Advances in Clinical Medicine
Vol.
13
No.
04
(
2023
), Article ID:
64594
,
9
pages
10.12677/ACM.2023.134923
多模态超声预测甲状腺乳头状癌颈部中央区 淋巴结转移的价值研究
李思瑶1,2*,薛杰2,王国运1,2,陈黄卓楠3,武志慧4,曹小丽2#
1青岛大学医学部,山东 青岛
2烟台毓璜顶医院超声医学科,山东 烟台
3潍坊医学院医学影像学院,山东 潍坊
4滨州医学院医学影像学院,山东 烟台
收稿日期:2023年3月24日;录用日期:2023年4月18日;发布日期:2023年4月27日

摘要
目的:探讨多模态超声在预测甲状腺乳头状癌颈部中央区淋巴结转移中的应用价值。方法:选取在我院就诊且病理证实为甲状腺乳头状癌的患者103例。根据颈部中央区淋巴结病理结果分为转移组和未转移组。采用单因素及多因素逻辑回归分析甲状腺乳头状癌的多模态超声图像特征与CLNM之间的关系,并绘制ROC曲线评估诊断效能。结果:单因素及多因素逻辑回归分析显示,男性、多灶性、微钙化、被膜侵犯及等–高增强是预测CLMN的危险因素(P < 0.05)。五者联合的诊断模型预测效能优于各因素单独预测(P < 0.05)。结论:男性、多灶性、微钙化、被膜侵犯及等–高增强是PTC患者CLNM的危险因素,五者联合时诊断效能较好,可为临床制定治疗方案及评估预后提供客观依据。
关键词
甲状腺乳头状癌,多模态超声,淋巴结

The Value of Multimodal Ultrasound in Predicting Lymph Node Metastasis in the Central Neck Region of Thyroid Papillary Carcinoma
Siyao Li1,2*, Jie Xue2, Guoyun Wang1,2, Huangzhuonan Chen3, Zhihui Wu4, Xiaoli Cao2#
1School of Medicine, Qingdao University, Qingdao Shandong
2Department of Ultrasound, Affiliated Yantai Yuhuangding Hospital, Yantai Shandong
3School of Medical Imaging, Weifang Medical University, Weifang Shandong
4School of Medical Imaging, Binzhou Medical University, Yantai Shandong
Received: Mar. 24th, 2023; accepted: Apr. 18th, 2023; published: Apr. 27th, 2023

ABSTRACT
Objective: To explore the value of multimodal ultrasound in predicting lymph node metastasis in the central region of the neck of thyroid papillary carcinoma. Methods: 103 patients with thyroid papillary carcinoma confirmed by pathology were selected. According to the pathological results of lymph nodes in the central region of the neck, they were divided into metastatic group and non-metastatic group. The relationship between the features of multimodal ultrasound images of thyroid papillary carcinoma and CLNM was analyzed by single factor and multiple factor logistic regression, and the ROC curve was drawn to evaluate the diagnostic efficacy. Results: Univariate and multivariate logistic regression analysis showed that male, multifocal, microcalcification, capsule invasion and iso-high enhancement were risk factors for predicting CLMN (P < 0.05). The predictive efficiency of the diagnostic model combined with the five factors was better than that of each factor alone (P < 0.05). Conclusion: Male, multifocal, microcalcification, capsule invasion and iso-high enhancement are the risk factors of CLNM in PTC patients. The combination of the five can provide an objective basis for clinical treatment planning and evaluation of prognosis.
