵循涨落–耗散定理,也就是 , ,因此体系符合正则平衡分布。
整个模拟在具有周期性边界条件的立方箱60 × 60 × 60中,采用NVT系综,使用GALAMOST软件包在NVIDIA GTX 680 GPU上进行计算的 [28] 。纳米粒子上所有的粗粒化粒子都受到刚体限制。 是虎克弹簧力,用来将相邻的粒子连接到一起来构筑聚合物链,弹簧力常数 。为了数值处理上的便利,物理量的单位采用了约化单位: 。在模拟当中,我们用GW-VV算法 [26] 来对粒子的运动方程进行积分,积分步长 。粒子的数密度 ,因此体系中总粒子数为648,000,包括1000个纳米粒子C30,8000个嵌段共聚物链AxBy,其余为溶剂S粒子。模拟中所采用的DPD粒子间相互作用参数列表1中。同种类粒子的相互作用参数,我们设定为 ;不同种类粒子之间的相互作用参数,我们视模型中二者的亲合程度而定。例如 表明我们定义的嵌段共聚物上的A段和纳米粒子C为疏水端, 表明B是亲水的, 表明B与A和C之间有相同大小的较弱的排斥效应。
Table 1. Interaction parameters αij between DPD beads
表1. DPD粒子αij间相互作用参数
3. 结果与讨论
3.1. 胶束的形成过程
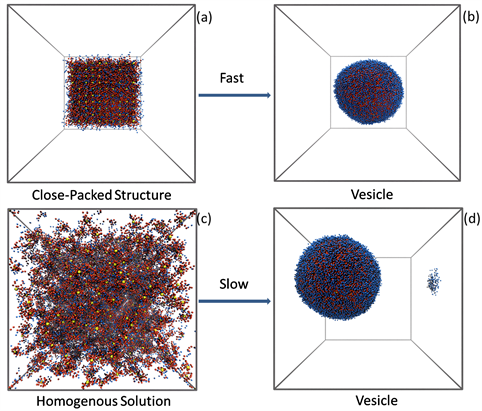
在具有周期性边界条件的60 × 60 × 60立方箱中,我们将1000个C30 (AxBy)8纳米粒子接枝AB两嵌段共聚物等距离摆放和无序摆放,都获得了胶束结构。从无序的初始结构出发(图2(a)),首先形成小的球状胶束(图2(b)),然后聚集形成棒状胶束(图2(c)),直到形成联通的蠕虫状胶束(图2(d))。AB两嵌段共聚物和纳米粒子形成胶束的过程,与传统的两嵌段共聚物形成胶束的机理一致。由于纳米粒子有一定的硬度,在这里起到支撑的作用;由于纳米粒子被疏水嵌段包裹,也是疏水的,从而促进了胶束的形成。需要指出的是,胶束的形成机制是唯一的,也就是从球状胶束到蠕虫状胶束的形成过程。
 Yellow: AuNP, red: hydrophobic, blue: hydrophilic.
Yellow: AuNP, red: hydrophobic, blue: hydrophilic.
Figure 2. The formation process of micelles
图2. 胶束的形成过程
3.2. 囊泡的形成过程
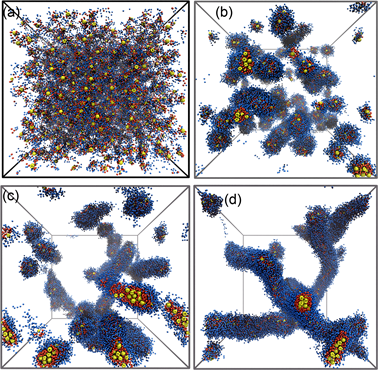
3.2.1. 囊泡的两种形成机理
我们发现囊泡有两种形成机制。对于初始状态是紧密排列的结构来说,会迅速形成囊泡(图3(a),图3(b));而对于初始状态是非均一排列的结构来说,形成囊泡是一个漫长的过程(图3(c),图3(d))。另外,我们发现,是否形成囊泡,不取决于初始结构的排列方式,而是取决于亲水端和疏水段的比例。当亲疏水比例大于2:1时,很容易形成胶束,亲疏水比例小于1:2时,则很容易形成囊泡。同时,我们发现两种机理得到的囊泡的尺寸、膜的厚度几乎是一样。
 Yellow: AuNP, red: hydrophobic, blue: hydrophilic.
