Journal of Organic Chemistry Research
Vol.05 No.04(2017), Article ID:23194,11
pages
10.12677/JOCR.2017.54022
Advances in the Study of Microwave Irradiation Efficient Construction of Heterocyclic Compounds with Enaminone or Enamino Ester as Building Blocks
Hairui Bai, Xiaojing Wang, Huangmei Fu, Ping Wang, Chao Huang*
*通讯作者。
Engineering Research Center of Biopolymer Functional Materials of Yunnan Province, School of Chemistry and Environment, Yunnan Minzu University, Kunming Yunnan

Received: Dec. 3rd, 2017; accepted: Dec. 19th, 2017; published: Dec. 27th, 2017

ABSTRACT
Nitrogen-containing heterocyclic structures often are ubiquitous scaffolds in many natural products and usually exhibit diverse biological activities. Microwave irradiation synthesis has been recognized as an important method for the environmentally-friendly preparations of novel heterocyclic compounds, and attracted the interest of some scientific researchers. In this review, the recent progress of microwave irradiation construction of heterocyclic compounds with enaminone or enamino ester is summarized, and a full vision of its development is made.
Keywords:Enaminone, Enamino Ester, Microwave Irradiation, N-Heterocyclic Compound, Green and Efficient
微波辅助以烯胺酮(酯)为砌块高效构筑含氮杂环化合物的研究进展
白海瑞,王晓晶,付黄梅,王平,黄超*
云南民族大学化学与环境学院,云南省生物高分子功能材料工程技术研究中心,云南 昆明

收稿日期:2017年12月3日;录用日期:2017年12月19日;发布日期:2017年12月27日

摘 要
诸多天然产物分子和合成化合物都含有氮杂环结构并具有潜在的药物活性。采用微波照射的方法高效构筑含氮的五元、六元、多元环化合物是绿色合成杂环类化合物的重要途径,也是当今有机合成化学领域研究的热点。本文以微波合成为主旨,综述了烯胺酮(酯)为合成砌块高效构筑含氮杂环化合物的研究进展。
关键词 :烯胺酮(酯),微波合成,含氮杂环,绿色高效

