Journal of Organic Chemistry Research
Vol.
09
No.
04
(
2021
), Article ID:
46854
,
10
pages
10.12677/JOCR.2021.94006
芳烃[5,5]-重排反应的研究进展
潘文静*,王梦瑶
浙江师范大学,化学与生命科学学院,浙江 金华
收稿日期:2021年10月11日;录用日期:2021年11月23日;发布日期:2021年11月30日

摘要
1,4-二取代芳烃结构广泛存在于许多天然产物及药物分子中。因此,如何快速有效地实现芳烃分子的对位选择性C-H官能团化是化学研究人员关注的热点问题之一。芳烃对位直接引入所需官能团是合成1,4-二取代芳烃最为直接的途径,但目前芳烃选择性对位C-H键官能团化依旧充满挑战。而芳烃[5,5]-重排为芳烃对位C-H键官能团化提供了独特的解决方案。本文主要针对Claisen型、联苯胺型及苯醌亚胺衍生物与苯酚的[5,5]-重排进行总结、归纳和分析,并对该重排的发展做出展望。
关键词
[5,5]-重排,C-H键官能团化,芳烃,区域选择性

The Progress of [5,5]-Rearrangement Reaction
Wenjing Pan*, Mengyao Wang
College of Chemistry and Life Science, Zhejiang Normal University, Jinhua Zhejiang
Received: Oct. 11th, 2021; accepted: Nov. 23rd, 2021; published: Nov. 30th, 2021

ABSTRACT
The ubiquity of 1,4-disubstituted arenes in natural products and drugs ensures a constant demand for their efficient and selective synthesis. Introduction of functionalities onto para-position of arenes is the most straightforward strategy for the synthesis of 1,4-disubstituted arenes. However, reports with respect to such transformation are rare, and developing an efficient method realizing remote para-selective C-H functionalization of arenes still remains a challenge. Aromatics [5,5]-rearrangement provides a unique solution for the functionalization of aromatic C-H bonds at the para-position. In this review, we will summarize and analyze Claisen-type, benzidine-type and reaction of phenol with iminobenzoquinone via [5,5]-rearrangement of aromatics, and make an outlook for the future development of this field.
Keywords:[5,5]-Rearrangement, C-H Bond Functionalization, Aromatics, Regional Selectivity

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
σ重排反应是符合Woodward-Hoffmann规则的一种周环反应 [1]。通过π电子及σ键的协同迁移重组构成新的分子结构。这类反应是有机化学中的一类重要反应,具有独特的区域选择性和立体选择性,并且可以实现分子结构重组及C-C键的形成。在各类σ重排反应中,以Claisen重排为代表的[3,3]-重排,由于化学、区域、立体选择性高,而广受化学家们的关注 [2] - [18]。在过去一百年间,化学家对该类反应的关注度从未减少,至今已有多种类型的[3,3]-重排反应被发展出来,包括Claisen重排 [19] [20]、Cope重排 [21] [22] [23]、Overman重排 [24] [25] [26] [27] 等多个人名反应。与此形成鲜明对比,[5,5]-重排反应研究较少。尽管利用前线轨道理论(FMO)对所涉及轨道对称性的分析表明,最高占据轨道HOMO中两个P轨道的对称性是匹配的,[5,5]-重排在热学上是允许的,反应是经过一个十元环的中间过渡态。但同时[3,3]-重排在热学上也是允许的,且是经过一个六元环椅式的中间过渡态,所以一般很难避免[3,3]-重排反应的竞争 [28]。基于此,本文主要讨论芳烃[5,5]-重排反应,包括Claisen型重排、联苯胺类的重排及苯醌亚胺衍生物与苯酚的重排。
2. Claisen型芳基二烯基醚重排
