Advances in Material Chemistry
Vol.06 No.03(2018), Article ID:24817,10
pages
10.12677/AMC.2018.63007
Research Progress on Durability and Evaluation Methods of Superhydrophobic Surface
Zeen Zhao, Shuhong Sun, Yongmao Hu, Yan Zhu*
Kunming University of Science and Technology, Kunming Yunnan

Received: Mar. 20th, 2018; accepted: May 3rd, 2018; published: May 10th, 2018

ABSTRACT
With the deepening of research in superhydrophobic surface, the superhydrophobic surface will gradually be applied in industry but not just in laboratory. In practical applications, durability is particularly important. This paper reviews the research progress on surface durability and its evaluation methods.
Keywords:Superhydrophobic Surface, Durability, Evaluation Method
超疏水表面耐用性及评价方法研究进展
赵泽恩,孙淑红,胡永茂,朱艳*
昆明理工大学,云南 昆明

收稿日期:2018年3月20日;录用日期:2018年5月3日;发布日期:2018年5月10日

摘 要
随着超疏水表面研究的日益深入,超疏水表面逐渐从理论走向生活乃至工业应用。而在实际应用中,对超疏水表面耐用性的考量尤为重要。本文从超疏水表面耐用性角度出发,综述了超疏水表面耐用性及评价方法的研究进展,并对其发展前景进行了展望。
关键词 :超疏水表面,耐用性,评价方法

Copyright © 2018 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
超疏水表面通常指静态水接触角大于150˚,并且其滚动角小于10˚的表面。受荷叶 [1] 、猪笼草 [2] 、壁虎皮 [3] 等植物优良的液体排斥作用的启发,科研工作者深入研究了人工制备超疏水表面的方法。人工制备超疏水表面的方法通常是降低粗糙表面的表面能,或者在疏水的材料表面构造出一定的粗糙结构。目前已通过溶胶–凝胶法 [4] 、化学腐蚀 [5] [6] [7] [8] [9] 、阳极处理 [10] [11] [12] 、电沉积 [13] 、热液反应 [14] 以及静电纺丝 [15] 等方法对人工制备超疏水表面进行了广泛的研究。人工制备出的超疏水表面具备了良好的抗腐蚀 [16] 、自清洁 [4] [16] [17] [18] 、抗结冰 [18] [19] [20] 以及低的表面液体阻力 [20] [21] 等优异性能。
虽然对超疏水表面的研究取得了很大进展,但目前人工制备的超疏水表面仍面临着耐久性差的难题。耐久性包括机械耐久性和化学耐久性两个方面。超疏水表面的机械耐久性主要通过线性直接摩擦或者持续冲击下的性能表现来衡量;而化学耐久性则主要通过在酸碱溶液、盐水、盐雾、紫外光照射或者高温等条件下的应用表现来衡量(如表1所示)。
2. 机械耐用性及评价方法
2.1. 耐磨性
目前砂纸线切割磨损的方法经常被用于测试超疏水表面的耐磨性。Lu等 [22] 将超疏水涂料涂于钢、棉纺、玻璃及纸张等各种不同物质的表面以获得超疏水表面。超疏水涂料以纳米颗粒TiO2、有机树脂以及1H,1H,2H,2H-全氟辛基三乙氧基硅烷按照一定配比混合而成。采用如图1(A)、图1(B)的方式将100 g的砝码置放在样品表面上,再将其放置在240目的砂纸上进行线性磨损测试,结果显示表面的超疏水状态并未有明显改变,由此推测超疏水涂层的耐磨性性能较为优异,但是该文中并没有研究线性磨损实验后的超疏水表面的滞后性,而对于超疏水的表征并不能简单的通过静态接触角测试来直观的表达,因此此文中的磨损实验之后的检测并不够全面。
Table 1. Durability and evaluation methods of superhydrophobic surface
表1. 超疏水表面耐用性及评价方法

Figure 1. Sandpaper abrasion tests. (A) (B) One cycle of the sandpaper abrasion test; (C) Plot of mechanical abrasion cycles and water contact angles after each abrasion test; (D) Water droplet traveling test after 40th cycle abrasion [22]
图1. 砂纸磨损试验。(A) (B) 砂纸磨损试验的一个周期;(C) 每次磨损试验后的机械磨损循环图和水接触角图;(D) 第40次循环磨损后的水滴流动试验 [22]
Tian等 [23] 在2016年的《Science》中也指出单纯用静态接触角表征是否失效并不准确,因为在此种失效形式下前进角变化不大,直接显示为静态接触角不会产生明显变化,而具体的改变则是体现在后退角的减小上,直观表示为滞后性变差。因此他们在文中建议,应该对磨损测试进行标准化,将线性磨损作为初步的试验,之后明确规定压力、磨损距离和磨损材料,并给出磨损引起的接触角滞后、后退接触角或滑动角的变化(如图2所示)。