Yellow: AuNP, red: hydrophobic, blue: hydrophilic.
Figure 3. Two different formation mechanisms of vesicles
图3. 囊泡的两种形成机理
3.2.2. 囊泡在均一溶剂中的组装机理
从无序的初始结构出发(图4(a)),首先形成小的球状胶束(图4(b),图4(c)),然后聚集形成小的蝶状胶束(图4(d)~(f)),直到形成大的蝶状胶束(图4(g)~(i))。经过漫长的动力学过程,大的蝶状胶束通过弯曲形成半球状结构。一旦形成半球状结构,就会迅速发生闭合,形成完整的囊泡(图4(j),图4(l))。这与普通两嵌段形成囊泡的组装机理一致,纳米粒子的引入,使得囊泡具有更好的力学性能,也更稳定。需要指出的是,以上结论全部建立在稀溶液体系中,纳米粒子复合体系的浓度如果超出稀溶液范围,则会形成连通结构,因此纳米粒子复合体系的浓度对最终的结构也有一定程度的影响。
4. 结论
本文利用耗散粒子动力学方法,我们系统研究了纳米粒子接枝AB两嵌段共聚物在稀溶液中的自组装过程,不仅得到了胶束和囊泡等有序结构,还探究了该类体系的设计规则。我们发现嵌段共聚物的亲疏水比例对有序结构起到决定性的影响,纳米粒子复合体系的浓度对最终的结构也有一定程度的影响。无论是形成胶束还是形成囊泡,都会先形成纳米簇的聚集体,进而聚集甚至弯曲形成更加有序的结构。
 Yellow: AuNP, red: hydrophobic, blue: hydrophilic
Yellow: AuNP, red: hydrophobic, blue: hydrophilic
Figure 4. The formation process of vesicles in homogeneous solution
图4. 囊泡在均一溶剂中的组装机理过程
结合实验现象,我们通过调控初始结构中纳米粒子的排列方式,发现囊泡有两种截然不同的形成机制。最后,我们总结了这类自组装的设计规则,即亲疏水比例大于2:1时,很容易形成胶束,亲疏水比例小于1:2时,则很容易形成囊泡。我们的研究为实验中快速制备胶束和囊泡,提供了有力的理论支持。
基金项目
吉林省科技发展计划(项目号20230508165RC)和吉林工商学院2022年度院级科研基金(批准号:院理科合字K[2022]第007号)资助。
文章引用
李 洋,王科飞,胡海燕,王志超,李延春. AB共聚物和纳米粒子组装形成有序结构的设计规则
Design Rules of Ordered Structure Formed by AB Diblock Copolymers and Nanoparticles in Dilute Solution[J]. 物理化学进展, 2023, 12(01): 13-20. https://doi.org/10.12677/JAPC.2023.121002
参考文献
- 1. Shim, M.K., Yang, S., Sun, I.-C. and Kim, K. (2022) Tumor-Activated Carrier-Free Prodrug Nanoparticles for Targeted Cancer Immunotherapy: Preclinical Evidence for Safe and Effective Drug Delivery. Advanced Drug Delivery Reviews, 183, Article ID: 114177. https://doi.org/10.1016/j.addr.2022.114177
- 2. Gagliardi, A., Giuliano, E., Venkateswararao, E., Fresta, M., Bulotta, S., Awasthi, V. and Cosco, D. (2021) Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Frontiers in Pharmacology, 12, Article 601626. https://doi.org/10.3389/fphar.2021.601626
- 3. Yan, L.-T. and Xie, X.-M. (2013) Computational Modeling and Simulation of Nanoparticle Self-Assembly in Polymeric Systems: Structures, Properties and External Field Effects. Progress in Polymer Science, 38, 369-405. https://doi.org/10.1016/j.progpolymsci.2012.05.001
- 4. Yi, C., Yang, Y., Liu, B., He, J. and Nie, Z. (2020) Polymer-Guided Assembly of Inorganic Nanoparticles. Chemical Society Reviews, 49, 465-508. https://doi.org/10.1039/C9CS00725C
- 5. Shifrina, Z.B., Matveeva, V.G. and Bronstein, L.M. (2020) Role of Polymer Structures in Catalysis by Transition Metal and Metal Oxide Nanoparticle Composites. Chemical Reviews, 120, 1350-1396. https://doi.org/10.1021/acs.chemrev.9b00137
- 6. Kakkar, A., Traverso, G., Farokhzad, O.C., Weissleder, R. and Langer, R. (2017) Evolution of Macromolecular Complexity in Drug Delivery Systems. Nature Reviews Chemistry, 1, Article No. 0063. https://doi.org/10.1038/s41570-017-0063