早在1968年,Frater和Schmid等人 [28] 发现N, N-二乙基苯胺与芳基二烯基醚在186℃下反应5小时,不仅能得到[3,3]-重排产物图1 (3) (24%),同时也意外得到了[5,5]-重排产物图1 (2) (37%)。
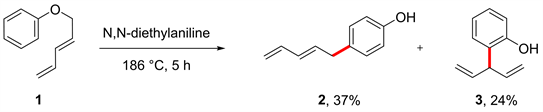
Figure 1. Rearrangement of aryl dienyl ether
图1. 芳基二烯基醚的重排
在1985年,Maruyama等 [29] 采用不同的路易斯酸对Frater和Schmid的反应条件进行优化,发现在BF3·Et2O的作用下芳基二烯基醚能高选择性地生成对位重排产物图2 (5a-5e),并发现苯环的邻位或对位取代基会对该重排反应的选择性产生较大的影响。当对位有烷基或是其它供电子基团时,重排反应优先发生在间位图2 (6a, 6b)。另外,当对位含有较大体积的官能团,如叔丁基和苯基时,重排反应优先发生在邻位图2 (7a, 7b)。
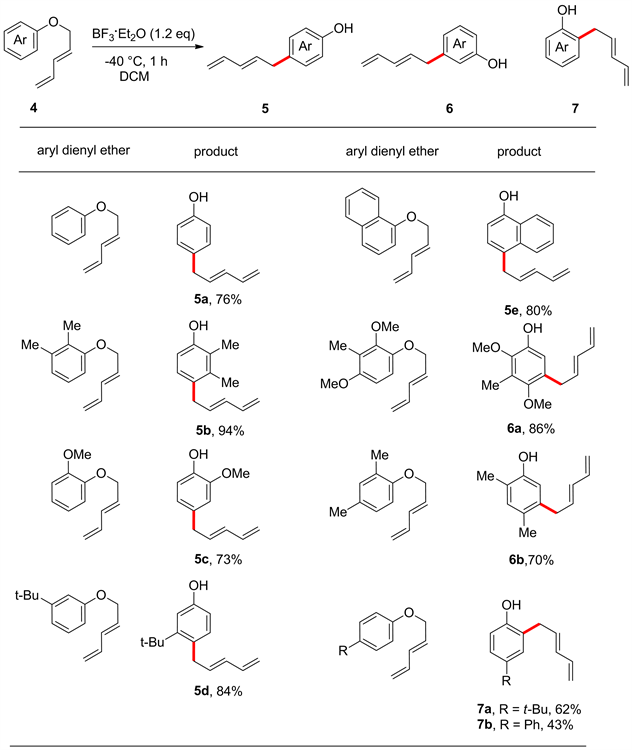
Figure 2. Claisen-type rearrangement of 2,4-pentadienyl phenyl ethers with different substituents
图2. 不同取代基的2,4-戊二烯基苯基醚的克莱森型重排
为了进一步探究该反应的机理,Maruyama [29] 进行了同位素标记实验,首先制备了三类不同结构的氘代2,4-戊二烯芳基醚,在BF3·Et2O的作用下发生重排。结果显示,对位无取代基或带有较小取代基(如,-OMe, -Me)时,反应都会经历[5,5]-重排过程,并生成相应的重排产物(见图3式1,式2),而当对位的取代基变为位阻较大的基团(如,-t-Bu)时,会抑制[5,5]-重排反应(见图3式3)。
对位无取代基或连有较小取代基的底物都需经历[5,5]-重排生成对应的重排产物。对位无取代基2,4-戊二烯芳基醚会经历中间体I,生成相应的[5,5]-重排产物,对位带有较小取代基的底物也会经历[5,5]-重排的过程生成中间体II,再通过[1,2]迁移生成相应的重排产物。Maruyama为了探究对位为较大位阻取代基时,进行了交叉实验,向反应中加入苯酚(见图3式4),发现苯酚在反应完后只剩58%,推测该反应可能不仅会发生分子内的反应,也会发生分子间的反应。
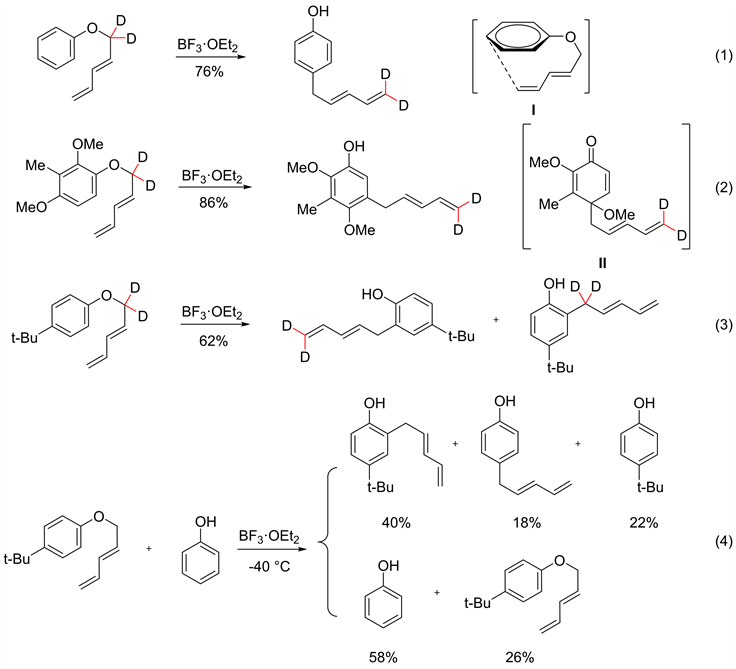
Figure 3. Deuterium rearrangement experiments of different 2,4-pentadiene aryl ethers
图3. 不同2,4-戊二烯芳基醚的氘代重排实验
Naruta等人通过一系列的条件优化大大提高了该重排反应的对位选择性,但遗憾的是它所报道的底物适用范围较窄,并没有展现出良好的官能团兼容性,因此该结果未获得更多的关注。
3. 联苯胺型的[5,5]重排
3.1. N-N联苯胺型的[5,5]重排
1863年,Hofmann在研究偶氮苯的氢化反应时得到了对联苯胺,发现了著名的联苯胺重排 [30]。但是该反应的选择性不好,除了生成对联苯胺,还会生成2,4'-联苯胺,邻联苯胺,邻半联胺和对半联胺,此外反应中还有少量的歧化产物偶氮苯和苯胺产生(见图4(A))。该结果在当时并未受到科学家的广泛关注。直到1963年,Badger等 [31] 在合成苯并[c]噌啉时,成功分离得到了35%联苯胺,但当时他们并没有意识到这是一种新型的[5,5]-重排反应,而把他们的实验结果归因于不确定的光化学反应。在2016年,Bouillon等 [32] 在Badger的反应基础上,采用对位有取代基的N,N-二芳基肼进行反应,拿到了28%的[5,5]-重排的产物,除此之外还拿到了16%的歧化产物和56%进一步重排的产物(见图4(B))。
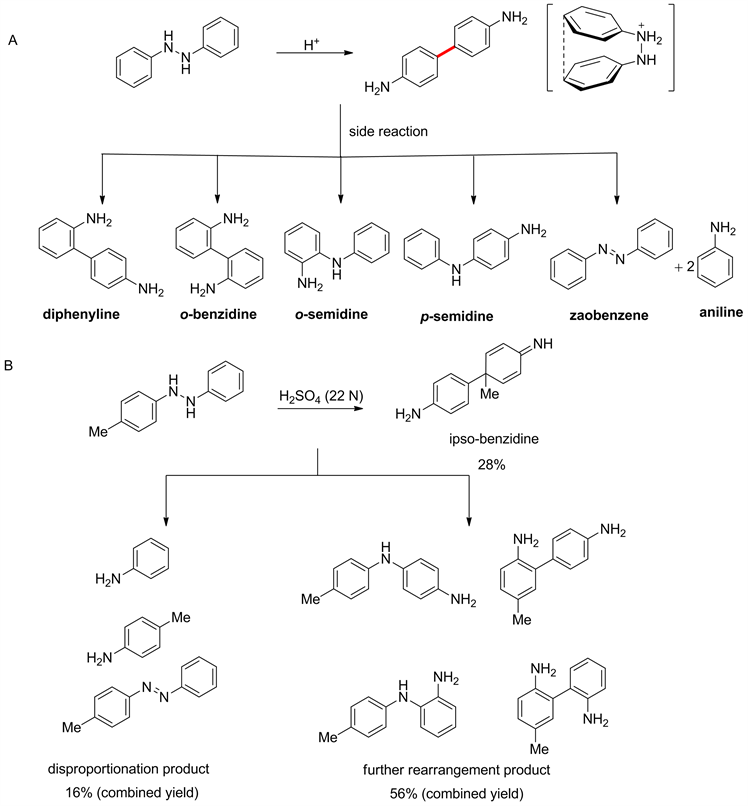
Figure 4. [5,5]-rearrangement of benzidine
图4. 联苯胺[5,5]-重排
在1957年,Wildgrube等 [33] 发现了第一例吡啶–芳基联苯胺的重排,由于该反应存在互变异构和一系列歧化产物(见图5式1),导致反应产率不高。2016年,Leung等 [34] 研究该反应时发现,在反应中加入三甲基碘硅烷有利于提高反应产率,这可能得益于吡啶环上的N效应。反应在三甲基碘硅烷的作用下,生成吡啶鎓盐(III),再在氯化锡的作用下生成杂联二芳基化合物(见图5式2)。研究发现:无取代的1,4-氨基吡啶图5 (11a,11b),能以中等产率拿到产物图5 (12a,12b)。当吡啶环图5 (11c-11e)或是芳环图5 (11f)的反应位点被取代时,[5,5]-重排的过程被阻断,不能生成相应[5,5]-重排的产物。当进行[5,5]-重排的两个位点都被取代时图5 (11g),[5,5]-重排的过程也没有发生,只有37%的图5 (14b)生成。

Figure 5. Rearrangement of pyridine-aryl benzidine
图5. 吡啶–芳基联苯胺的重排
3.2. N-杂原子的联苯胺型的[5,5]重排
2013年,Kürti等 [35] 在研究硝基苯和芳基格式试剂的反应时,也发现了有少量[5,5]-重排的产物(见图6式1)。另外,最近王梅祥课题组 [36] 在一锅法制备多取代联芳烃的过程中,也发现N-O连接的二芳基羟胺在碱性条件下会发生[5,5]-重排,但同时也会伴随着[3,3]-重排反应(见图6式2)。