Keywords:Papillary Thyroid Carcinoma, Multimodal Ultrasound, Lymph Node

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
甲状腺乳头状癌(papillary thyroid carcinoma, PTC)是甲状腺中最常见的恶性肿瘤,部分PTC患者易发生颈部中央区淋巴结转移(central lymph node metastasis, CLNM),转移率约50%~64%,而术前超声诊断率低于30%,为避免临床治疗不完全,目前临床常规行预防性中央区淋巴结清扫术(preventive central lymph node dissection, pCLND) [1] [2] 。但2015年ATA明确指出临床淋巴结阴性(clinical lymph node negative, cN0)患者无需行pCLND [3] 。因此,临床需要更有效的手段于术前精准预测颈部中央区淋巴结病理状态,以期制定个体化治疗方案。基于此,本文旨在探讨PTC患者颈部CLNM与PTC在常规超声、超声造影(contrast-enhanced ultrasound, CEUS)及剪切波弹性成像(shear wave elastography, SWE)的多模态超声特征之间的关系,并基于此建立术前诊断模型以评估PTC患者发生颈部CLNM的风险,从而辅助临床医生进行术前决策及预后评估。
2. 资料与方法
2.1. 临床资料
选取2020年9月至2022年12月在我院就诊且病理证实为PTC的103例患者,根据颈部中央区淋巴结病理结果将纳入病例分为转移组及未转移组。纳入标准:1) 术前1个月内的多模态超声检查资料均完整;2) 均行甲状腺切除术 + 颈部中央区淋巴结清扫术。排除标准:1) 既往有甲状腺手术史;2) 其他恶性肿瘤病史;3) 妊娠或哺乳期妇女。本研究经烟台毓璜顶医院伦理委员会审核批准。
2.2. 检查方法
采用Philips EPIQ Elite超声多普勒超声诊断仪,4~18 MHz线阵探头。受检者取仰卧位,充分暴露颈部,对甲状腺及颈部淋巴结进行检查。记录常规超声图像资料,包括病灶数目、最大径、纵横比、内部回声、微钙化、被膜侵犯及血流。在二维图像上选取结节的最大切面,切换至SWE模式进行图像采集,取样框内包含目标结节及周围正常腺体组织,待取样框中颜色填充稳定后同时启动计时器并留存动态图像,选取成像效果最好的图像进行分析(见图1)。分析剪切波弹性成像图像资料(避开结节内的钙化及囊性部分,在病灶内重复测量5次取其平均值),包括杨氏模量平均值(Emean)及杨氏模量比值(Eratio,病灶杨氏模量平均值/正常组织杨氏模量平均值)。再次切换至甲状腺造影模式并双幅显示,向肘静脉内团注造影剂混悬液2.4 ml后立即推注5.0 ml的0.9%氯化钠溶液冲管,同时启动计时器并留存动态图像,连续观察至少90 s后结束检查(见图2)。分析超声造影图像资料,包括病灶增强模式、增强均匀性、增强强度、增强边界、造影后大小(同一切面较二维大小相比)、强化开始时间(较正常组织相比)、峰值强度(PI)、达峰时间(TP)及曲线下面积(AUC)。
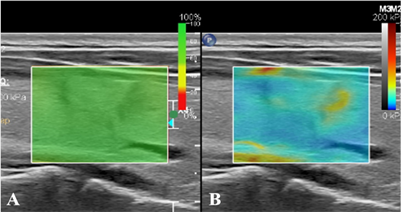
Figure 1. (A) SWE image credibility is 100%; (B) In SWE mode, the hardness of nodules is greater than that of normal glands at the same depth
图1. (A) SWE图像可信度为100%;(B) SWE模式下显示结节硬度大于同深度正常腺体
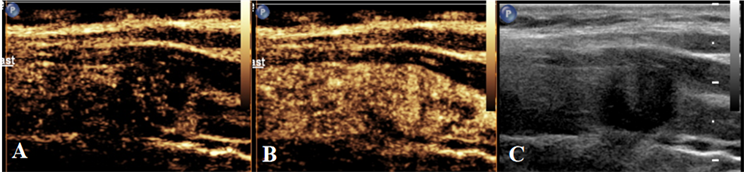
Figure 2. Schematic diagram of centrifugal enhancement of nodules in CEUS mode. (A) When the contrast agent enters 10 s, the center of the nodule intensifies before the surrounding area; (B) When the contrast agent enters 17 s, the nodules show uneven iso-high enhancement; (C) Two-dimensional ultrasound nodule map during CEUS dual imaging
图2. CEUS模式下结节呈离心性增强示意图。(A) 造影剂进入10 s时,结节中心先于周围强化;(B) 造影剂进入17 s时,结节呈不均匀等–高增强;(C) CEUS双幅成像时二维超声结节图
2.3. 统计学分析
采用SPSS 25.0软件进行统计学分析。对计量资料分布进行正态检验后,正态分布的资料使用x ± s,非正态分布的资料使用M (Q1, Q3)表示,两组间比较采用曼–惠特尼U检验,对计数资料以例(%)表示,组间比较采用c2检验。各资料进行单因素Logistic回归分析后,将可能存在相关性的变量进行多因素Logistic回归分析,筛选出独立危险因素并绘制各独立危险因素及联合模型预测PTC患者颈部CLNM的受试者工作特征(ROC)曲线,在最佳临界值下计算相应的曲线下面积(AUC)、敏感度和特异性并比较各个指标间的诊断效能。
3. 结果
3.1. 颈部中央区淋巴结转移的单因素Logistic回归分析