Copyright © 2017 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
天然产物是大自然给予人类的馈赠,长期作为药物的主要来源为人类的健康发挥着重要的作用 [1] [2] 。含氮杂环结构是天然产物分子中重要组成部分,其种类繁多,常在合成药物、中草药活性成分中出现 [3] [4] 。含氮杂环化合物作为药物或药物先导结构呈现出独特的生物活性例如抗菌 [5] 、抗人类免疫缺陷病毒(HIV) [6] 、抗癌 [7] 、抗炎 [8] 等,其在合成染料 [9] 、感光材料 [10] 、表面活性剂 [11] 、新型催化剂 [12] 等中也有着广泛的用途。
烯胺酮(酯)是有机化学中经典的活性反应结构之一,是合成杂环化合物如吡啶、嘧啶、吡唑、哒嗪、异噻唑等的常用原料 [13] [14] 。烯胺酮一词由Greenhill [15] 首先提出,泛指含N-C=C-C=O的共轭结构的1,3-二酮、β-酮酯或类似的1,3双官能团化合物,也通常被称为烯胺基酮或β-氨基-α,β-不饱和酮 [16] 。烯胺酮(酯)存在共轭结构,物理及化学性质均具有显著的特点 [17] 。该化合物的α-C的HOMO轨道能量较普通亲核试剂醛酮更高,使得其具有较强的亲核性 [18] [19] 。当烯胺酮(酯) α-C连有吸电子基团如硝基、三氟甲基或其它强吸电子结构,双键电子云进一步极化,从而使连有吸电子基团的β-C亲电活性及α-C亲核性进一步增强 [20] [21] 。此外,烯胺酮(酯)的双键在一定条件下可作亲双烯体参与Diels-Alder [22] 环加成反应,并且结构中氨基也是活泼的亲核基团(图1) [23] [24] 。近年来,基于烯胺酮(酯)多反应位点的
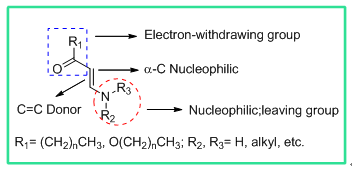
Figure 1. Structure of versatile reactive sites enaminone
图1. 烯胺酮(酯)活性位点
特点 [25] ,采用新的合成技术以其为合成砌块构筑杂环化合物的工作不断被报道。
微波辅助有机合成是有机化学中一个新的热点研究领域,逐渐发展成为一门新兴交叉学科微波化学(Microwave Chemistry)也可以叫做微波诱导催化有机反应化学 [26] [27] 。研究表明,微波条件下一些反应的反应能垒降低导致反应更容易进行 [28] [29] 。同时利用微波加热快速、均质等 [30] 优点可大幅度的缩短有机反应时间和减少催化剂的使用从而使反应条件变得更加绿色温和 [31] 。随着微波技术的普及,关于利用微波辅助合成杂环化合物的反应不断出现 [32] 。本文结合文献报道和我们课题组研究兴趣及相关工作,对近年来微波条件下高效构筑烯胺酮(酯)合成砌块和含氮杂环化合物相关研究进行了综述。
2. 微波辅助构筑烯胺酮(酯)合成砌块
烯胺酮最常用的合成方法是用具有乙酮结构的各类化合物和N,N-二甲基甲酰胺二甲基缩醛(DMF-DMA)反应 [33] [34] 。2009年Saleh Mohammed Al-Mousawi等 [35] 报道了用含烯胺酮结构化合物1和DMF-DMA反应生成具有烯胺酮结构的化合物2继续和萘-1,4-二酮3反应得到新的具有呋喃环萘醌衍生物4 (图2)。第一步在合成烯胺酮化合物2的反应中,使用连续微波照射使得反应温和进行,和传统加热方法相比收率有所提高。
Utpalparna Kalita等 [36] 报道了以苯乙酮衍生物5为原料分别和DMF-DMA、DMA-DMA反应得到β-烯胺酮中间体6和7进而在微波辅助照射下和金刚烷反应生成具有抗癌、抗炎活性的新化合物9 (图3)。由于微波辅助作用使得合成最终目标产物9的反应时间由传统加热64 h缩短为15 min,并且产率由原来的70%提高到了85%。
Imen Erraye等 [37] 报道了微波辅助照射下通过α-碳烯丙基化反应在烯胺酮α-碳位置引入烯丙基合成化合物12 (图4)。该反应在钯作催化剂的条件下合成了一系列β-烯胺酮衍生物12,这些化合物保留了烯胺酮的化学性质,为后续合成含氮杂环化合物提供了原料。而且,当另一种反应物为乙酸丙烯脂时,该反应可能存在两种途径:1) β-烯胺酮通过氮烯丙基化及胺基先被取代再通过[3,3]-分子内重排得到最终

Figure 2. Synthesis of enaminone building block 2
图2. 合成烯胺酮合成砌块2

Figure 3. Synthetic scheme for enaminone
图3. 烯胺酮合成方案
产物12;2) 直接通过碳烯丙基化形成中间体再消除得到最终产物12 (图5)。
3. 微波辅助以烯胺酮(酯)构筑含氮杂环化合物
3.1. 构筑含氮五元杂环化合物
Lidia De Luc等 [38] 通过优化反应条件选择微波辅助照射下合成吡唑及异恶唑17。反应以胺类化合物13、吡唑化合物14、1,3-二羰基化合物15为原料反应生成烯胺酮砌块继续加入胺类化合物进行环化反应生成具有潜在药物活性的吡唑化合物17 (图6),值得一提的是该反应在开放环境下进行HPLC检测第一步产率高达95%第二步高达98%。
David Kralj等 [39] 通过对含有烯胺酮结构化合物18和具有烷基取代的水合肼19用乙醇做溶剂,功率为300 W微波照射下温度为120˚C反应30 min得到化合物20,进而酸解脱掉Boc两步一锅反应得到吡唑衍生物21 (图7)。
2012年Tamer S. Saleh等 [40] 利用金属催化剂以烯胺酮化合物23为原料在微波辅助条件下进行1,3-偶极环加成反应,生成了化合物24 (图8),反应产率高达90%以上。反应利用了微波辅助合成的优点,使反应在较短时间获得高收益,并具有环境友好、原子利用率高等特点。
2016年Zhang Xiaoyan等 [41] 报道了用廉价易得的原料25,简单有效的方法在微波辅助照射下高效

