Advances in Microbiology
Vol.05 No.03(2016), Article ID:18520,11
pages
10.12677/AMB.2016.53004
The Effects of Rapeseed Oil and Linseed Oil on Biomass and Metabolites of Lachnum sp.
Shuai Zong1, Moyuan Zhao2, Jinglei Li1, Man Huang1, Liuqing Yang1, Ming Ye1*
1Institute of Microbiology and Food, College of Food Science and Technology, Hefei University of Technology, Hefei, Anhui
2Faculty of Medicine, Nursing, and Health Sciences, Monash University, Melbourne, Australia

Received: Aug. 18th, 2016; accepted: Sep. 1st, 2016; published: Sep. 12th, 2016
Copyright © 2016 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



ABSTRACT
The research mainly investigated the effects of different concentrations of rapeseed oil and linseed oil on biomass and metabolites of Lachnum sp. Different concentrations of 0.5%, 1%, 2%, 3% and 4% rapeseed oil or linseed oil were added into culture medium respectively and fermented for 10 days, it was found that the biomass of 1% rapeseed oil and 2% linseed oil were 4.26 g/L and 4.27 g/L, which were almost equal to that absence of lipids (4.29 g/L), while the biomass with rapeseed oil and linseed oil of other concentrations was inhibited at different degrees. However, there was no significant difference in the biomass of the same oil among different concentrations. Rapeseed oil and linseed oil in different concentrations could inhibit exopolysaccharides (LEP) production significantly. The fermentation broth with 1% rapeseed oil and 2% linseed oil was selected, then metabolites in chloroform and ethyl acetate extraction components of fermentation broth and the original one were analyzed by Liquid Chromatograph Mass Spectrometer (LC-MS), the results showed that 27 kinds of substances were detected in the fermentation broth with 1% rapeseed oil and 20 kinds of substances were detected in the one with 2% linseed oil, only 4 same substances were detected in the fermentation broth added with these two kinds of lipids, which suggested that different kinds of lipids had significant influences on metabolites. 3 same substances were detected in the fermentation broth with 1% rapeseed oil after extracted with two extraction solvents, while 4 same substances were detected in the fermentation broth (with 2% flax seed oil) extractions, which illustrated that the test results of different broth extractions were obviously different. The results of the research indicate that different kinds of lipids have inhibiting effects on biomass and LEP production of Lachnum and the inhibition effects of different concentrations of oil are different; different kinds of lipids can affect the types of metabolites of Lachnum.
Keywords:Lachnum, Biomass, Metabolites, LC-MS, Lipid

菜籽油和亚麻籽油对粒毛盘菌生物量 和代谢产物的影响
宗帅1,赵墨元2,李井雷1,黄曼1,杨柳青1,叶明1*
1合肥工业大学食品科学与工程学院,微生物与食品研究所,安徽 合肥
2莫纳什大学医药、护理与健康科学系,澳大利亚 墨尔本

收稿日期:2016年8月18日;录用日期:2016年9月1日;发布日期:2016年9月12日

摘 要
本文主要研究了不同浓度的菜籽油和亚麻籽油对粒毛盘菌YM406生物量和代谢产物的影响。向培养基中分别添加0.5%、1%、2%、3%和4%的菜籽油和亚麻籽油进行发酵10天,发现当菜籽油浓度为1%,亚麻籽油浓度为2%时,生物量与未添加油脂的平均生物量(4.29 g/L)差距不大,分别是4.26 g/L和4.27 g/L,而其他浓度都会不同程度地抑制生物量。但同一油脂不同浓度间生物量没有显著性差异。不同浓度的菜籽油和亚麻籽油对胞外多糖(LEP)产量均有明显的抑制作用。选择添加1%菜籽油和添加2%亚麻籽油的发酵液,采用液相色谱-质谱联用技术(LC-MS),分别检测了经氯仿、乙酸乙酯萃取及未萃取的发酵液中代谢产物,检测结果显示添加1%菜籽油发酵液共检测出27种物质,添加2%亚麻籽油发酵液共检测出20种物质,添加这两种油脂的发酵液中检测出的物质只有4种相同,这说明不同类型的油脂对代谢产物类型有显著影响。添加1%菜籽油发酵液经两种萃取剂萃取后只有3种相同物质,而添加2%亚麻籽油后只有4种,说明不同萃取剂萃取后发酵液的检测结果差异明显。本研究结果表明,不同类型的油脂对粒毛盘菌YM406生物量和多糖产量具有抑制作用,并且不同浓度的油脂抑制作用不同;不同类型的油脂会影响粒毛盘菌YM406代谢产物种类。
关键词 :粒毛盘菌,生物量,代谢产物,LC-MS,油脂

