Advances in Clinical Medicine
Vol.
13
No.
04
(
2023
), Article ID:
64762
,
9
pages
10.12677/ACM.2023.134951
鱼鳞胶对MC3T3-E1细胞成骨性能的影响
王亚辉1,董冰子2,乔梦菲1,王芳2
1青岛大学,医学部,山东 青岛
2青岛大学附属医院,内分泌与代谢病科,山东 青岛
收稿日期:2023年3月26日;录用日期:2023年4月21日;发布日期:2023年4月28日

摘要
研究目的:用含有鱼鳞胶的培养基培养小鼠胚胎成骨细胞(MC3T3-E1细胞),探究鱼鳞胶对MC3T3-E1细胞成骨性能的影响,为临床应用鱼鳞胶治疗骨质疏松症提供理论基础。研究方法:1. 用鱼鳞胶分别以5 mg/mL、10 mg/mL、20 mg/mL的浓度培养MC3T3-E1细胞,同时设置空白对照组和阳性对照组。在培养的第14 d,通过ALP染色检测碱性磷酸酶(ALP)的活性,imageJ软件计算反映蓝紫色沉淀单位面积浓度的平均光密度(AOD)值,并进行组间比较。2. 上述浓度诱导MC3T3-E1细胞,在培养的第21 d,通过茜素红染色(ARS)评估钙结节形成情况。3. 设置空白对照组,处理组,含20 mg/mL鱼鳞胶的处理组,培养MC3T3-E1细胞48 h后,通过逆转录–实时荧光定量PCR (RT-qPCR)分别检测上述三组MC3T3-E1细胞的碱性磷酸酶(ALP),Runx相关转录因子2 (Runx2),骨钙素(OCN) mRNA相对表达量的变化。研究结果:1. ALP染色实验结果显示,不同浓度鱼鳞胶干预MC3T3-E1细胞14 d,与对照组相比,10 mg/mL鱼鳞胶组,20 mg/mL鱼鳞胶组和阳性对照组的AOD均比空白对照组增加(P < 0.05)。2. 不同浓度的鱼鳞胶体外培养MC3T3-E1细胞21 d时,茜素红染色结果显示,鱼鳞胶组与空白对照组相比,各组均形成钙化结节,且存在剂量依赖性,即鱼鳞胶浓度越大,结节越明显。3. RT-qPCR结果表明,体外培养MC3T3-E1细胞48 h,处理组和含鱼鳞胶的处理组ALP、OCN mRNA表达量均上调,与空白对照组相比有统计学意义(P < 0.01),且两组间均有统计学差异(P < 0.05)。处理组和含鱼鳞胶的处理组Runx2 mRNA表达量较空白对照组上调(P < 0.05),两组间没有统计学差异(P > 0.05)。结论及意义:鱼鳞胶能够显著促进MC3T3-E1细胞的成骨分化成熟、矿化,从而加快骨基质矿化过程,促进骨形成。这些体外实验数据证实了鱼鳞胶对骨质疏松症具有潜在的治疗作用,为全面评价其在临床上防治骨质疏松症的药效作用提供实验参考依据。
关键词
MC3T3-E1细胞,鱼鳞胶,成骨,骨质疏松症

The Effect of Fish Scale Glue on the Osteogenic Performance of MC3T3-E1 Cells
Yahui Wang1, Bingzi Dong2, Mengfei Qiao1, Fang Wang2
1Medical College, Qingdao University, Qingdao Shandong
2Department of Endocrinology and Metabolism, The Affiliated Hospital of Qingdao University, Qingdao Shandong
Received: Mar. 26th, 2023; accepted: Apr. 21st, 2023; published: Apr. 28th, 2023

ABSTRACT
Research aim: To culture mouse embryonic osteoblasts (MC3T3-E1 cells) in a medium containing fish scale glue, and investigate the effect of fish scale glue on the osteogenic performance of MC3T3-E1 cells, providing a theoretical basis for the clinical application of fish scale glue in the treatment of osteoporosis. Methods: 1. MC3T3-E1 cells were cultured with fish scale glue at concentrations of 5 mg/mL, 10 mg/mL, and 20 mg/mL, respectively, while a blank control group and a