Figure 4. Pd-mediated allylation of enamine derivatives
图4. 钯催化合成烯胺酮衍生物

Figure 5. Possible mechanistic paths toward the allylated product
图5. 合成烯胺酮(酯)砌块可能存在的路径
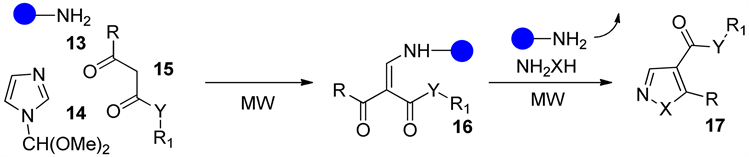
Figure 6. Microwave irradiation synthesis of pyrazoles derivatives
图6. 微波辅助合成吡唑衍生物

Figure 7. One-pot synthesis of pyrazol derivatives
图7. 一锅法合成吡唑衍生物

Figure 8. Microwave irradiation regioselective 1,3-dipolar cycloaddition
图8. 微波条件下1,3-偶极环加成反应
实现α-芳基吡咯类化合物27 (图9)的合成。反应以化合物烯胺酯25和乙酸炔丙酯26作为原料,微波辅助照射下加入CuBr作为催化剂收益可达到90%以上,符合绿色化学高原子经济性要求。该反应在敞开体系中进行,空气对反应无较大影响,为吡咯类化合物工业化生产提供新的高效低成本合成途径。
3.2. 构筑含氮六元杂环化合物
Eman M. H.等 [42] 报道了微波辅助下通过两条途径高效的合成含氮稠杂环化合物33 (图10),该反应以极性溶剂AcOH作溶剂,烯胺酮砌块28和杂环化合物29反应一步成环得到目标化合物33。在此基础上,研究者发现烯胺酮砌块28可由化合物31和DMF-DMA合成,于是设计了三组分的连级反应,在微波条件下三组分化合物迅速反应生成目标化合物。新设计三组分连级反应可以减少反应步骤,提高原子利用率,并且使得原本复杂的原料变为普通简单易得原料,降低了合成反应成本。
同时该课题组还研究了烯胺酮31在微波照射下以醋酸为溶剂醋酸胺为底物分别和下列化合物反应生成一系列稠杂环化合物34、35、36、37 (图11)。
2003年Andrea Porcheddu等 [43] 报道了微波辅助合成嘧啶衍生物40 (图12)的方法,反应以烯胺酮38和胍类化合物39为原料在微波加热条件下,反应温度为80˚C,反应29 min,快速的合成化合物40,产率达到90%以上。若用传统条件65˚C加热2 h可得到与微波条件相同的效果,但微波加热条件下极大的缩短了反应时间提高合成效率。
2004年Sarah Almazroaa等 [44] 报道了一种微波辅助照射下合成具有抗菌活性喹诺酮类化合物42的新方法(图13)。反应以烯胺酮衍生物41为原料微波辅助照射下短时间内环化生成喹诺酮类化合物,该方法为重要药物中间体喹诺酮骨架的合成提供了新的合成思路,并且该方法有望被应用于工业生产喹诺酮类药物当中。
2004年Tu Shujiang等 [45] 设计了多组分串联反应,该反应在微波辅助照射下实现,并且具有无催化剂、反应时间短、产率高等优点。反应以环状烯胺酮43、醛类化合物44和1,3-二羰基化合物45为底物醋酸作为溶剂,在极短时间内实现了高收益的得到具有潜在生物活性的化合物46 (图14)。

Figure 9. Microwave irradiation for synthesis of pyrroles
图9. 微波合成吡咯衍生物

Figure 10. Multicomponent reactions for synthesis of polyheterocyclic 33
图10. 多组分反应合成稠杂环化合物33