103例PTC患者,转移组46例,未转移组57例,约45.8%的患者发生CLNM。研究对象中位数年龄44 (35, 52)岁,范围22~65岁。转移组与未转移组比较,性别、病灶数目、微钙化、被膜侵犯、增强强度及造影后大小的差异具有统计学意义(P < 0.05,表1),而年龄、最大径、纵横比、内部回声、血流、增强模式、增强均匀性、增强边界、强化开始时间、TP、PI、AUC、Emean及Eratio在两组间的差异无统计学意义(P > 0.05,见表1)。
Table 1. Single factor logistic regression analysis of lymph node metastasis in the central region of the neck
表1. 颈部中央区淋巴结转移的单因素Logistic回归分析
3.2. 颈部中央区淋巴结转移的多因素Logistic回归分析
多因素Logistic回归模型分析结果显示男性(OR = 6.721, P = 0.005),多灶性(OR = 4.288, P = 0.011)、微钙化(OR = 4.090, P = 0.007)、被膜侵犯(OR = 6.197, P = 0.003)及等–高增强(OR = 6.547, P = 0.001)是PTC患者颈部CLNM的独立危险因素(P < 0.05,见表2)。
Table 2. Multivariate logistic regression analysis of related factors of cervical CLNM in patients with PTC
表2. 多因素Logistic回归分析PTC患者颈部CLNM的相关因素
3.3. 单独指标及联合指标对中央区淋巴结转移的诊断效能分析
ROC曲线分析结果显示联合指标预测PTC患者颈部CLNM的AUC为0.855,灵敏度为89.1%,特异度为70.2%。联合指标诊断效能优于男性、多灶性、微钙化、被膜侵犯及等–高增强单独预测的诊断效能(见图3)。联合指标与男性,多灶性、微钙化、被膜侵犯及等–高增强之间的诊断效能差异性具有统计学意义(P < 0.001),但各单独指标之间的诊断效能差异无统计学意义(P > 0.05) (见表3,图1)。
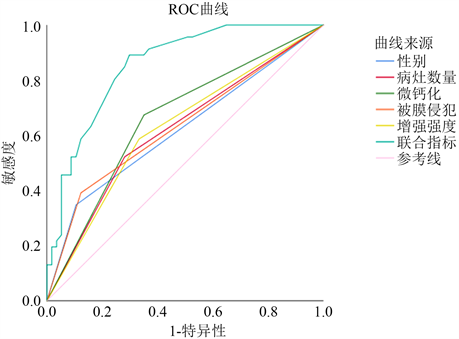 注:Logit(P) = −3.224 + 1.905 × 性别 + 1.456 × 病灶数目 + 1.409 × 微钙化 + 1.824 × 被膜侵犯 + 1.879 × 增强强度。
注:Logit(P) = −3.224 + 1.905 × 性别 + 1.456 × 病灶数目 + 1.409 × 微钙化 + 1.824 × 被膜侵犯 + 1.879 × 增强强度。
Figure 3. ROC curve for evaluating CLNM risk using single and combined indicators
图3. 单指标及联合指标评估CLNM风险的ROC曲线
Table 3. Analysis of predictive value of risk factors alone and combined indicators for lymph node metastasis in the central region of the neck
表3. 危险因素单独及联合指标对颈部中央区淋巴结转移的预测价值分析
4. 讨论
近年来,随着甲状腺超声检查及细针穿刺活检技术的普遍开展,甲状腺乳头状癌检出率逐年上升 [4] 。随着微波、射频及激光等越来越多的微创技术应用于颈部淋巴结阴性PTC患者的治疗,全球学者对低风险PTC的管理更加趋向于保守治疗或主动检测 [5] 。在本研究中术后病理证实约55.3%的PTC患者未发生CLNM,这表明pCLND将会导致近半数患者过度治疗。因此,术前准确评估颈部中央区淋巴结状态对PTC患者至关重要,有助于临床制定治疗方案和评估预后。
本研究显示男性、多灶性、微钙化均是CLNM的危险因素。在本研究中男性患者中央区淋巴结的转移率高达70.6%,是女性患者转移率的3倍,男性PTC患者更易发生CLNM。可能是由于在男性患者中,雌激素通过促进PTC病灶的上皮细胞–间质转化和血管生成获取较高的迁移性与侵袭性,由此导致男性患者复发、转移及死亡率高于女性 [6] 。我们发现多灶性也是PTC患者颈部中央区淋巴结转移的危险因素之一。目前观点认为多灶性PTC可能由单个恶性克隆的癌细胞腺内转移或多个独立原发肿瘤组成,二者之间的区别主要在于生物学行为方面,腺内转移可能预示着拥有较强的侵袭性行为 [7] 。微钙化常发生于弥漫硬化型和实性型PTC中 [8] ,这说明存在微钙化的PTC发生CLNM也可能与肿瘤病理分型相关。因此,考虑微钙化是否能够作为预测PTC淋巴结转移的危险因素之一时,可以依据PTC病理亚分型分组进行讨论。当侵袭性较强的甲状腺恶性结节靠近被膜时易发生被膜侵犯。Chen等研究 [9] 发现侵犯被膜的PTC患者更易发生颈部CLNM,在本研究中,侵犯被膜者的CLNM率为72.2% (26/36),未侵犯者转移率为34.2% (27/79),与本研究结果一致,即侵犯被膜是CLNM的危险因素之一。考虑可能是由于甲状腺被膜由薄纤维组织构成,包含骨骼肌、血管和纤维组织及丰富的淋巴管,侵犯被膜者易侵犯淋巴管向周围组织延伸,进而导致腺外侵犯 [1] 。