1. 引言
粒毛盘菌属(Lachnum Retz.)始建于1769年,隶属于盘菌纲(Discomycetes)柔膜菌目(Helotiales)晶杯菌科(Hyaloscyphaceae),是一类腐生性真菌。据统计,就粒毛盘菌属(Lachnum Retz)而言,全世界己知大约有170种 [1] ,中国己知48种 [2] ,而且新种不断被发现 [3] 。先前已经有研究表明,从粒毛盘菌属的深层培养液中提取的物质具有杀线虫和抗微生物作用的生物活性 [4] [5] 。近几年,我们对于粒毛盘菌代谢产物进行了更加深入的研究,发现一些粒毛盘菌在深层发酵的条件下能够产生多糖、色素、多酚等活性物质并且研究表明粒毛盘菌多糖具有抗慢性肾衰竭 [6] 、抗氧化 [7] 、抗肿瘤 [8] 、抗衰老 [9] 、降血糖 [10] 、肝保护 [11] 等作用;粒毛盘菌黑色素具有抗菌 [12] 、抗衰老 [13] 、抗氧化 [14] 、抗辐射 [15] 、抗贫血 [16] 等作用。
我们先前的研究已经表明,碳源、氮源、生长因子、pH、发酵时间对粒毛盘菌胞内和胞外多糖的产量具有重要影响 [17] ,另有研究表明油脂也会影响微生物的代谢产物,如王梦之等通过添加豆油等四种油脂,研究了不同饱和程度的油脂对瘤胃微生物体外产气及辅酶F420动态变化的影响 [18] 。谭韵雅等以油樟油作为诱导物,研究了三株油樟内生真菌在不同培养条件下对菌株中活性化合物的影响 [19] 。Yang等人通过向灵芝菌丝深层培养基中添加薏苡仁油,研究了薏苡仁油对三萜类化合物、多糖等活性代谢产物产量的影响 [20] 。在同一培养条件下,不同种微生物代谢产物往往不同 [21] ,因此代谢产物可以作为鉴定菌种的依据 [22] 。菜籽油和亚麻籽油作为我国主要食用油,分布广泛,廉价易得,并且其中油酸,亚油酸,亚麻酸等物质对微生物生长和代谢具有刺激作用 [23] - [25] 。但菜籽油和亚麻籽油对粒毛盘菌其他代谢产物的影响尚未报道。此外,采用LC-MS检测微生物发酵液中代谢产物多样性也少见报道。
本研究首次选用粒毛盘菌YM406,通过向培养基中添加不同浓度的菜籽油和亚麻籽油后,分别使用氯仿和乙酸乙酯对发酵液进行萃取,通过液相色谱–质谱联用技术(LC-MS)检测萃取后发酵液中的代谢产物,分析油脂对于粒毛盘菌YM406生物量和代谢产物多样性的影响。本研究为微生物代谢产物多样性研究、代谢产物调控以及菌种鉴定提供了重要的理论依据。
2. 材料与方法
2.1. 材料
2.1.1. 菌株
粒毛盘菌YM406子实体采集于安徽黄山,由合肥工业大学微生物资源与应用研究室分离保藏。
2.1.2. 培养基
平板活化培养基:葡萄糖20 g/L,蛋白胨5 g/L,酵母膏5 g/L,琼脂15 g/L,自然pH。基础发酵培养基:葡萄糖20 g/L,蛋白胨5 g/L,酵母膏5 g/L,自然pH。
2.2. 实验方法
2.2.1. 粒毛盘菌YM406的活化
将斜面保藏菌种接种于平板活化培养基上于25℃活化培养5天,作为摇瓶发酵的种子。
2.2.2. 油脂添加实验