Figure 11. Multicomponent reactions for synthesis of heterocyclic 37
图11. 多组分反应合成稠杂环化合物37
为了研究合成砌块烯胺酮的反应性质,微波对烯胺酮构筑杂环化合物的影响,李明等 [46] 利用取代烯胺酮47与3-甲硫基-4-氰基-5-氨基-1-H-吡唑48,分别用传统方法和微波合成法合成文献未曾报道的2-甲硫基-3-氰基-7-取代苯基吡唑并嘧啶49 (图15)。将微波催化合成与传统催化剂催化相比,解决了催化剂

Figure 12. Synthesis of pyrimidine
图12. 多组分反应合成嘧啶化合物
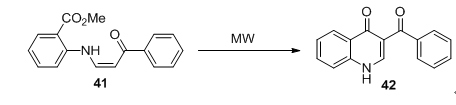
Figure 13. Microwave irradiation synthesis of quinolone
图13. 微波合成喹诺酮化合物
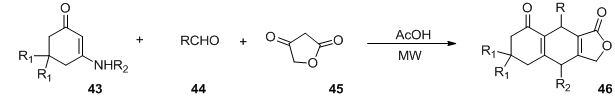
Figure 14. Microwave irradiation synthesis of heterocyclic 46
图14. 微波反应合成杂环化合物46

Figure 15. Microwave irradiation synthesis of heterocyclic 49
图15. 微波反应合成杂环化合物49
回收循环利用带来的一系列问题。
2009年Zhu Songlei等 [47] 设计了微波辅助照射下以烯胺酮化合物50、丙二腈51、吲哚化合物52为原料的多组分反应(MCRs)合成了具有生物活性的螺环含喹啉和吲哚结构的化合物53 (图16)。该反应利用了微波辅助照射使得反应时间缩短收率提高,并且采取了“一锅法”的设计思路,多步反应可连续进行,无须分离出中间体,不产生相应的废弃物,可免去各步后处理和分离带来的消耗和污染。该反应的特点是微波辅助照射下一个反应瓶内连续进行的多步串联反应合成复杂含氮杂环化合物分子。是一类环境友好反应,并且具有原料简单、操作容易、节省溶剂、反应步骤少、降低消耗等优点。
2009年林军等 [48] 以微波辅助无溶剂法合成了一系列具有良好生物活性的异喹啉酮、异喹啉亚胺衍生物57 (图17)。以廉价易得的多取代的间苯二甲腈55为原料在微波条件下与杂环烯胺酮(酯) 54发生芳基化反应,随后在碱性条件下,氰基加成、分子内环化得到一系列异喹啉亚胺衍生物,异喹啉亚胺在酸性条件下水解得到异喹啉酮衍生物。
2012年Alya M. Al-Etaibi等 [49] 用烯胺酮化合物58和吡唑衍生物59合成了含氮杂环化合物60 (图18)。
西班牙有机化学家Rosalyn Pena等 [50] 报道了在微波条件下利用一锅法以醌类化合物61、烯胺酮化合物62和醛类化合物63为底物合成了具有抗葡萄球菌活性的二氢吡啶的衍生物64 (图19)。
4. 结论与展望
综上所述,烯胺酮(酯)作为构筑含氮杂环化合物的重要合成砌块,引起了化学家的广泛关注。近年来
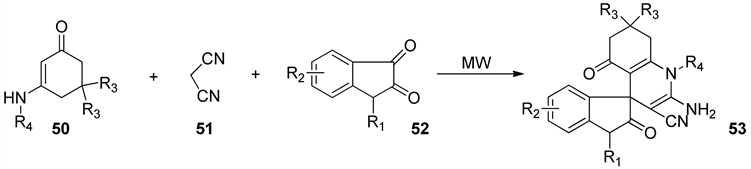
Figure 16. Microwave irradiation synthesis of heterocyclic 53
图16. 微波反应合成杂环化合物53
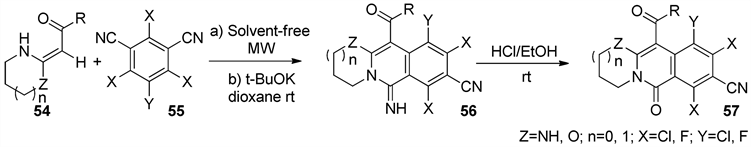
Figure 17. Microwave irradiation synthesis of quinolin
图17. 微波反应合成异喹啉衍生物