2.2. 耐冲击性
在实际应用中,线性磨损仅仅是最简单的磨损机制,材料还有可能受到物质的直接冲击。通常会采用水冲甚至是沙冲来的冲击实验直观体现耐冲击性。图3所示为砂冲击实验,而水冲击实验则可以选用同样的实验装置,只需将载荷更换为水。
在冲击过程中,主要考虑流体的冲击速度,冲击高度来间接表征冲击过程中流体的能量,以便比较冲击能量的变化给超疏水表面带来的变化。Xiong等 [25] 选取0.7 m/s和1.98 m/s的冲击速度,分别从2.5 cm和20 cm的高度对超疏水表面进行简单的冲击实验,结果显示随着冲击速度和冲击高度的增大,流体的能量变大,对超疏水表面的冲击效果更为强烈,导致超疏水性能更容易丢失。Zhang等 [26] 将下落流体与超疏水表面的接触大小以及高度作为研究冲击的参数,即水下落时的出水口直径以及其与表面的垂直距离,并将水压控制在10~100 KPa内,研究表明随着水压的增大,初始接触角变化不大,但是代表滞后性的滚动角显著增大,而直至水压加至某数值后,接触角亦开始变小,据此,说明在一定的水压范围内,冲击对滞后性的影响较为明显。
Xu等 [27] 采用的是基于多孔二氧化硅胶囊制备超疏水涂层的简单方法,该超疏水涂层的静态接触角为160˚,滑动角小于5˚。选取30厘米的高度,用100~300微米大小的砂粒撞击该超疏水表面后,接触角并未发生明显变化。Tang等 [28] 研究了一种基于纳米碳酸钙–聚合物复合材料制备超疏水表面的简单方法,通过改变纳米碳酸钙–聚合物复合材料的重量比调节表面的疏水性。该方法简单可靠,便于在普通基底上制备超疏水表面。并且文中通过沙冲实验对超疏水表面进行冲击测试,其中沙粒的直径约为200微米,而冲击高度为15厘米,经过5分钟的持续冲击之后,接触角略微降低,约为150˚。

Figure 2. Wearing out a nonwetting surface. A superhydrophobic surface generally loses its liquid repellency after mechanical abrasion. (A) A water droplet rolls on a superhydrophobic surface, where the liquid is suspended by a solid-air composite interface; (B) A setup for linear abrasion test; (C) A droplet gets stuck on the same surface after abrasion because of the failure of the composite interface [23]
图2. 超疏水表面在机械磨损后通常会失去其液体排斥性。(A) 水滴在超疏水表面上滚动,其中液体被固体–空气复合界面悬浮;(B) 线性磨损试验的设置;(C) 由于复合界面的失效,液滴在磨损后被粘在同一表面上 [23]
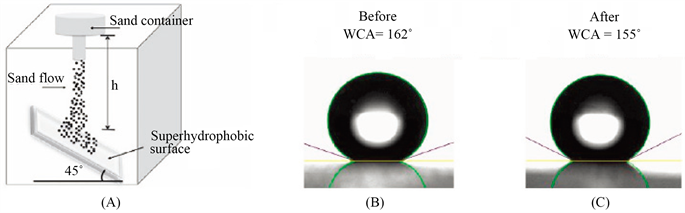
Figure 3. Mechanical robustness assessed by sand impact abrasion. (A) Sketch of the setup for the sand impact abrasion test. WCAs of the glass substrate with coating B before (B) and after (C) impact abrasion from a 100 cm height [24]
图3. 用砂冲击磨损评定机械稳定性。(A) 冲砂磨损试验装置示意图;静态水接触角冲击前(B)和冲击后(C) [24]
3. 化学耐用性及评价方法
3.1. 酸碱溶液环境
工业生产应用中,酸碱环境显然是较为常见的,而对于超疏水材料而言尤其是金属基的超疏水表面,其稳定性尤为重要,而这部分评价则主要通过简单浸没实验,将制备出的超疏水表面乃至材料置放于不同pH值下的溶液环境中,通过对比浸没前后表面的变化情况来展现酸碱溶液环境对超疏水性能的影响。Liu等 [29] 人对自己所制备的超疏水表面进行了酸碱溶液浸没测试,经过24小时的浸泡,静态接触角并没有发生明显变化,结果显示超疏水表面在酸碱环境下依然能表现出较好的疏水性。而Takahiro等 [30] 人通过等离子处理以及气相沉积制备出活泼的镁基超疏水表面,并研究其在三种不同pH值(4、7和10)的溶液环境中超疏水表面接触角变化情况(如图4所示),结果表明酸性以及碱性都在不同程度上影响了超疏水表面的性能,而在中性溶液中超疏水性能较为稳定。通过对比,说明酸碱性对超疏水表面会产生一定影响。

Figure 4. Changes in static contact angles of the super-hydrophobic film deposited for 30 min on AZ31 as a function of immersion time in aqueous solutions at pH of 4.0, 7.0, and 10.0 [30]
图4. 在pH分别为4.0、7.0和10.0的水溶液中浸泡30 min后,超疏水膜的静态接触角随浸泡时间的变化而变化 [30]
3.2. 盐水环境