在基础发酵培养基中分别加入0.5%、1%、2%、3%、4%的菜籽油和亚麻籽油制作成添加不同油脂的培养基,并与不添加油脂的空白组进行对照。每种浓度设置3个平行。
2.2.3. 发酵培养
在250 mL的三角瓶中,装入150 mL不同的发酵培养基,用打孔器接入直径为8 mm的菌块,在发酵条件下进行培养(发酵培养基,发酵温度25℃,发酵时间10天,摇床转速160 r∙min−1)。
2.2.4. 生物量测定
发酵结束后,将发酵液抽滤,菌丝体用蒸馏水清洗2至3次后于50℃干燥箱中烘干至恒重,称重换算为菌体干重浓度(菌体干重浓度=菌体干重/发酵液体积)。
2.2.5. LEP产量测定
取10 mL发酵液并加入3倍体积的95%乙醇,于4℃下沉淀12 h,然后4000 r∙min−1离心10 min,除去上清液,沉淀物用蒸馏水溶解至10 mL,参考Vinarta等 [26] 利用苯酚-硫酸法测定多糖质量浓度。
2.2.6. 代谢产物检测
取添加量为1%菜籽油和2%亚麻籽油的培养基中的发酵液,每瓶发酵液分装于三个小锥形瓶中编号为1、2、3,分别对1和2进行氯仿萃取和乙酸乙酯萃取,保留有机相。处理后,对发酵液进行LC-MS检测。
LC-MS检测条件:样品分离使用Agilent Eclipse XDB-C18 colunm (5 μm, 2.1 mm × 150 mm),进样体积5 ul,柱温25℃,流速0.3 ml,流动相:A = 0.1%甲酸水溶液,B = 0.1%甲酸的乙腈,洗脱条件:0~3 min,5%B,3~20 min,5~50%B,20~45 min,50~100%B,45~55 min,100%B。质谱阳离子模式条件:氮气用作干燥气,氮气温度350℃,流速12 L/min,雾化气压35 psi;毛细管电压:阳离子4000 V,阴离子3500 V;碎裂电压:阳离子215 V,阴离子170 V,分离器电压60 V;质量采集范围:阳离子50~1500 Da,阴离子50~1500 Da。
3. 结果与分析
3.1. 油脂对生物量的影响
图1表明,在菜籽油组中,当添加浓度为1%时,生物量最大,为4.26 g/L;在亚麻籽油组中,当添加浓度为2%时,生物量最大,为4.27 g/L。添加两种油脂后的最大生物量与未添加油脂时的平均生物量(4.29 g/L)没有明显差异;而添加0.5%、4%等浓度油脂会明显降低最大生物量。这可能是由于不同种类和浓度的油脂对细胞的刺激作用不同 [27] ,及1%的菜籽油和2%的亚麻籽油能够作为特定的表面活性剂改变细胞膜成分,改善膜的渗透性从而缓解油脂中某些脂肪酸对微生物的毒害作用,而其他浓度的油脂对膜渗透性的改善作用不同,故表现出不同的抑制作用 [28] - [30] 。
3.2. 油脂对LEP产量的影响
图2表明,浓度为1%和2%的亚麻籽油能降低粒毛盘菌YM406胞外多糖LEP产量(P < 0.05),并且当亚麻籽油浓度为2%时,LEP产量最大,为0.81 g/L。菜籽油也能降低LEP产量,但作用不明显(P > 0.05)。这与谢通慧等人报道的甘油能够提高小球藻还原糖产量 [31] 以及Yang等人报道的薏苡仁油能提高灵芝菌