Figure 18. Microwave irradiation synthesis of heterocycle 60
图18. 微波反应合成杂环化合物60

Figure 19. Microwave irradiation synthesis of heterocyclic 64
图19. 微波反应合成杂环化合物64
随着绿色化学兴起,微波加热成为促进绿色合成的重要手段之一。其减少反应时间、降低催化剂用量、清洁绿色高效等特点在杂环构筑方面得以很好的体现。随着对烯胺酮(酯)结构的开发和微波辅助合成的深入研究,该方法将在医药、生物、天然产物及全合成等领域得到广泛应用,甚至有望将其推广至工业化生产。
致谢
感谢国家自然科学基金(Nos. 21202142, 21662046)。
文章引用
白海瑞,王晓晶,付黄梅,王 平,黄 超. 微波辅助以烯胺酮(酯)为砌块高效构筑含氮杂环化合物的研究进展
Advances in the Study of Microwave Irradiation Efficient Construction of Heterocyclic Compounds with Enaminone or Enamino Ester as Building Blocks[J]. 有机化学研究, 2017, 05(04): 164-174. http://dx.doi.org/10.12677/JOCR.2017.54022
参考文献 (References)
- 1. Clardy, J. and Walsh, C. (2004) Lessons from Natural Molecules. Nature, 432, 829-837.
https://doi.org/10.1038/nature03194 - 2. Li, J.W.H. and Vederas, J.C. (2009) Drug Discovery and Natural Products: End of an Era or an Endless Frontier. Science, 325, 161-165.
https://doi.org/10.1126/science.1168243 - 3. Eicher, T., Hauptmann, S. and Speicher, A. (2013) The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications. John Wiley & Sons, Hoboken, New Jersey.
- 4. Ma, J., Li, Y., Ye, Q., et al. (2000) Constituents of Red Yeast Rice, a Traditional Chinese Food and Medicine. Journal of Agricultural and Food Chemistry, 48, 5220-5225.
https://doi.org/10.1021/jf000338c - 5. Meyer, E., Schwab, F., Gastmeier, P., et al. (2007) Antifungal Use in Intensive Care Units. Journal of Antimicrobial Chemotherapy, 60, 619-624.
https://doi.org/10.1093/jac/dkm255 - 6. Sax, P.E., DeJesus, E., Mills, A., et al. (2012) Co-Formulated Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir versus Co-Formulated Efavirenz, Emtricitabine, and Tenofovir for Initial Treatment of HIV-1 Infection: A Randomised, Double-Blind, Phase 3 Trial, Analysis of Results after 48 Weeks. The Lancet, 379, 2439-2448.
https://doi.org/10.1016/S0140-6736(12)60917-9 - 7. Xia, Y., Yang, Z.Y., Xia, P., et al. (2001) Antitumor Agents. 211. Fluorinated 2-Phenyl-4-Quinolone Derivatives as Antimitotic Antitumor Agents. Journal of Medicinal Chemistry, 44, 3932-3936.
https://doi.org/10.1021/jm0101085 - 8. Candel, F.J. and Peñuelas, M. (2017) Delafloxacin: Design, Development and Potential Place in Therapy. Drug Design, Development and Therapy, 11, 881.
https://doi.org/10.2147/DDDT.S106071 - 9. McNulty, J. and Still, I.W.J. (2000) Synthetic Approaches to the Eudistomin Marine Alkaloids. Current Organic Chemistry, 4, 121-138.
https://doi.org/10.2174/1385272003376319 - 10. Ovsianikov, A., Viertl, J., Chichkov, B., et al. (2008) Ultra-Low Shrinkage Hybrid Photosensitive Material for Two-Photon Polymerization Microfabrication. Acs Nano, 2, 2257-2262.
https://doi.org/10.1021/nn800451w - 11. El-Sayed, R. (2008) Surface Active Properties and Biological Activity of Novel Nonionic Surfactants Containing Pyrimidines and Related Nitrogen Heterocyclic Ring Systems. Grasas Y Aceites, 59, 110-120.