联苯胺类型的[5,5]-重排为合成多官能团联芳烃提供了新的思路,但该反应通常面临选择性差的问题,同时还伴随着一些副反应,除了会生成[3,3]-重排产物,还有歧化产物生成,如对联苯胺,对半联胺,邻半联胺,邻联苯胺和邻对联苯胺。此外,它的反应机理曾长期困扰着化学家们,直到20世纪80年代,Shine等通过同位素标记动力学实验才证实了对联苯胺的形成是通过[5,5]-迁移实现的。
4. 苯酚与苯醌亚胺衍生物的重排
2017年,许庆龙 [37] [38] [39] [40] 课题组发现苯醌亚胺衍生物与苯酚组装形成重排中间体,在DPP (磷酸二苯酯)催化下,发生[5,5]-重排反应,生成苯酚对位C-H官能团化产物。
Figure 6. N-O benzidine type [5,5] rearrangement
图6. N-O联苯胺型的[5,5]重排
该反应对不同取代基的底物具有良好的耐受性。值得注意的是,具有一定空间位阻的3,5-二甲氧基苯酚参与的反应,仍然能拿到相应的对位C-H胺化产物图7 (17e),产率为76%。该反应还耐受带有二羟基基团的底物,如邻苯二酚能得到89%直接对位C-H胺化产物。通过该反应可以实现苯酚对位C-N键的构建,为咔唑衍生物的合成提供了思路。该反应具有原料易得、反应条件温和、酸催化剂简单、催化剂负载低等特点,但该反应同样受限于结构特殊的底物。

Figure 7. Rearrangement of benzoquinone imine derivatives and phenol
图7. 苯醌亚胺衍生物跟苯酚的重排
5. 结论
在过去的一个多世纪里,科学家们从未停止对芳烃[5,5]-重排反应的研究,并取得了一些显著的研究成果,为1,4-二取代芳烃的合成提供了借鉴。但是通过我们深入分析可以发现以上所报道的几类[5,5]-重排反应大都具有一定的反应局限性:1) 由于重排反应研究对象通常为相对稳定的重排前体,致使许多稳定且结构多样的重排前提较难制备,从而限制了该重排反应的广泛应用;2) 反应的选择性不好,常常伴随[3,3]-重排或分子间歧化等反应。因此,发展高效的[5,5]-重排反应仍然是合成化学的重要任务之一。
文章引用
潘文静,王梦瑶. 芳烃[5,5]-重排反应的研究进展
The Progress of [5,5]-Rearrangement Reaction[J]. 有机化学研究, 2021, 09(04): 40-49. https://doi.org/10.12677/JOCR.2021.94006
参考文献
- 1. Huang, X., Zhang, Y.G., Zhang, C.S., Zhang, L., Xu, Y., Kong, L.C., Wang, Z.X. and Peng, B. (2019) The ortho-difluoroalkylation of Aryliodanes with Enol Silyl Ethers: Rearrangement Enabled by a Fluorine Effect. Angewandte Chemie International Edition, 58, 5956-5961. https://doi.org/10.1002/anie.201900745
- 2. Yuan, H.R., Du, Y.B., Liu, F.T., Guo, L.R., Sun, Q.Y., Feng, L. and Gao, H.Y. (2020) Tandem Approach to NOBIN Analogues from Arylhydroxylamines and Diaryliodonium Salts via [3,3]-Sigmatropic Rearrangement. Chemical Communications, 56, 8226-8229. https://doi.org/10.1039/D0CC02919J
- 3. Zhang, J.W., Qi, L.W., Li, S.Y., Xiang, S.H. and Tan, B. (2020) Direct Construction of NOBINs via Domino Arylation and Sigmatropic Rearrangement Reactions. Chinese Journal of Chemistry, 38, 1503-1514. https://doi.org/10.1002/cjoc.202000358
- 4. Shafir, A. (2016) The Emergence of Sulfoxide and Iodonio-Based Redox Arylation as a Aynthetic Tool. Tetrahedron Letters, 57, 2673-2682. https://doi.org/10.1016/j.tetlet.2016.05.013
- 5. Pulis, A.P. and Procter, D.J. (2016) C-H Coupling Reactions Directed by Sulfoxides: Teaching an Old Functional Group New Tricks. Angewandte Chemie International Edition, 55, 9842-9860. https://doi.org/10.1002/anie.201601540