- 7. Boles, M.A., Engel, M. and Talapin, D.V. (2016) Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chemical Reviews, 116, 11220-11289. https://doi.org/10.1021/acs.chemrev.6b00196
- 8. Mai, Y. and Eisenberg, A. (2010) Controlled Incorporation of Particles into the Central Portion of Vesicle Walls. Journal of the American Chemical Society, 132, 10078-10084. https://doi.org/10.1021/ja1024063
- 9. Guo, Y.Y., Harirchian-Saei, S, Izumi, C.M.S. and Moffitt, M.G. (2011) Block Copolymer Mimetic Self-Assembly of Inorganic Nanoparticles. ACS Nano, 5, 3309-3318. https://doi.org/10.1021/nn200450c
- 10. Song, J.B., Cheng, L., Liu, A.P., Yin, J., Kuang, M. and Duan, H.W. (2011) Plasmonic Vesicles of Amphiphilic Gold Nanocrystals: Self-Assembly and External-Stimuli-Triggered Destruction. Journal of the American Chemical Society, 133, 10760-10763. https://doi.org/10.1021/ja204387w
- 11. Hu, J.M., Wu, T., Zhang, G.Y. and Liu, S.Y. (2012) Efficient Synthesis of Single Gold Nanoparticle Hybrid Amphiphilic Triblock Copolymers and Their Controlled Self-Assembly. Journal of the American Chemical Society, 134, 7624- 7627. https://doi.org/10.1021/ja302019q
- 12. He, J., Liu, Y.J., Babu, T., Wei, Z.J. and Nie, Z.H. (2012) Self-Assembly of Inorganic Nanoparticle Vesicles and Tubules Driven by Tethered Linear Block Copolymers. Journal of the American Chemical Society, 134, 11342-11345. https://doi.org/10.1021/ja3032295
- 13. Yuan, S.L., Cai, Z.T. and Xu, G.Y. (2002) Dynamic Simulation of Aggregation Morphology in Surfactant Solution. Acta Chimica Sinica, 60, 241-245.
- 14. Zhao, Y., Liu, Y.-T., Lu, Z.-Y. and Sun, C.-C. (2008) Effect of Molecular Architecture on the Morphology Diversity of the Multicompartment Micelles: A Dissipative Particle Dynamics Simulation Study. Polymer, 49, 4899-4909. https://doi.org/10.1016/j.polymer.2008.08.063
- 15. Liu, Y.-T., Zhao, Y., Liu, H., Liu, Y.-H. and Lu, Z.-Y. (2009) Spontaneous Fusion between the Vesicles Formed by A2n(B2)n Type Comb-Like Block Copolymers with a Semiflexible Hydrophobic Backbone. Journal of Physical Chemistry B, 113, 15256-15262. https://doi.org/10.1021/jp903570w
- 16. Zhao, Y., You, L.-Y., Lu, Z.-Y. and Sun, C.-C. (2009) Dissipative Particle Dynamics Study on the Multicompartment Micelles Self-Assembled from the Mixture of Diblock Copolymer Poly(Ethyl Ethylene)-Block-Poly(Ethylene Oxide) and Homopolymer Poly(Propylene Oxide) in Aqueous Solution. Polymer, 50, 5333-5340. https://doi.org/10.1016/j.polymer.2009.09.014
- 17. Zhao, Y., Xie, Y., Lu, Z.-Y. and Sun, C.-C. (2009) Dissipative Particle Dynamics Simulation of Physical Gelation Behavior of P123(PEO20-PPO70-PEO20) Block Copolymer Aqueous Solution. Chemical Journal of Chinese Universities, 30, 2455-2459.