https://doi.org/10.3989/gya.2008.v59.i2.498 - 12. Brannock, K.C., Burpitt, R.D., Goodlett, V.W., et al. (1963) Enamine Chemistry. II. Reactions with Acetylenedicarboxylates. The Journal of Organic Chemistry, 28, 1464-1468.
https://doi.org/10.1021/jo01041a006 - 13. Katritzky, A.R., Oniciu, D.C., O’Ferrall, R.A.M., et al. (1997) Study of the Enol-Enaminone Tautomerism of α-Heterocyclic Ketones by Deuterium Effects on 13C Chemical Shifts. Journal of the Chemical Society, Perkin Transactions, 2, 2605-2608.
https://doi.org/10.1039/a705235i - 14. Li, M., Guo, W., Wen, L., et al. (2006) Synthesis of Enaminones and Their Utility in Organic Synthesis. Chinese Journal of Organic Chemistry, 26, 1192.
- 15. Greenhill, J.V. (1977) Enaminones. Chemical Society Reviews, 6, 277-294.
https://doi.org/10.1039/cs9770600277 - 16. Brannock, K.C., Bell, A., Burpitt, R.D., et al. (1964) Enamine Chemistry. IV. Cycloaddition Reactions of Enamines Derived from Aldehydes and Acyclic Ketones. The Journal of Organic Chemistry, 29, 801-812.
https://doi.org/10.1021/jo01027a009 - 17. Kascheres, C.M. (2003) The Chemistry of Enaminones, Diazocarbonyls and Small Rings: Our Contribution. Journal of the Brazilian Chemical Society, 14, 945-969.
https://doi.org/10.1590/S0103-50532003000600012 - 18. Shengjiao, Y., Yulan, C., Liu, L., et al. (2010) Three-Component Solvent-Free Synthesis of Highly Substituted Bicyclic Pyridines Containing a Ring-Junction Nitrogen. Green Chemistry, 12, 2043-2052.
https://doi.org/10.1039/c0gc00373e - 19. Mabkhot, Y.N., Aldawsari, F.D., Al-Showiman, S.S., et al. (2015) Novel Enaminone Derived from Thieno [2, 3-b] Thiene: Synthesis, X-Ray Crystal Structure, HOMO, LUMO, NBO Analyses and Biological Activity. Chemistry Central Journal, 9, 1-11.
- 20. Stork, G., Brizzolara, A., Landesman, H., et al. (1963) The Enamine Alkylation and Acylation of Carbonyl Compounds. Journal of the American Chemical Society, 85, 207-222.
https://doi.org/10.1021/ja00885a021 - 21. Edafiogho, I.O., Moore, J.A., Alexander, M.S., et al. (1994) Nuclear Magnetic Resonance Studies of Anticonvulsant Enaminones. Journal of Pharmaceutical Sciences, 83, 1155-1170.
https://doi.org/10.1002/jps.2600830817 - 22. Notz, W., Tanaka, F. and Barbas, C.F. (2004) Enamine-Based Organocatalysis with Proline and Diamines: The Development of Direct Catalytic Asymmetric Aldol, Mannich, Michael, and Diels-Alder Reactions. Accounts of Chemical Research, 37, 580-591.
https://doi.org/10.1021/ar0300468 - 23. Chen, X., Bai, H., Huang, C. (2017) Concise Synthesis of Quinolinone Derivatives. Chinese Journal of Organic Chemistry, 37, 881-888.
- 24. Slosse, P. and Hootelé, C. (1979) Stereospecific Addition of Nucleophiles to Enaminones and the Synthesis of Myrtine and 4-Epimyrtine. Tetrahedron Letters, 20, 4587-4588.
https://doi.org/10.1016/S0040-4039(01)86656-9 - 25. Elassar, A.Z.A. and El-Khair, A.A. (2003) Recent Developments in the Chemistry of Enaminones. Tetrahedron, 59, 8463-8480.