- 6. Yanagi, T., Nogi, K. and Yorimitsu, H. (2018) Recent Development of ortho-C-H Functionalization of Aryl Sulfoxides through [3,3]-sigmatropic Rearrangement. Tetrahedron Letters, 59, 2951-2959. https://doi.org/10.1016/j.tetlet.2018.06.055
- 7. Khatri, H.R. and Zhu, J. (2012) Synthesis of Complex ortho-allyliodoarenes by Employing the Reductive Iodonio-Claisen Rearrangement. Chemistry—A European Journal, 18, 12232-12236. https://doi.org/10.1002/chem.201202049
- 8. Ochiai, M., Ito, T. and Masaki, Y.Z. (1992) Ipso Selectivity in the Reductive Iodonio-Claisen Rearrangement of Allenyl(p-methoxyaryl)iodinanes. Journal of the Chemical Society, Chemical Communications, 24, 15-16. https://doi.org/10.1039/c39920000015
- 9. Ochiai, M., Kida, M. and Okuyama, T. (1998) On the Mechanism of Nucleophilic Substitution of Allenyl(aryl)iodine(III): Formation of Propargyl Cation and Competition with Sigmatropic Rearrangement. Tetrahedron Letters, 39, 6207-6210. https://doi.org/10.1016/S0040-4039(98)01276-3
- 10. Izquierdo, S., Bouvet, S., Wu, Y.C., Molina, S. and Shafir, A. (2018) The Coming of Age in Iodane-Guided ortho C-H Propargylation: From Insight to Synthetic Potential. Chemistry—A European Journal, 24, 15517-15521. https://doi.org/10.1002/chem.201804058
- 11. Izquierdo, S., Essafi, S., Rosal, I.D., Vidossich, P., Pleixats, R., Vallribera, A., Ujaque, G., Lledos, A. and Shafir, A. (2016) Acid Activation in Phenyliodine Dicarboxylates: Direct Observation, Structures, and Implications. Journal of the American Chemical Society, 138, 12747-12750. https://doi.org/10.1021/jacs.6b07999
- 12. Nguyen, H., Khatri, H.R. and Zhu, J. (2013) Reductive Iodonio-Claisen Rearrangement of Iodothiophene Diacetates with Allylsilanes: Formal Synthesis of Plavix. Tetrahedron Letters, 54, 5464-5466. https://doi.org/10.1016/j.tetlet.2013.07.138
- 13. Khantri, H.R., Nguyen, H., Dunaway, J.K. and Zhu, J.L. (2015) Fluoroalcohol-Mediated Reductive Iodonio-Claisen Rearrangement: Synthesis of Complex ortho-Substituted-Allyl Iodoarenes. Frontiers of Chemical Science and Engineering, 9, 359-368. https://doi.org/10.1007/s11705-015-1530-6