- 18. Zhu, P.F., Li, Y., Li, Q.W., Song, X.W., Cao, X.L. and Li, Z.Q. (2011) Mesoscopic Simulation of the Interfacial Behavior of Biosurfactant Rhamnolipids and the Synergistic Systems. Acta Chimica Sinica, 69, 2420-2426.
- 19. Li, Y.C., Wang, Y.L., Li, Z.W., Liu, H. and Lu, Z.Y. (2013) Towards Larger Spatiotemporal Scales in Polymer Simulations. Chinese Science Bulletin, 58, 3595-3599. https://doi.org/10.1007/s11434-013-5981-1
- 20. Sun, N.N., Li, Y.M., Wang, D.X., Bao, M.T. and Tong, L.J. (2013) Mesoscopic Simulation Studies on the Self-Assembly of Pluronic at Oil/Water Interface. Acta Chimica Sinica, 71, 186-192. https://doi.org/10.6023/A12121023
- 21. He, L.L., Zhang, L.X. and Liang, H.J. (2010) Mono- or Bidisperse Nanorods Mixtures in Diblock Copolymers. Polymer, 51, 3303-3314. https://doi.org/10.1016/j.polymer.2010.05.026
- 22. Yue, T.T., Li, S.Y., Zhang, X.R. and Wang, W.C. (2010) The Relationship between Membrane Curvature Generation and Clustering of Anchored Proteins: A Computer Simulation Study. Soft Matter, 6, 6109-6118. https://doi.org/10.1039/c0sm00418a
- 23. Ding, H.-M., Tian, W.-D. and Ma, Y.-Q. (2012) Designing Nanoparticle Translocation through Membranes by Computer Simulations. Acs Nano, 6, 1230-1238.. https://doi.org/10.1021/nn2038862
- 24. Huang, M.X., Li, Z.Q. and Guo, H.X. (2012) The Effect of Janus Nanospheres on the Phase Separation of Immiscible Polymer Blends via Dissipative Particle Dynamics Simulations. Soft Matter, 8, 6834-6845. https://doi.org/10.1039/c2sm25086a
- 25. Español, P. and Warren, P. (1995) Statistical Mechanics of Dissipative Particle Dynamics. Europhysics Letters, 30, 191-196. https://doi.org/10.1209/0295-5075/30/4/001
- 26. Groot, R.D. and Warren, P.B. (1997) Dissipative Particle Dynamics: Bridging the Gap between Atomistic and Mesoscopic Simulation. Journal of Chemical Physics, 107, 4423-4435. https://doi.org/10.1063/1.474784
- 27. Qian, H.-J., Lu, Z.-Y., Chen, L.-J., Li, Z.-S. and Sun, C.-C. (2005) Computer Simulation of Cyclic Block Copolymer Microphase Separation. Macromolecules, 38, 1395-1401. https://doi.org/10.1021/ma0478658
- 28. Zhu, Y.-L., Liu, H., Li, Z.-W., Qian, H.-J., Milano, G. and Lu, Z.-Y. (2013) GALAMOST: GPU-Accelerated Large- Scale Molecular Simulation Toolkit. Journal of Computational Chemistry, 34, 2197-2211. https://doi.org/10.1002/jcc.23365
NOTES
*通讯作者。