由于超疏水表面的抗腐蚀性能以及其对水有较低的吸附作用,可以减少表面在水中的运动阻力,使得将超疏水表面应用到海上的船舰表面具有重要的价值。因此需要加强分析盐水环境对超疏水性能的影响。Lv等 [31] 将制备超疏水表面的方法分成两步考虑:一、制备一定表面粗糙度的表面;二、修饰上低表面能物质。并以三种样品互为参照对象,包括未经过任何处理的铝板、修饰有低表面能物质的铝表面以及进行超疏水化处理的铝板(即不仅具有粗糙结构也修饰有低表面能物质),将它们放置在同样的NaCl溶液(3.5 wt%)中7天,之后观察三种表面,会发现未经过处理的表面出现明显的腐蚀裂纹,疏水性的铝表面出现了絮状颗粒,而超疏水铝片并未发生变化。通过EDS测试发现具有超疏水性能的铝板并未发生明显的变化,而其他两种都不同程度上由于盐水的腐蚀,发生了铝被刻蚀的现象。
3.3. 潮湿环境
一般而言,在环境温度较高或者湿度越大的条件下,超疏水表面的稳定性也就越差,超疏水表面甚至可能会转变成高疏水或是亲水性表面,尤其当超疏水表面同时处在湿热环境时,这种转变将更为明显。Wang等 [32] 人通过溶胶–凝胶法使得碳钢表面超疏水化,并比较进行超疏水处理后的表面与未处理的碳钢以及表面粗糙程度不同的样品,将它们置于相对湿度为90%并且温度为30℃的试验箱中24 h后,发现超疏水化处理的表面形貌基本未发生变化,而未经处理的钢片表面出现密集的腐蚀锈点。与此同时还发现在表面粗糙度大的情况下,表面小水滴易于互相接触,可以部分连接在一起,因此电解质膜则会在表面突起上形成,从而导致腐蚀,如图5所示。
同样,Rao等 [33] 人则是简单研究了超疏水铜表面在湿热环境中的变化,主要关注了在湿度是95%,温度为35℃环境下表面润湿性的改变,实验表明在90天内超疏水性能不会发生明显的改变。

Figure 5. Model for water condensation on the surface of different roughness [32]
图5. 在不同粗糙度表面上的水凝模型 [32]
3.4. 盐雾环境
盐雾实验同样也是一种衡量超疏水表面腐蚀性能的重要实验。一般将盐雾环境下的耐用性能作为考核涂层材料长期应用性能的主要指标之一。Wang等 [34] 人将疏水涂层喷涂在经过砂纸打磨后的低碳钢表面,静置24小时之后进行盐雾测试,发现盐雾处理会直接导致坑状蚀锈的形成,并且锈蚀的微观结构由铁纳米板组成,并由此使得超疏水性能变差,而直接表征盐雾带来的超疏水表面变化如图6所示。Ananda等 [35] 人研究了一种新型的智能型涂料,该涂料由超疏水咪唑包覆空心磷酸锌纳米粒子组成,而整个方法涉及到多孔纳米粒子在环氧树脂中的分散。之后通过系统的长期盐雾试验对涂层的防腐性能进行了评估,并与普通磷酸锌复合环氧涂层和纯环氧涂层进行了比较,结果表明:在监控盐雾室中,涂覆了超疏水咪唑负载的中空磷酸锌纳米粒子涂层在盐雾暴露下500小时后才失去超疏水性能,而普通磷酸锌涂层和整齐的环氧涂层的低碳钢板则分别只有100小时和48小时的寿命,这主要是由于超疏水性能带来的良好性能表现。
3.5. 紫外光照环境
紫外光照作为户外应用的重要条件,是超疏水表面甚至是涂层材料长期在户外应用的主要考核指标之一。超疏水涂料在光照条件下会发生老化,从而导致超疏水性能的丧失。Feng等 [36] 人在研究定向氧化锌纳米棒润湿性的过程中,观察到此种超疏水材料在紫外照射下向超亲水性转变(如图7所示)。Xiu等 [37] 将异丁基三甲氧基硅烷引入二氧化硅层中,由于疏水异丁基表面基团的存在,使表面产生了疏水性。这样制备出的超疏水表面具有极好的抗紫外照射性能,实验中经过5500 h的紫外光照射后,制备出的表面水接触与滚动角均没有发生退化现象。
3.6. 高温环境
由于超疏水表面多是采取极性有机物进行修饰后获得疏水性能,而有机物在高温环境下易于发生变化,因此高温环境亦是衡量超疏水表面化学稳定性能的一个重要指标。Lin等 [38] 人制备的透明超疏水纳

Figure 6. Rust areal ratio and average size of pit-etching evolution with salt spray time [34]
图6. 盐雾时间对腐蚀坑平均尺寸和锈蚀面积的影响 [34]

Figure 7. (A) Photographs of water droplet shape on the aligned ZnO nanorod films before (left) and after (right) UV illumination; (B) Reversible super-hydrophobic-super-hydrophilic transition of the as-prepared films under the alternation of UV irradiation and dark storage [36]
图7. (A) 紫外照射前(左)后(右)ZnO纳米棒膜水滴形状的照片;(B) 紫外光照射和暗存储交替作用下制备的ZnO纳米棒膜的可逆超疏水–超亲水转变 [36]
米涂层具有良好的耐高温性,该文重点研究了纳米涂层的热稳定性,结果显示(如图8所示):在高于500℃的温度下由超疏水转变为超亲水。同样Liu等 [39] 人也发现了相似的情况,在高于300℃也发生了同样的转变。其研究发现表面形貌在此温度下并未发生改变,而发生润湿性变化的原因仅仅是超疏水表面涂层被氧化。

Figure 8. Thermal stability of as-obtained nanocoating [38]
图8. 纳米涂层的热稳定性示意图 [38]
4. 展望