- 14. Shi, W., Li X.H., Liang, C. and Mo, D.L. (2017) Base-Free Selective o-Arylation and Sequential [3,3]-Rearrangement of Amidoximes with Diaryliodonium Salts: Synthesis of 2-Substituted Benzoxazoles. Advanced Synthesis & Catalysis, 359, 4129-4135. https://doi.org/10.1002/adsc.201700906
- 15. Claisen, L. (1912) Über Umlagerung von Phenol-allyläthern in C-allyl-phenole. Berichte der Deutschen Chemischen Gesellschaft, 45, 3157-3166. https://doi.org/10.1002/cber.19120450348
- 16. Claisen, L. and Eisleb, O. (1913) Über die Umlagerung von Phenolallyläthern in Die Isomeren Allylphenole. Justus Liebigs Annalen der Chemie, 401, 21-119. https://doi.org/10.1002/jlac.19134010103
- 17. Cope, A.C. and Hardy, E.M. (1940) The Introduction of Substituted Vinyl Groups. V. A Rearrangement Involving the Migration of an Allyl Group in a Three-Carbon System. Journal of the American Chemical Society, 62, 441-444. https://doi.org/10.1021/ja01859a055
- 18. Khojandi, M., Zahedi, E., Seif, A., Taghvamanesh, A. and Karimkhani, M. (2021) A Theoretical Study on the Degenerate Cope Rearrangement of Hypostrophene Using the RRKM Theory and Topological Approaches. ChemistrySelect, 6, 1607-1615. https://doi.org/10.1002/slct.202004495
- 19. Woodward, R.B. and Hoffmann, R. (1965) Selection Rules for Sigmatropic Reactions. Journal of the American Chemical Society, 87, 2511-2513. https://doi.org/10.1021/ja01089a050
- 20. Feng, X.W., Wei, Q.Y., Li, S.P., Wei, X.Z., Yang, X.F., Song, Z.L., Li, Z.Q., Zhang, J. and Yan, M. (2020) Organic-Inorganic Photoelectrochemical Sensor Based on Aza-Cope Rearrangement Reaction for Formaldehyde. Sensors and Actuators B Chemical, 330, Article ID: 129342. https://doi.org/10.1016/j.snb.2020.129342
- 21. Ferguson, M.L., Senecal, T.D., Groendyke, T.M. and Mapp, A.K. (2006) [3,3]-Rearrangements of Phosphonium Ylides. Journal of the American Chemical Society, 128, 4576-4577. https://doi.org/10.1021/ja058746q
- 22. Anderson, C.E. and Overman, L.E. (2003) Catalytic Asymmetric Rearrangement of Allylic Trichloroacetimidates. A Practical Method for Preparing Allylic Amines and Congeners of High Enantiomeric Purity. Journal of the American Chemical Society, 125, 12412-12413. https://doi.org/10.1021/ja037086r
- 23. Álvaro, V.R., Eric, J.A., Makoto, Y., Austin, C.W. and Brian, M.S. (2019) Stereospecific Overman Rearrangement of Substituted Cyclic Vinyl Bromides: Access to Fully Substituted α-Amino Ketones. Organic Letters, 21, 8962-8965. https://doi.org/10.1021/acs.orglett.9b03347