Figure 1. The effects of lipid on biomass
图1. 油脂对生物量的影响

Figure 2. The effects of lipid on LEP
图2. 油脂对LEP产量的影响

Figure 3. LC-MS chromatogram of chloroform extraction of fermentation broth with 1% rapeseed oil
图3. 添加1%菜籽油发酵液经氯仿萃取后LC-MS色谱图
丝体胞外多糖产量 [20] 相反,而与Yang等人报道的亚油酸能够强烈抑制多糖的产生 [27] 及Park等人报道的亚油酸能抑制冬虫夏草菌丝体生长和胞外生物聚合物的产生 [32] 一致。这可能是由于植物和微生物种类不同,对于油脂的应答也有所不同 [18] 。并且,这也与油脂对于微生物的毒害作用和油脂对细胞膜及葡萄糖转移酶活性的影响是密不可分的 [24] [30] 。
3.3. 油脂对粒毛盘菌YM406代谢产物的影响
粒毛盘菌YM406添加1%菜籽油的发酵液分别经氯仿、乙酸乙酯萃取,萃取后有机相及未萃取的发酵液经LC-MS检测,色谱图分别为图3、图4、图5,对应检测物质见表1,“+”表示该物质检测出。
未萃取发酵液中时共检测出14种代谢产物。经氯仿萃取后检测出11种物质,其中5种在未萃取培养基中检出。经乙酸乙酯萃取后检测出13种物质,其中6种在未萃取培养基中检出。结果表明,发酵液在经这两种不同萃取剂萃取后代谢产物差异较大。对比氯仿与乙酸乙酯两种物质萃取后的发酵液可看出,发酵液在经两种不同萃取剂萃取后剩余产物差异较大。这可能是由于水相和有机相的检测、洗脱条件不同,导致检测出的物质不同。

Figure 4. LC-MS chromatogram of ethyl acetate extraction of fermentation broth with 1% rapeseed oil
图4. 添加1%菜籽油发酵液经乙酸乙酯萃取后LC-MS色谱图

Figure 5. LC-MS chromatogram of fermentation broth with 1% rapeseed oil
图5. 添加1%菜籽油发酵液LC-MS色谱图
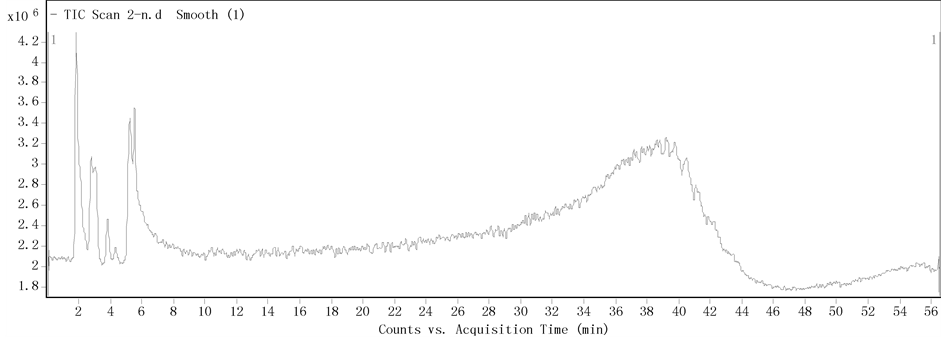
Figure 6. LC-MS chromatogram of chloroform extraction of fermentation broth with 2% linseed oil
图6. 添加2%亚麻籽油发酵液经氯仿萃取后LC-MS色谱图
Table 1. Results of LC-MS of fermentation broth with 1% rapeseed oil
表1. 添加1%菜籽油发酵液LC-MS检测结果
3.4. 亚麻籽油对粒毛盘菌YM406代谢产物的影响
粒毛盘菌YM406添加菜籽油的发酵液分别经氯仿、乙酸乙酯萃取,萃取后有机相及未萃取的发酵液
经LC-MS检测,色谱图分别为图6、图7、图8,对应检测物质见表2,“+”表示该物质检测出。
发酵液经氯仿萃取后共检测出9种物质,其中3种物质在未萃取培养基中检出。经乙酸乙酯萃取后

Figure 7. LC-MS chromatogram of ethyl acetate extraction of fermentation broth with 2% linseed oil
图7. 添加2%亚麻籽油发酵液经乙酸乙酯萃取后LC-MS色谱图