https://doi.org/10.1016/S0040-4020(03)01201-8 - 26. Kappe, C.O. and Dallinger, D. (2006) The Impact of Microwave Synthesis on Drug Discovery. Nature Reviews Drug Discovery, 5, 51-63.
https://doi.org/10.1038/nrd1926 - 27. Bose, A.K., Manhas, M.S., Ghosh, M., et al. (1991) Microwave-Induced Organic Reaction Enhancement Chemistry. 2. Simplified Techniques. The Journal of Organic Chemistry, 56, 6968-6970.
https://doi.org/10.1021/jo00025a004 - 28. Kappe, C.O. (2008) Microwave Dielectric Heating in Synthetic Organic Chemistry. Chemical Society Reviews, 37, 1127-1139.
https://doi.org/10.1039/b803001b - 29. Adam, D. (2003) Microwave Chemistry: Out of the Kitchen. Nature, 421, 571-572.
https://doi.org/10.1038/421571a - 30. Bose, A.K., Manhas, M.S., Banik, B.K., et al. (1994) Microwave-Induced Organic Reaction Enhancement (More) Chemistry: Techniques for Rapid, Safe and Inexpensive Synthesis. Research on Chemical Intermediates, 20, 1-11.
https://doi.org/10.1163/156856794X00027 - 31. Varma, R.S. (1999) Solvent-Free Organic Syntheses. Using Supported Reagents and Microwave Irradiation. Green Chemistry, 1, 43-55.
https://doi.org/10.1039/a808223e - 32. Mingos, D.M.P. and Baghurst, D.R. (1991) Tilden Lecture. Applications of Microwave Dielectric Heating Effects to Synthetic Problems in Chemistry. Chemical Society Reviews, 20, 1-47.
https://doi.org/10.1039/cs9912000001 - 33. Gomha, S.M. and Abdel-Aziz, H.A. (2012) Enaminones as Building Blocks in Heterocyclic Preparations: Synthesis of Novel Pyrazoles, Pyrazolo[3,4-d]pyridazines, Pyrazolo[1,5-a]pyrimidines, Pyrido[2,3-d]pyrimidines Linked to Imidazo[2,1-b]thiazole System. Heterocycles, 85, 2291-2303.
https://doi.org/10.3987/COM-12-12531 - 34. Saleh, T.S., A Al-Omar, M.A. and Abdel-Aziz, H.A. (2010) One-Pot Synthesis of Enaminones Using Gold’s Reagent. Letters in Organic Chemistry, 7, 483-486.
https://doi.org/10.2174/157017810791824793 - 35. Al-Mousawi, S.M. and El-Apasery, M.A. (2009) Azolyacetones as Precursors to Indoles and Naphthofurans Facilitated by Microwave Irradiation with Simultaneous Cooling. Molecules, 14, 2976-2984.
https://doi.org/10.3390/molecules14082976 - 36. Kalita, U., Kaping, S., Nongkynrih, R., et al. (2015) Synthesis, Structure Elucidation, and Anti-Inflammatory/ Anti-Cancer/Anti-Bacterial Activities of Novel (Z)-3-Adamantyl-1-aryl-prop/but-2-en-1-ones. Medicinal Chemistry Research, 24, 32-50.
https://doi.org/10.1007/s00044-014-1086-x - 37. Erray, I., Rezgui, F., Oble, J., et al. (2014) Microwave-Assisted Palladium-Catalyzed Allylation of β-Enaminones. Synlett, 25, 2196-2200.
https://doi.org/10.1055/s-0034-1378540 - 38. De Luca, L., Giacomelli, G., Porcheddu, A., et al. (2003) Cellulose Beads: A New Versatile Solid Support for Microwave-Assisted Synthesis. Preparation of Pyrazole and Isoxazole Libraries. Journal of Combinatorial Chemistry, 5, 465-471.
https://doi.org/10.1021/cc0201187 - 39. Kralj, D., Novak, A., Dahmann, G., et al. (2008) One-Pot Parallel Solution-Phase Synthesis of 1-Substituted 4-(2-Aminoethyl)-1H-Pyrazol-5-ols. Journal of Combinatorial Chemistry, 10, 664-670.