随着国内外研究者对超疏水表面的研究逐渐深入,超疏水的制备乃至应用将会飞速发展,然而目前针对超疏水表面耐用性的评价方法却莫衷一是,这种大量的参差不齐的性能表征方法容易混淆人们对超疏水性能的判断,为了可以更直观的表征超疏水表面的各项性能以及更有利于超疏水表面的应用扩展,制定一系列的超疏水表面耐用性能的表征及测试标准尤为重要,也是当务之急。
基金项目
国家自然科学基金(No.11764003)。
文章引用
赵泽恩,孙淑红,胡永茂,朱艳. 超疏水表面耐用性及评价方法研究进展
Research Progress on Durability and Evaluation Methods of Superhydrophobic Surface[J]. 材料化学前沿, 2018, 06(03): 56-65. https://doi.org/10.12677/AMC.2018.63007
参考文献
- 1. Patankar, N.A. (2011) On the Modeling of Hydrophobic Contact Angles on Rough Surfaces. Langmuir, 19, 1249-1253. https://doi.org/10.1021/la026612+
- 2. Nguyen, S.H., Webb, H.K., Mahon, P.J., et al. (2014) Natural Insect and Plant Micro-/Nanostructsured Surfaces: An Excellent Selection of Valuable Templates with Superhydrophobic and Self-Cleaning Properties. Molecules, 19, 13614-13630. https://doi.org/10.3390/molecules190913614
- 3. Watson, G.S., Green, D.W., .Lin, S., et al. (2015) A Gecko Skin Micro/Nano Structure—A Low Adhesion, Superhydrophobic, Anti-Wetting, Self-Cleaning, Biocompatible, Antibacterial Surface. Acta Biomaterialia, 21, 109-122. https://doi.org/10.1016/j.actbio.2015.03.007
- 4. Liang, J., Hu, Y., Wu, Y., et al. (2014) Facile Formation of Su-perhydrophobic Silica-Based Surface on Aluminum Substrate with Tetraethylorthosilicate and Vinyltriethoxysilane as Co-Precursor and Its Corrosion Resistant Performance in Corrosive NaCl Aqueous Solution. Surface & Coatings Technology, 240, 145-153. https://doi.org/10.1016/j.surfcoat.2013.12.028
- 5. Zang, D., Zhu, R., Zhang, W., et al. (2014) Stearic Acid Modified Aluminum Surfaces with Controlled Wetting Properties and Corrosion Resistance. Corrosion Science, 83, 86-93. https://doi.org/10.1016/j.corsci.2014.02.003
- 6. Li, X., Zhang, Q., Guo, Z., et al. (2015) Low-Cost and Large-Scale Fabrication of Superhydrophobic 5052 Aluminum Alloy Surface with Enhanced Corrosion Resistance. RSC Advances, 5, 29639-29646. https://doi.org/10.1039/C5RA00324E
- 7. Poorebrahimi, S. and Norouzbeigi, R. (2015) A Facile Solu-tion-Immersion Process for the Fabrication of Superhydrophobic Gibbsite Films with a Binary Micro-Nano Structure: Effective Factors Optimization via Taguchi Method. Applied Surface Science, 356, 157-166. https://doi.org/10.1016/j.apsusc.2015.07.172
- 8. Wan, B., Ou, J., Lv, D., et al. (2016) Superhydrophobic Ceria on Aluminum and Its Corrosion Resistance. Surface & Interface Analysis, 48, 173-178. https://doi.org/10.1002/sia.5940