Figure 8. LC-MS chromatogram of fermentation broth with 2% linseed oil
图8. 添加2%亚麻籽油发酵液LC-MS色谱图
Table 2. Results of LC-MS of fermentation broth with 2% linseed oil
表2. 添加2%亚麻籽油发酵液LC-MS检测结果
续表
共检测出9种物质,其中5种物质在未萃取培养基中检出。对比表1与表2可知,两表中检测出相同物质有4种,由此可推断粒毛盘菌YM406在分别添加菜籽油和亚麻籽油培养后都会产出这4种物质,并且添加这两种油脂后发酵液中代谢产物有明显差异。这些差异可能是由于油脂作为碳源会直接影响微生物代谢产物 [33] ,以及不同种类的油脂成分、饱和度不同对微生物物质合成相关酶的刺激作用不同 [34] 。
4. 结语
本研究首次通过向发酵培养基中添加不同浓度菜籽油和亚麻籽油,结合液相色谱–质谱联用技术(LC-MS),研究油脂对粒毛盘菌YM406生物量和代谢产物的影响。实验分别选取了0.5%、1%、2%、3%和4%五种浓度的菜籽油和亚麻籽油,结果表明菜籽油浓度为1%以及亚麻籽油浓度为2%时,生物量与未添加油脂时的平均生物量(4.29 g/L)相差不大,分别为4.26 g/L和4.27 g/L,但其他浓度会明显降低生物量;同样,两种油脂对胞外多糖(LEP)产量也有明显的抑制作用。此外,还使用LC-MS检测了氯仿、乙酸乙酯萃取后发酵液及未萃取的发酵液中的代谢产物,发现添加这两种油脂后未萃取的发酵液中产物差异明显,并且添加同种油脂发酵后采用不同萃取剂萃取出的代谢产物也有较大差异,表明不同类型的油脂能显著的改变粒毛盘菌YM406代谢产物种类且萃取剂对LC-MS物质检测有显著影响。本研究结果不仅对深度挖掘微生物资源,开发新型次级代谢产物,发现新型活性化合物具有重要意义,而且为通过代谢途径调控相关物质合成及通过代谢产物进行菌种鉴定奠定了基础。在后续研究中,我们将检测更多粒毛盘菌菌种的代谢产物,为真菌菌种鉴定提供辅助依据。
基金项目
国家自然科学基金项目(31470146)资助。
文章引用
宗 帅,赵墨元,李井雷,黄 曼,杨柳青,叶 明. 菜籽油和亚麻籽油对粒毛盘菌生物量和代谢产物的影响
The Effects of Rapeseed Oil and Linseed Oil on Biomass and Metabolites of Lachnum sp.[J]. 微生物前沿, 2016, 05(03): 26-36. http://dx.doi.org/10.12677/AMB.2016.53004
参考文献 (References)
- 1. 戴芳澜. 中国真菌总汇[M]. 北京: 科学出版社, 1979: 50-85.
- 2. 庄文颖. 中国粒毛盘菌属研究[J]. 中国菌物学会菌物学学术讨论会, 2003: 440-441.
- 3. Ye, M. and Zhuang, W.Y. (2003) New Taxa of Lachnum (Helotiales, Hyaloscyphaceae) from Temperate China. Nova Hedwigia, 76, 443-450. http://dx.doi.org/10.1127/0029-5035/2003/0076-0443
- 4. Stadler, M., Anke, H. and Sterner, O. (1995) New Metabolites with Nematicidal and Antimicrobial Activities from the Ascomycete Lachnum papyraceum (Karst.) Karst. VII. Structure Determination of Brominated Lachnumon and Mycorrhizin A Derivatives. Japanese Journal of Antibiotics, 48, 158-161. http://dx.doi.org/10.7164/antibiotics.48.158
- 5. Shan, R., Stadler, M., Sterner, O. and Anke, H. (1996) New Metabolites with Nematicidal and Antimicrobial Activities from the Ascomycete Lachnum papyraceum (Karst.) Karst. VIII. Isolation, Structure Determination and Biological Activities of Minor Metabolites Structurally Related to Mycorrhizin A. Journal of Antibiotics, 49, 447-452. http://dx.doi.org/10.7164/antibiotics.49.447
- 6. Wu, Y.N., Ye, M., Du, Z.Z., Jing, L.Y., Surahio, M. and Yang, L. (2014) Carboxymethylation of an Exopolysaccharide from Lachnum and Effect of Its Derivatives on Experimental Chronic Renal Failure. Carbohydrate Polymers, 114, 190-195. http://dx.doi.org/10.1016/j.carbpol.2014.07.075
- 7. Chen, W.X., Cai, J.M., Qiu, T., Zhang, L.B. and Ye, M. (2011) Optimization of Fermentation Conditions of Extracellular Polysaccharide by Lachnum YM328 and Its Antioxidant Activity. Journal of Hefei University of Technology, 34, 300-303.