- 24. Yuya, O., Mayu, K., Erina, K., Sayaka, K., Koki, I., Kenta, M., Kazuki, M., Siro, S., Takaaki, S. and Noritaka, C. (2021) Seven-Step Synthesis of All-Nitrogenated Sugar Derivatives Using Sequential Overman Rearrangements. Angewandte Chemie International Edition, 60, 5193-5198. https://doi.org/10.1002/anie.202015141
- 25. Andy, A.T., Kishor, L.H. and Robert, A.B. (2020) Stereocontrolled Microwave-Assisted Domino [3,3]-sigmatropic Reactions: A Winstein-Overman Rearrangement for the Formation of Differentiated Contiguous C-N Bonds. Organic Letters, 22, 3050-3055. https://doi.org/10.1021/acs.orglett.0c00801
- 26. Li, G.Q., Gao, H.Y., Keene, C., Devonas, M., Daniel, H.E. and László, K. (2013) Organocatalytic Aryl-aryl Bond Formation: An Atroposelective [3,3]-Rearrangement Approach to Binam Derivatives. Journal of the American Chemical Society, 135, 7414-7417. https://doi.org/10.1021/ja401709k
- 27. Fráter, G. and Schmid, H. (1968) Thermische Umwandlung von Phenyl-penta-2,4-dienylthern in 4-[penta-2,4-dienyl]-phenole als Beispiel für [5,5]-sigmatropische Umlagerungen. Vorlufige Mitteilung. Helvetica Chimica Acta, 51, 190-193. https://doi.org/10.1002/hlca.19680510120
- 28. Maruyama, K., Nagai, N. and Naruta, Y. (1985) Lewis Acid Mediated Claisen-Type Rearrangement of Aryl Dienyl Ethers. Tetrahedron Letters, 26, 5149-5152. https://doi.org/10.1016/S0040-4039(00)98888-9
- 29. Hofmann, A.W. (1863) Notes of Researches on the Polyammonias, No. XXIII-Hydrazobenzol, a New Compound Isomeric with Benzidin. Proceedings of the Royal Society of London, 12, 576-578. https://doi.org/10.1098/rspl.1862.0127
- 30. Badger, G., Drewer, R. and Lewis, G. (1963) Photochemical Reactions of azo Compounds. II. Photochemical Cyclodehydrogenations of Methyl- and Dimethylazobenzenes. Australian Journal of Chemistry, 16, 1042-1050. https://doi.org/10.1071/CH9631042
- 31. Bouillon, M.E. and Meyer, H.H. (2016) The 4.4’-Benzidine Rearrangement of 4-Alkyl Substituted Hydrazobenzenes. Tetrahedron, 72, 3151-3161. https://doi.org/10.1016/j.tet.2016.03.103
- 32. Hans, B., Hans, J.H. and Wolfgang, W. (1958) Über Pyridinabkömmlinge, II. Benzidinartige UAmlagerungen in der Pyridinreihe. European Journal of Inorganic Chemistry, 91, 247-256. https://doi.org/10.1002/cber.19580910202