- 9. Li, X., Zhang, Q., Guo, Z., et al. (2015) Fabrication of Superhydrophobic Surface with Improved Corrosion Inhibition on 6061 Aluminum Alloy Substrate. Applied Surface Science, 342, 76-83. https://doi.org/10.1016/j.apsusc.2015.03.040
- 10. Zheng, S., Li, C., Fu, Q., et al. (2016) Fabrication of Mi-cro-Nanostructured Superhydrophobic Aluminum Surface with Excellent Corrosion Resistance and Anti-Icing Perfor-mance. RSC Advances, 6, 79389-79400. https://doi.org/10.1039/C6RA13447E
- 11. Lu, Z., Wang, P. and Zhang, D. (2015) Super-Hydrophobic Film Fab-ricated on Aluminium Surface as a Barrier to Atmospheric Corrosion in a Marine Environment. Corrosion Science, 91, 287-296. https://doi.org/10.1016/j.corsci.2014.11.029
- 12. Vengatesh, P. and Kulandainathan, M.A. (2015) Hierarchically Ordered Self-Lubricating Superhydrophobic Anodized Aluminum Surfaces with Enhanced Corrosion Resistance. ACS Applied Materials & Interfaces, 7, 1516-1526. https://doi.org/10.1021/am506568v
- 13. Liu, Y., Yin, X., Zhang, J., et al. (2014) A Electro-Deposition Process for Fabrication of Biomimetic Super-Hydrophobic Surface and Its Corrosion Resistance on Magnesium Alloy. Elec-trochimica Acta, 125, 395-403. https://doi.org/10.1016/j.electacta.2014.01.135
- 14. Wang, Z., Gong, J., Ma, J., et al. (2014) In Situ Growth of Hierarchical Boehmite on 2024 Aluminum Alloy Surface as Superhydrophobic Materials. RSC Advances, 4, 14708-14714. https://doi.org/10.1039/C4RA00160E
- 15. Lin, J., Cai, Y., Wang, X., et al. (2011) Fabrication of Biomimetic Superhydrophobic Surfaces Inspired by Lotus Leaf and Silver Ragwort Leaf. Nanoscale, 3, 1258-1262. https://doi.org/10.1039/c0nr00812e
- 16. Fürstner, R., Barthlott, W., Neinhuis, C., et al. (2005) Wetting and Self-Cleaning Properties of Artificial Superhydrophobic Surfaces. Langmuir the ACS Journal of Surfaces & Colloids, 21, 956-961. https://doi.org/10.1021/la0401011
- 17. Feng, L., Yan, Z., Qiang, X., et al. (2015) Polystyrene-Grafted Al Surface with Excellent Superhydrophobicity and Corrosion Resistance. Surface & Interface Analysis, 47, 506-513. https://doi.org/10.1002/sia.5740
- 18. Cao, L., Jones, A.K., Sikka, V.K., et al. (2009) Anti-Icing Superhydrophobic Coatings. Langmuir, 25, 12444-12448. https://doi.org/10.1021/la902882b
- 19. Ruan, M., Li, W., Wang, B., et al. (2013) Preparation and Anti-Icing Be-havior of Superhydrophobic Surfaces on Aluminum Alloy Substrates. Langmuir the ACS Journal of Surfaces & Colloids, 29, 8482-8491. https://doi.org/10.1021/la400979d