positive control group were set up. On the 14th day of culture, the activity of alkaline phosphatase (ALP) was detected by ALP staining, and the average optical density (AOD) value reflecting the concentration per unit area of blue-violet precipitates was calculated by imageJ software and compared between groups. 2. MC3T3-E1 cells were induced at the above concentrations. On the 21st day of culture, the formation of calcium nodules was evaluated by alizarin red staining (ARS). 3. Set up a blank control group, a treatment group and a treatment group containing 20 mg/mL fish scale glue. After culturing MC3T3-E1 cells for 48 hours, detect the changes in the relative expression of alkaline phosphatase (ALP), Runx related transcription factor 2 (Runx2), and osteocalcin (OCN) mRNA in the above three groups of MC3T3-E1 cells by reverse transcription real-time fluorescence quantitative PCR (RT-qPCR). Results: The results of ALP staining experiment showed that the AOD of MC3T3-E1 cells in the 10 mg/mL fish scale glue group, the 20 mg/mL fish scale glue group, and the positive control group increased compared to the blank control group after 14 days of intervention with different concentrations of fish scale glue (P < 0.05). When MC3T3-E1 cells were cultured in vitro with different concentrations of fish scale colloid for 21 days, the results of alizarin red staining showed that compared with the blank control group, calcified nodules were formed in each group, and there was a dose dependent relationship. That is, the greater the concentration of fish scale colloid, the more obvious the nodules were. The results of RT-qPCR showed that the expression of ALP and OCN mRNA in MC3T3-E1 cells cultured in vitro for 48 hours was upregulated in both the treatment group and the treatment group containing fish scale gum, which was statistically significant compared to the blank control group (P < 0.01), and there was a significant difference between the two groups (P < 0.05). The expression of Runx2 mRNA in the treatment group and the treatment