除常规超声征象外,一些研究表明CEUS可以评估甲状腺结节的生物学行为 [10] [11] [12] 。在本研究中,呈等或高增强的PTC患者发生CLNM的患病率显著高于无或低增强PTC患者,多因素分析显示PTC呈等或高增强是颈部CLNM的独立预测因子,其他研究也指出转移组早期增强强度明显高于未转移组 [13] 。造影呈等或高增强时说明肿瘤生长较快,其内部血供丰富、新生血管多且管壁较薄、渗透性较高,因此癌细胞更易侵入微血管导致转移发生。但Zhang等认为PTC呈不均匀性低增强是预测CLNM最有价值的参数 [14] ,造成这种差异的原因可能是其纳入的PTC直径较大,肿瘤较大内部易出现液化坏死,此区域造影呈灌注缺陷,常被观察到异质性低灌注。因此,利用CEUS定性指标评估CLNM转移风险时,应将肿瘤大小纳入考虑范围。在甲状腺结节的临床评估中,结节硬度的增加与恶性风险的增高密切相关。Li等指出CLNM组PTC病灶硬度大于非CLNM组 [15] ,但本研究中发现Emean及Eratio不是CLNM的危险因素,可能是因为癌结节生长过程中成分变化复杂多样,影响其硬度值的混杂因素较多,SWE不能反映病灶细胞及纤维胶原成分含量所呈现的真实硬度,因此利用SWE 预测CLNM的有效性有待深入探究。
本研究存在一定局限性,我们仅对CLNM与否进行观察,并未对其转移数量进行关注,后续研究可根据转移数量进行分组讨论。
5. 结论
男性、多灶性、微钙化、被膜侵犯及等–高增强是PTC患者CLNM的独立危险因素,五者联合指标构建的多模态超声预测模型诊断效能优于单独指标预测模型,且有较高的敏感度和特异度,可为临床制定治疗方案及评估预后提供客观依据。
文章引用
李思瑶,薛 杰,王国运,陈黄卓楠,武志慧,曹小丽. 多模态超声预测甲状腺乳头状癌颈部中央区淋巴结转移的价值研究
The Value of Multimodal Ultrasound in Predicting Lymph Node Metastasis in the Central Neck Region of Thyroid Papillary Carcinoma[J]. 临床医学进展, 2023, 13(04): 6585-6593. https://doi.org/10.12677/ACM.2023.134923
参考文献
- 1. Kim, S.K., Chai, Y.J., Park, I., et al. (2017) Nomogram for Predicting Central Node Metastasis in Papillary Thyroid Car-cinoma. Journal of Surgical Oncology, 115, 266-272. https://doi.org/10.1002/jso.24512
- 2. Solorzano, C.C., Carneiro, D.M., Ramirez, M., Lee, T.M. and Irvin, G.L. (2004) Surgeon-Performed Ultrasound in the Management of Thyroid Malignancy. The American Surgeon, 70, 576-580. https://doi.org/10.1177/000313480407000703
- 3. Haugen, B.R., Alexander, E.K., Bible, K.C., et al. (2016) 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thy-roid Cancer. Thyroid, 26, 1-133. https://doi.org/10.1089/thy.2015.0020
- 4. Chen, W., Zheng, R., Baade, P.D., et al. (2016) Cancer Statistics in China, 2015. CA, 66, 115-132. https://doi.org/10.3322/caac.21338
- 5. Jeong, S.Y., Baek, J.H., Choi, Y.J., Chung, S.R., Sung, T.Y., Kim, W.G., Kim, T.Y. and Lee, J.H. (2018) Radiofrequency Ablation of Primary Thyroid Carcinoma: Efficacy According to the Types of Thyroid Carcinoma. International Journal of Hyperthermia, 34, 611-616. https://doi.org/10.1080/02656736.2018.1427288
- 6. Zane, M., Parello, C., Pennelli, G., et al. (2017) Estrogen and Thyroid Cancer Is a Stem Affair: A Preliminary Study. Biomedicine & Pharmacotherapy, 85, 399-411. https://doi.org/10.1016/j.biopha.2016.11.043
- 7. Lu, Z., Sheng, J., Zhang, Y., et al. (2016) Clonality Analysis of Multifocal Papillary Thyroid Carcinoma by Using Genetic Profiles. The Journal of Pathology, 239, 72-83. https://doi.org/10.1002/path.4696