- 8. Ye, M., Yuan, R.Y., He, Y.L., Du, Z.Z. and Ma, X.J. (2013) Phosphorylation and Anti-Tumor Activity of Exopolysaccharide from Lachnum YM120. Carbohydrate Polymers, 97, 690-694. http://dx.doi.org/10.1016/j.carbpol.2013.05.033
- 9. Ye, M., Chen, W.X., Qiu, T., Yuan, R.Y., Ye, Y.W. and Cai, J.M. (2012) Structural Characterisation and Anti-Ageing Activity of Extracellular Polysaccharide from a Strain of Lachnum sp. Food Chemistry, 132, 338-343. http://dx.doi.org/10.1016/j.foodchem.2011.10.087
- 10. Chen, T., Zhang, M., Li, J., Surhio, M., Li, B. and Ye, M. (2016) Structural Characterization and Hypoglycemic Activity of Trichosanthes Peel Polysaccharide. LWT—Food Science and Technology, 70, 55-62. http://dx.doi.org/10.1016/j.lwt.2016.02.024
- 11. Qiu, T., Ma, X., Ye, M., Yuan, R. and Wu, Y. (2013) Purification, Structure, Lipid Lowering and Liver Protecting Effects of Polysaccharide from Lachnum YM281. Carbohydrate Polymers, 98, 922-930. http://dx.doi.org/10.1016/j.carbpol.2013.07.014
- 12. Wang, Y., Song, S., Ye, M., Lu, Y., Li, L. and Li, J.H. (2013) Extraction Technology, Structural Characteristics and Antibacterial Activity of Melanin from a Strain of Lachnum. Journal of Pure & Applied Microbiology, 7, 285-292.
- 13. Lu, Y., Ye, M., Song, S., Li, L., Shaikh, F. and Li, J. (2014) Isolation, Purification, and Anti-Aging Activity of Melanin from Lachnum singerianum. Applied Biochemistry & Biotechnology, 174, 762-771. http://dx.doi.org/10.1007/s12010-014-1110-0
- 14. Ye, M., Wang, Y., Qian, M., Chen, X. and Hu, X.Q. (2011) Preparation and Properties of the Melanin from Lachnum singerianum. International Journal of Basic & Applied Sciences, 11, 51-58.
- 15. Ye, M., Guo, G.Y., Lu, Y., Song, S., Wang, H.Y.andYang, L. (2014) Purification, Structure and Anti-Radiation Activity of Melanin from Lachnum YM404. International Journal of Biological Macromolecules, 63, 170-176. http://dx.doi.org/10.1016/j.ijbiomac.2013.10.046
- 16. Song, S., Yang, L., Ye, M., Chen, X., Shi, F. and Shaikh, F. (2016) Antioxidant Activity of a Lachnum YM226 Melanin-Iron Complex and Its Influence on Cytokine Production in Mice with Iron Deficiency Anemia. Food & Function, 7, 1508-1514. http://dx.doi.org/10.1039/C5FO01274K
- 17. Ye, M., Qiu, T., Yuan, R.Y. and Yang, L. (2011) Simultaneous Improvement of Yields of Intracellular Polysaccharide and Extracellular Polysaccharide in Submerged Cultivation from a Strain of Lachnum sp. International Journal of Basic & Applied Sciences, 11, 25-31.
- 18. 王梦芝, 王曙, 潘晓花, 王洪荣, 王加启. 4种油脂对瘤胃微生物体外产气及辅酶F420的影响[J]. 动物营养学报, 2011, 23(10): 1819-1825.
- 19. 谭韵雅, 卢红, 李群, 魏琴. 油樟油对油樟内生真菌中活性化合物影响[J]. 天然产物研究与开发, 2015(6): 1070-1075.