group containing fish scale glue was upregulated compared to the blank control group (P < 0.05), and there was no statistical difference between the two groups (P > 0.05). Conclusion and significance: Fish scale glue can significantly promote the osteogenic differentiation, maturation, and mineralization of MC3T3-E1 cells, thereby accelerating the mineralization process of bone matrix and promoting bone formation. These in vitro experimental data confirm the potential therapeutic effect of fish scale glue on osteoporosis, providing experimental reference for comprehensive evaluation of its clinical efficacy in preventing and treating osteoporosis.
Keywords:MC3T3-E1 Cells, Fish Scale Gum, Osteogenesis, Osteoporosis

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 背景及目的
骨质疏松症(Osteoporosis, OP)是常见的骨骼疾病,是一种以骨量减少、骨组织微结构损伤、易发生脆性骨折为特征的全身性骨病 [1] 。人体骨骼的完整性需要一系列不断重复的骨重建过程维持,即由成骨细胞(osteoblast, OB)介导的骨形成和由破骨细胞(osteoclast, OC)介导的骨吸收之间的动态平衡。骨代谢紊乱会导致骨质疏松症、骨关节炎等一系列骨代谢疾病的发生,严重威胁人体特别是中老年人的身体健康 [2] 。流行病学调查显示,我国50岁以上人群骨质疏松症患病率为19.2%,其中女性为32.1%,男性为6.9%;60岁以上人群骨质疏松症患病率为32%,其中女性为51.6%,男性为10.7% [3] ,呈现随总人口年龄增加而逐渐增加的趋势。目前临床上治疗骨质疏松的药物主要有骨吸收抑制剂、骨形成促进剂、双重作用药物、其他机制类药物及中成药 [3] ,其中骨吸收抑制剂应用较为广泛,如双膦酸盐、降钙素、雌激素类药物,而骨形成促进药物可供选择的种类有限,亟待更多的基础研究成果向临床转化 [4] ,本课题研究天然产物鱼鳞胶对小鼠胚胎成骨细胞(MC3T3-E1细胞)成骨性能的影响,以期为临床应用鱼鳞胶治疗骨质疏松症提供理论基础。
鱼鳞的结构分为两层:外层由羟基磷灰石晶体形成骨质层,内层由I型胶原纤维排布形成胶合板层 [5] [6] [7] ,其结构和成分与骨细胞外基质相似 [8] [9] ,具有良好的生物相容性 [10] [11] 。整体来看,鱼鳞表面有放射状的凹槽和环脊,类似于微拓扑结构 [12] ,而细胞外基质的微拓扑结构是调节细胞粘附和细胞骨架组装的重要生物物理信号 [13] [14] 。因此,鱼鳞的拓扑结构可能会影响细胞粘附、增殖、迁移等重要的细胞行为。Rena Matsumoto等人研究了鱼鳞胶原纤维对人间充质干细胞(hMSCs)成骨细胞分化的影响,实验发现,hMSCSs更易粘附在鱼鳞的胶原纤维上,体外细胞培养过程中显著加速了hMSCs的早期成骨细胞分化。而且人间充质干细胞(hMSCs)在鱼鳞胶原蛋白表面培养时,早期成骨细胞分化阶段,碱性磷酸酶活性、骨桥蛋白和骨形态发生蛋白2等成骨分化标志物水平显著上调 [15] ,这提示鱼鳞胶原蛋白可能具有干细胞分化诱导潜力。
Wei Wu等人制造了脱钙的鱼鳞衍生支架,脱钙后,胶原纤维暴露,更有利于胶原蛋白的释放。实验观察了在鱼鳞衍生支架上培养的大鼠骨髓间充质干细胞(BMSCs)迁移趋势,发现BMSCs可以很好地粘附在鱼鳞衍生支架表面,良好的粘附性使BMSCs在鳞片上更好地增殖,从而在鳞片上形成细胞层。实验进一步分析了鱼鳞衍生支架对细胞增殖的影响,结果表明,直接或间接接触鱼鳞的BMSCs增殖显著高于对照组,说明鱼鳞材料可以促进BMSCs的增殖 [12] 。
鱼鳞的高生物相容性和细胞培养作用展示了其在促进成骨细胞分化中的应用潜力。然而,现有研究均未探讨鱼鳞对成骨细胞成骨分化的影响。本课题旨在进一步研究鱼鳞制品鱼鳞胶对成骨细胞分化的影响,为临床应用鱼鳞胶治疗骨质疏松症提供理论基础。