- 8. Luster, M., Aktolun, C., Amendoeira, I., et al. (2019) European Perspective on 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differen-tiated Thyroid Cancer: Proceedings of an Interactive International Symposium. Thyroid, 29, 7-26. https://doi.org/10.1089/thy.2017.0129
- 9. Chen, J., Li, X.L., Zhao, C.-K., Wang, D., Wang, Q., Li, M.-X., Wei, Q., Ji, G. and Xu, H.-X. (2018) Conventional Ultrasound, Immunohistochemical Factors and BRAFV600E Mutation in Predicting Central Cervical Lymph Node Metastasis of Papillary Thyroid Carcinoma. Ultrasound in Medicine & Biology, 44, 2296-2306. https://doi.org/10.1016/j.ultrasmedbio.2018.06.020
- 10. Wei, X., Li, Y., Zhang, S. and Gao, M. (2014) Prediction of Thyroid Extracapsular Extension with Cervical Lymph Node Metastases (ECE-LN) by CEUS and BRAF Expression in Papillary Thyroid Carcinoma. Tumour Biology, 35, 8559-8564. https://doi.org/10.1007/s13277-014-2119-2
- 11. Zhan, J., Zhang, L.H., Yu, Q., et al. (2020) Prediction of Cervical Lymph Node Metastasis with Contrast-Enhanced Ultrasound and Association between Presence of BRAFV600E and Ex-trathyroidal Extension in Papillary Thyroid Carcinoma. Therapeutic Advances in Medical Oncology, 12. https://doi.org/10.1177/1758835920942367
- 12. Zhan, J., Diao, X., Chen, Y., Wang, W. and Ding, H. (2019) Pre-dicting Cervical Lymph Node Metastasis in Patients with Papillary Thyroid Cancer (PTC)—Why Contrast-Enhanced Ul-trasound (CEUS) Was Performed before Thyroidectomy. Clinical Hemorheology and Microcirculation, 72, 61-73. https://doi.org/10.3233/CH-180454
- 13. Liu, Y., Zhou, H., Yang, P., Zhou, Y., Wu, J., Chen, C.Y., Ye, M. and Luo, J. (2017) Contrast-Enhanced Ultrasonography Features of Papillary Thyroid Carcinoma for Predicting Cervical Lymph Node Metastasis. Experimental and Therapeutic Medicine, 14, 4321-4327. https://doi.org/10.3892/etm.2017.5087
- 14. Zhang, Y., Luo, Y.K., Zhang, M.B., Li, J., Li, C., Tang, J. and Li, J.-L. (2017) Values of Ultrasound Features and MMP-9 of Papillary Thyroid Carcinoma in Predicting Cervical Lymph Node Metastases. Scientific Reports, 7, Article No. 6670. https://doi.org/10.1038/s41598-017-07118-7
- 15. Li, T., Li, H., Xue, J., Miao, J. and Kang, C. (2021) Shear Wave Elastography Combined with Gray-Scale Ultrasound for Predicting Central Lymph Node Metastasis of Papillary Thyroid Carcinoma. Surgical Oncology, 36, 1-6. https://doi.org/10.1016/j.suronc.2020.11.004
NOTES
*第一作者。
#通讯作者。