- 20. Jung, Y.C. and Bhushan, B. (2010) Biomimetic Structures for Fluid Drag Reduction in Laminar and Turbulent Flows. Journal of Physics Condensed Matter, 22, Article ID: 035104. https://doi.org/10.1088/0953-8984/22/3/035104
- 21. Daniello, R.J., Waterhouse, N.E. and Rothstein, J.P. (2009) Drag Reduction in Turbulent Flows over Superhydrophobic Surfaces. Physics of Fluids, 21, Article ID: 085103. https://doi.org/10.1063/1.3207885
- 22. Lu, Y., Sathasivam, S., Song, J., et al. (2015) Repellent Materials. Robust Self-Cleaning Surfaces That Function When Exposed to Either Air or Oil. Science, 347, 1132-1135. https://doi.org/10.1126/science.aaa0946
- 23. Tian, X., Verho, T. and Ras, R.H. (2016) Moving Superhydrophobic Surfaces toward Real-World Applications. Science, 352, 142-143. https://doi.org/10.1126/science.aaf2073
- 24. Ren, T. and He, J. (2017) Substrate-Versatile Approach to Robust Antireflective and Superhydrophobic Coatings with Excellent Self-Cleaning Property in Varied Environments. ACS Applied Materials & Interfaces, 9, 34367-34376. https://doi.org/10.1021/acsami.7b11116
- 25. Xiong, L., Kendrick, L.L., Heusser, H., et al. (2014) Spray-Deposition and Photopolymerization of Organic-Inorganic Thiolene Resins for Fabrication of Superamphiphobic Surfaces. ACS Applied Materials & Interfaces, 6, 10763-10774. https://doi.org/10.1021/am502691g
- 26. Zhang, Y., Ge, D. and Yang, S. (2014) Spray-Coating of Superhydro-phobic Aluminum Alloys with Enhanced Mechanical Robustness. Journal of Colloid & Interface Science, 423, 101-107. https://doi.org/10.1016/j.jcis.2014.02.024
- 27. Deng, X., Mammen, L., Zhao, Y.F., et al. (2011) Transparent, Thermally Stable and Mechanically Robust Superhydrophobic Surfaces Made from Porous Silica Capsules. Advanced Materials, 23, 2962-2965. https://doi.org/10.1002/adma.201100410
- 28. Tang, X., Yu, F., Guo, W., et al. (2014) A Facile Procedure to Fabricate Nano Calcium Carbonate-Polymer-Based Superhydrophobic Surfaces. New Journal of Chemistry, 38, 2245-2249. https://doi.org/10.1039/C3NJ01592K
- 29. Liu, H., Szunerits, S., Xu, W., et al. (2009) Preparation of Superhydrophobic Coatings on Zinc as Effective Corrosion Barriers. ACS Applied Materials & Interfaces, 1, 1150-1153. https://doi.org/10.1021/am900100q