2. 研究方法
2.1. 实验细胞、主要试剂和仪器
小鼠胚胎成骨细胞(MC3T3-E1细胞)购自赛百慷(上海)生物技术股份有限公司,用含10%胎牛血清的α-MEM培养基,置于37℃、5% CO2培养箱中培养,收集对数生长期细胞用于实验,鱼鳞胶来自山东省青岛市融智汇实验室。α-MEM培养基及胎牛血清购自大连美仑生物技术有限公司,抗坏血酸(日本和光纯药工业株式会社)、β-甘油磷酸盐Sigma-Aldrich (美国)、地塞米松(北京索莱宝科技有限公司)用于配制阳性对照组及处理组培养基。ALP染色试剂盒购自无锡菩禾生物有限公司,茜素红S染液购自北京索莱宝科技有限公司。组织细胞RNA抽提液TRIzol,cDNA逆转录试剂盒,ChamQ Universal SYBR qPCR Master Mix购自南京诺唯赞生物科技股份有限公司。
2.2. 实验溶液配制
① 完全培养基(空白对照组):向丝口瓶中分别加入89% α-MEM培养基,10%胎牛血清,及1%青、链霉素;
② 实验组:鱼鳞胶粉末用完全培养基稀释成5 mg/mL、10 mg/mL、20 mg/mL;
③ 阳性对照组:完全培养基中加入10 mM β-甘油磷酸盐、50 μg/mL抗坏血酸和10 nM地塞米松;
④ 处理组:同③;
⑤ 含鱼鳞胶的处理组:溶液③中加入20 mg/mL的鱼鳞胶。
2.3. ALP染色检测鱼鳞胶对MC3T3-E1细胞胞外基质影响
将对数生长期MC3T3-E1细胞分别以5 mg/mL、10 mg/mL、20 mg/mL的鱼鳞胶浓度培养,同时设置空白对照组和阳性对照组,分别诱导MC3T3-E1细胞分化,每3天更换一次培养基,细胞培养至第14 d时,使用ALP染色试剂盒进行碱性磷酸酶(ALP)染色,通过imageJ软件计算反映蓝紫色沉淀单位面积浓度的平均光密度(AOD)值,并进行组间比较。
2.4. 茜素红染色检测鱼鳞胶对MC3T3-E1细胞钙化的影响
细胞分组同“1.3”,分别诱导MC3T3-E1细胞分化,每3天更换一次培养基,细胞培养至第21 d时,进行茜素红染色检测钙化结节,多聚甲醛固定后加入茜素红染色,双蒸水清洗数次,直到染液不再脱落,洗液比较澄清为止,保证每个孔板清洗次数一致,洗涤后,开盖晾干,显微镜下观察钙结节并拍照。
2.5. RT-qPCR法检测鱼鳞胶对MC3T3-E1细胞成骨分化的影响
Table 1. Primer sequences for RT-qPCR
表1. RT-qPCR各引物序列
将对数生长期MC3T3-E1细胞分别接种在处理组,含鱼鳞胶的处理组培养基中,并设置空白对照组,诱导48 h后,使用Trizol法提取各组细胞的总RNA,并测量RNA的浓度和纯度,以提取的RNA为模板,使用Hiscript试剂将RNA逆转录为cDNA,获得的cDNA储存于−20℃条件下,qPCR反应时原液稀释5倍进行后续步骤。使用ChamQ Universal SYBR qPCR Master Mix试剂进行实时荧光定量PCR检测,反应条件:95℃预变性30 s;95℃、10 s,60℃、30 s,共40个循环;溶解曲线95℃、15 s,60℃、60 s,95℃、15 s。根据两步法扩增程序,得到Ct循环阈值,并通过公式2-ΔΔCt求出不同组别RNA的相对表达量,甘油醛-3-磷酸脱氢酶(Glycerol-3-Phosphate Dehydrogenase, GAPDH)用作内部对照。各引物序列见表1。
2.6. 统计学分析
采用GraphPad Prism 9.0统计软件进行统计学分析,实验数据表示为平均值 ± 标准差,组间两两比较采用t检验,多组间结果比较采用单因素方差分析,以P < 0.05为差异有统计学意义。
3. 实验结果
3.1. ALP染色检测鱼鳞胶对MC3T3-E1细胞胞外基质影响
含不同浓度鱼鳞胶培养基培养MC3T3-E1细胞14 d后,行ALP染色检测各实验组及对照组中碱性磷酸酶活性,染色结果见图1。采用Image J软件计算反映蓝紫色沉淀单位面积浓度的AOD值,与空白对照组相比,10 mg/mL鱼鳞胶组,20 mg/mL鱼鳞胶组和阳性对照组的AOD均明显增加(P < 0.01),将20 mg/mL鱼鳞胶组和阳性对照组进行两组间比较,阳性对照组较鱼鳞胶组碱性磷酸酶活性(P < 0.05),见图2。
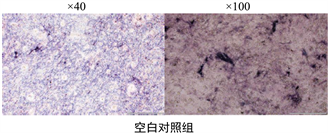

Figure 1. The results of ALP staining
图1. ALP染色结果
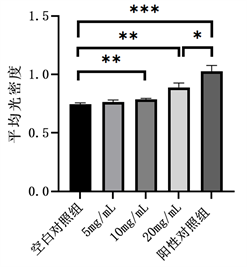
Figure 2. The AOD of ALP staining
图2. ALP染色的AOD值
3.2. 茜素红染色检测鱼鳞胶对MC3T3-E1细胞钙化的影响