https://doi.org/10.1021/cc8000794 - 40. Saleh, T.S., Narasimharao, K., Ahmed, N.S., et al. (2013) Mg-Al Hydrotalcite as an Efficient Catalyst for Microwave Assisted Regioselective 1,3-Dipolar Cycloaddition of Nitrilimines with the Enaminone Derivatives: A Green Protocol. Journal of Molecular Catalysis A: Chemical, 367, 12-22.
https://doi.org/10.1016/j.molcata.2012.11.009 - 41. Zhang, X.Y., Yang, Z.W., Chen, Z., et al. (2016) Tandem Copper-Catalyzed Propargylation/Alkyne Azacyclization/Isomerization Reaction under Microwave Irradiation: Synthesis of Fully Substituted Pyrroles. The Journal of Organic Chemistry, 81, 1778-1785.
https://doi.org/10.1021/acs.joc.5b02429 - 42. Abbas, E.M.H., Gomha, S.M. and Farghaly, T.A. (2014) Multicomponent Reactions for Synthesis of Bioactive Polyheterocyclic Ring Systems under Controlled Microwave Irradiation. Arabian Journal of Chemistry, 7, 623-629.
https://doi.org/10.1016/j.arabjc.2013.11.036 - 43. Porcheddu, A., Giacomelli, G., De Luca, L., et al. (2004) A “Catch and Release” Strategy for the Parallel Synthesis of 2,4,5-Trisubstituted Pyrimidines. Journal of Combinatorial Chemistry, 6, 105-111.
https://doi.org/10.1021/cc034024o - 44. Almazroa, S., Elnagdi, M.H. and El-Din, A.M.S. (2004) Studies with Enaminones: The Reaction of Enaminones with Aminoheterocycles. A Route to Azolopyrimidines, Azolopyridines and Quinolones. Journal of Heterocyclic Chemistry, 41, 267-272.
https://doi.org/10.1002/jhet.5570410219 - 45. Tu, S., Zhang, Y., Jiang, B., et al. (2006) One-Pot Synthesis of N-Substituted Azapodophyllotoxin Derivatives under Microwave Irradiation. Synthesis, No. 22, 3874-3882.
https://doi.org/10.1055/s-2006-950297 - 46. Li, M., Guo, A., et al. (2006) Synthesis of Enaminones and Their Utility in Organic Synthesis. Chinese Journal of Organic Chemistry, 26, 1192-1207.
- 47. Wang, J., Li, J., Liu, H., et al. (2015) A Facile and Efficient Synthesis of Spiro[indoline-3,5’-pyrido[2,3-d] Pyrimidine] Derivatives via Microwave-Assisted Multicomponent Reactions. Letters in Organic Chemistry, 12, 62-66.
https://doi.org/10.2174/157017861201150112124526 - 48. Yan, S., Huang, C., Su, C., et al. (2009) Facile Route to 1,3-Diazaheterocycle-Fused [1,2b]Isoquinolin-1(2H)-One Derivatives via Substitution-Cyclization Reactions. Journal of Combinatorial Chemistry, 12, 91-94.
https://doi.org/10.1021/cc900121c - 49. Al-Etaibi, A.M., El-Apasery, M.A., Ibrahim, M.R., et al. (2012) A Facile Synthesis of New Monoazo Disperse Dyes Derived from 4-Hydroxyphenylazopyrazole-5-Amines: Evaluation of Microwave Assisted Dyeing Behavior. Molecules, 17, 13891-13909.
https://doi.org/10.3390/molecules171213891 - 50. Pena, R., Jiménez-Alonso, S., Feresin, G., et al. (2013) Multicomponent Synthesis of Antibacterial Dihydropyridin and Dihydropyran Embelin Derivatives. The Journal of Organic Chemistry, 78, 7977-7985.
https://doi.org/10.1021/jo401189x