- 20. Yang, H., Min, W., Bi, P., Zhou, H. and Huang, F. (2013) Stimulatory Effects of Coix lacryma-jobi Oil on the Mycelial Growth and Metabolites Biosynthesis by the Submerged Culture of Ganoderma lucidum. Biochemical Engineering Journal, 76, 77-82. http://dx.doi.org/10.1016/j.bej.2013.04.012
- 21. 李军伟. 两种不同来源的微生物次生代谢产物研究[D]: [硕士学位论文]. 北京: 协和医学院, 2014.
- 22. Hung, R., Lee, S. and Bennett, J.W. (2015) Fungal Volatile Organic Compounds and Their Role in Ecosystems. Applied Microbiology & Biotechnology, 99, 3395-3405. http://dx.doi.org/10.1007/s00253-015-6494-4
- 23. Fukushima, Y., Itoh, H., Fukase, T. and Motai, H. (1991) Stimulation of Protease Production by Aspergillus oryzae with Oils in Continuous Culture. Applied Microbiology & Biotechnology, 34, 586-590. http://dx.doi.org/10.1007/BF00167904
- 24. 王梦芝, 喻礼怀, 王洪荣, 卜登攀. 4种不同油脂对瘤胃微生物营养成分的影响[J]. 中国畜牧杂志, 2013, 49(13): 55-58.
- 25. 祁宏伟, 闫晓刚, 于维, 陶浩. 不同培养条件对乳酸菌发酵代谢产物CLA的影响[J]. 安徽农学通报, 2011, 17(21): 38-40.
- 26. Vinarta, S.C., Molina, O.E., Figueroa, L.I.C. and Farina, J.I. (2006) A Further Insight into the Practical Applications of Exopolysaccharides from Sclerotium rolfsii. Food Hydrocolloids, 20, 619-629. http://dx.doi.org/10.1016/j.foodhyd.2005.05.006
- 27. Yang, F.C., Ke, Y.F. and Kuo, S.S. (2000) Effect of Fatty Acids on the Mycelial Growth and Polysaccharide Formation by Ganoderma lucidum in Shake Flask Cultures. Enzyme & Microbial Technology, 27, 295-301. http://dx.doi.org/10.1016/S0141-0229(00)00213-1
- 28. Dehority, B.A., Tirabasso, P.A. and Grifo Jr., G.A. (1989) Most-Probable-Number Procedures for Enumerating Ruminal Bacteria, Including the Simultaneous Estimation of Total and Cellulolytic Numbers in One Medium. Applied & Environmental Microbiology, 55, 2789-2792.
- 29. Keweloh, H. and Heipieper, H.J. (1996) Trans Unsaturated Fatty Acids in Bacteria. Lipids, 31, 129-137. http://dx.doi.org/10.1007/BF02522611
- 30. Stasinopoulos, S.J. and Seviour, R.J. (1990) Stimulation of Exopolysaccharide Production in the Fungus Acremonium Persicinum with Fatty Acids. Biotechnology & Bioengineering, 36, 778-782. http://dx.doi.org/10.1002/bit.260360804
- 31. 谢通惠, 张永奎. 甘油对小球藻生长及代谢产物的影响[J]. 中国生物柴油, 2011(5): 11-14.
- 32. Park, J.P., Kim, S.W., Hwang, H.J., Cho, Y.J. and Yun, J.W. (2002) Stimulatory Effect of Plant Oils and Fatty Acids on the Exo-Biopolymer Production in Cordyceps militaris. Enzyme & Microbial Technology, 31, 250-255. http://dx.doi.org/10.1016/S0141-0229(02)00099-6
- 33. Benincasa, M. and Accorsini, F.R. (2008) Pseudomonas Aeruginosa LBI Production as an Integrated Process Using the Wastes from Sunflower-Oil Refining as a Substrate. Bioresource Technology, 99, 3843-3849. http://dx.doi.org/10.1016/j.biortech.2007.06.048
- 34. Reese, E.T. and Maguire, A. (1969) Surfactants as Stimulants of Enzyme Production by Microorganisms. Applied Microbiology, 17, 242-245.