- 30. Ishizaki, T., Hieda, J., Saito, N., et al. (2010) Corrosion Resistance and Chemical Stability of Super-Hydrophobic Film Deposited on Magnesium Alloy AZ31 by Microwave Plasma-Enhanced Chemical Vapor Deposition. Electrochimica Acta, 55, 7094-7101. https://doi.org/10.1016/j.electacta.2010.06.064
- 31. Lv, D., Ou, J., Xue, M., et al. (2015) Stability and Corrosion Resistance of Superhydrophobic Surface on Oxidized Aluminum in NaCl Aqueous Solution. Applied Surface Science, 333, 163-169. https://doi.org/10.1016/j.apsusc.2015.02.012
- 32. Wang, N. and Xiong, D. (2014) Superhydrophobic Membranes on Metal Substrate and Their Corrosion Protection in Different Corrosive Media. Applied Surface Science, 305, 603-608. https://doi.org/10.1016/j.apsusc.2014.03.142
- 33. Rao, A.V., Latthe, S.S., Mahadik, S.A., et al. (2011) Mechanically Stable and Corrosion Resistant Superhydrophobic Sol-Gel Coatings on Copper Substrate. Applied Surface Science, 257, 5772-5776. https://doi.org/10.1016/j.apsusc.2011.01.099
- 34. Wang, L., Yang, J., Zhu, Y., et al. (2016) A Study of the Me-chanical and Chemical Durability of Ultra-Ever Dry Superhydrophobic Coating on Low Carbon Steel Surface. Colloids & Surfaces a Physicochemical & Engineering Aspects, 497, 16-27. https://doi.org/10.1016/j.colsurfa.2016.02.022
- 35. Jadhav, A.J., Holkar, C.R. and Pinjari, D.V. (2018) Anticor-rosive Performance of Super-Hydrophobic Imidazole Encapsulated Hollow Zinc Phosphate Nanoparticles on Mild Steel. Progress in Organic Coatings, 114, 33-39. https://doi.org/10.1016/j.porgcoat.2017.09.017
- 36. Feng, X., Feng, L., Jin, M., et al. (2004) Reversible Su-per-Hydrophobicity to Super-Hydrophilicity Transition of Aligned ZnO Nanorod Films. Journal of the American Chemical Society, 126, 62-63. https://doi.org/10.1021/ja038636o
- 37. Xiu, Y., Hess, D.W. and Wong, C.P. (2008) UV and Thermally Stable Superhydrophobic Coatings from Sol-Gel Processing. Journal of Colloid & Interface Science, 326, 465-470. https://doi.org/10.1016/j.jcis.2008.06.042
- 38. Lin, J., Chen, H., Fei, T., et al. (2013) Highly Transparent Super-hydrophobic Organic-Inorganic Nanocoating from the Aggregation of Silica Nanoparticles. Colloids & Surfaces a Physicochemical & Engineering Aspects, 421, 51-62. https://doi.org/10.1016/j.colsurfa.2012.12.049
- 39. Niu, L. and Kang, Z. (2017) A Facile Approach for the Fab-rication of 3D Flower-Like Cu2S Nanostructures on Brass Mesh with Temperature-Induced Wetting Transition for Ef-ficient Oil-Water Separation. Applied Surface Science, 422, 456-468.
NOTES
*通讯作者。