- 33. Leung, G.Y., William, A.D. and Johannes, C.W. (2014) Improved Synthesis of Pyridyl-Biaryl Ring Systems via Benzidine Rearrangements. Tetrahedron Letters, 55, 3950-3953. https://doi.org/10.1016/j.tetlet.2014.04.126
- 34. Gao, H.Y., Daniel, H.E., Muhammed, Y. and Kürti, L. (2013) Transition-Metal-Free Direct Arylation: Synthesis of Halogenated 2-Amino-2’-hydroxy-1,1’-biaryls and Mechanism by DFT Calculations. Journal of the American Chemical Society, 135, 7086-7089. https://doi.org/10.1021/ja400897u
- 35. Shang, L., Chang, Y.H., Luo, F., He, J.N., Huang, X., Zhang, L., Kong, L.C., Li, K.X. and Peng, B. (2017) Redox-Neutral Alpha-Arylation of Alkyl Nitriles with Aryl Sulfoxides: A Rapid Electrophilic Rearrangement. Journal of the American Chemical Society, 139, 4211-4217. https://doi.org/10.1021/jacs.7b00969
- 36. Liang, D.D., Guo, S.Y., Tong, S. and Wang, M.X. (2021) Polyfunctionalized Biaryls Accessed by a One-Pot Nucleophilic Aromatic Substitution and Sigmatropic Rearrangement Reaction Cascade under Mild Conditions. Tetrahedron, 83, Article ID: 131966. https://doi.org/10.1016/j.tet.2021.131966
- 37. Liu, L., Chen, K., Wu, W.Z., Wang, P.F., Song, H.Y., Sun, H.B., Wen, X.A. and Xu, Q.L. (2017) Organocatalytic para-Selective Amination of Phenols with Iminoquinone Monoacetals. Organic Letters, 19, 3823-3826. https://doi.org/10.1021/acs.orglett.7b01700
- 38. Kamitanaka, T., Morimoto, K., Tsuboshima, K., Koseki, D., Takamuro, H., Toshifumi, D. and Yasuyuki, K. (2016) Efficient Coupling Reaction of Quinone Monoacetal with Phenols Leading to Phenol Biaryls. Angewandte Chemie International Edition, 128, 15764-15768. https://doi.org/10.1002/ange.201608013
- 39. Ichijima, S., Fukunaga, H. and Koga, N. (1999) A Theoretical Study on Reaction Mechanism of Oxidative Coupling Reaction of p-phenylenediamine with Phenol: A Proposal of the Route via a [5,5]-Sigmatropic Rearrangement. Journal of Molecular Structure, 461-462, 429-438. https://doi.org/10.1016/S0166-1280(98)00454-0
- 40. Tong, L.K.J. and Glesmann, M.C. (1968) Kinetics and Mechanism of Oxidative Coupling of p-Phenylenediamines. Journal of the American Chemical Society, 90, 5164-5173. https://doi.org/10.1021/ja01021a019
NOTES
*通讯作者。