空白对照组 5 mg/mL鱼鳞胶培养基组
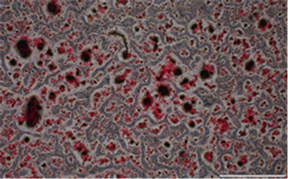
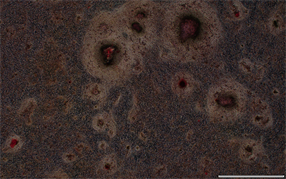 10 mg/mL鱼鳞胶培养基组 20 mg/mL鱼鳞胶培养基组
10 mg/mL鱼鳞胶培养基组 20 mg/mL鱼鳞胶培养基组 阳性对照组
阳性对照组
Figure 3. The results of ARS
图3. 茜素红染色结果
含不同浓度鱼鳞胶培养基培养MC3T3-E1细胞21 d后,行茜素红染色检测鱼鳞胶对MC3T3-E1细胞钙化的影响,染色结果见图3,结果显示,与空白对照组相比,各组均形成钙化结节,且钙结节面积存在剂量依赖性,即鱼鳞胶浓度越大,结节越明显。说明鱼鳞胶能促进成骨细胞钙结节的形成从而促进成骨分化。
3.3. RT-qPCR法检测鱼鳞胶对MC3T3-E1细胞成骨分化的影响

Figure 4. The relative expressions of mRNA
图4. 各组间mRNA相对表达量
与空白对照组相比,处理组和含鱼鳞胶的处理组成骨相关因子ALP、Runx2、OCN mRNA相对表达量均升高,且差异有统计学意义(P < 0.01)。处理组与含鱼鳞胶的处理组两组间比较,ALP和OCN mRNA有统计学差异,含鱼鳞胶的处理组MC3T3-E1细胞ALP和OCN mRNA表达量均升高(P < 0.05),含鱼鳞胶的处理组Runx2 mRNA表达量较处理组上调,但没有统计学差异(P > 0.05),见图4。
4. 讨论
流行病学调查显示,骨质疏松症已成为我国重要的公共健康问题。目前治疗骨质疏松症的药物疗效显著,但同时需注意其副作用。例如,使用双膦酸盐类药物时需警惕双膦酸盐类相关性下颌骨坏死 [16] [17] 、非典型股骨骨折 [18] 的发生;而长期使用降钙素类药物可能与恶性肿瘤风险增加相关 [19] ;启动绝经激素治疗时需格外关注子宫内膜癌、乳腺癌 [20] 、心血管疾病病史及血栓的风险 [21] ;另外一项动物实验发现,大剂量、长时间使用甲状旁腺素类似物特立帕肽增加了大鼠骨肉瘤的发生率 [22] 。迄今为止,大多数抗骨质疏松症药物机制为抑制破骨细胞活性,从而抑制骨吸收。甲状旁腺素类似物是当前促骨形成的代表性药物,其能刺激成骨细胞活性,促进骨形成,增加骨密度,改善骨质量 [23] 。
为此,我们期待更多的毒副作用小而且效果显著的骨形成剂,以刺激新骨形成并纠正受损骨小梁的微结构。天然产物相对于药物对人体产生的不良反应少,更适合长期服用,受到国内外的广泛关注和研究。本课题以成骨细胞为重点,选用小鼠MC3T3-E1细胞,以天然产物鱼鳞胶进行诱导,探讨了其具有的潜在促进骨形成的作用。
细胞成骨分化可以通过多种方式进行检测和评估,ALP染色和茜素红染色通常是提示成骨分化早期 [24] 和晚期 [25] 的标志性实验方法。成骨细胞能够分泌碱性磷酸酶,合成I型胶原等细胞外基质,从而进一步矿化形成骨组织。在碱性磷酸酶催化下,ALP染色试剂盒能形成不溶性的蓝紫色沉淀。我们的实验于14 d进行ALP染色,实验结果表明用鱼鳞胶干预的MC3T3-E1细胞表现出更高的ALP活性水平,10mg/mL鱼鳞胶组和20mg/mL鱼鳞胶组ALP活性水平显著高于空白对照组,这说明鱼鳞胶能够增强MC3T3-E1细胞ALP活性,促进MC3T3-E1细胞中早期分化。成骨细胞矿化的一个特征是钙结节的形成,可用于确定成骨细胞发生矿化的程度。茜素红染色是检验成骨晚期标志物钙化结节的经典实验,本实验于21 d进行茜素红染色,结果提示添加了鱼鳞胶的培养基形成的钙化结节较空白对照组多,染色面积大,且存在剂量依赖性。这些结果提示鱼鳞胶能够促进MC3T3-E1细胞成骨分化及矿化能力。
成骨细胞的分化分为两个阶段,第一个阶段,细胞增殖并伴随细胞基质的成熟,与骨细胞表型相关的特定蛋白质,例如碱性磷酸酶(ALP)可以在细胞增殖和基质成熟期被检测 [26] 。成骨的第二阶段是基质的矿化,产生晚期骨标志物,例如骨钙蛋白(OCN) [27] 。其中多种合成代谢信号通路积极参与控制骨形成,例如BMP,Wnt和Runx2通路,其中Runx2通路调控的具体例子包括与DLX蛋白和ATF4的相互作用以及ERK/MAP激酶通路的磷酸化等 [28] 。我们设置了空白对照组,处理组,及含鱼鳞胶的处理组,通过RT-qPCR在RNA水平对成骨相关基因ALP、Runx2及OCN的表达进行分析,以进一步验证鱼鳞胶对MC3T3-E1细胞成骨分化的影响。本研究结果表明,与不含鱼鳞胶的处理组相比,含鱼鳞胶的处理组提高了ALP mRNA和OCN mRNA的水平,对Runx2 mRNA水平没有影响。这些结果表明鱼鳞胶在RNA水平促进了MC3T3-E1细胞成骨分化,增强了其ALP和OCN基因的表达,但可能并不是通过Runx2通路发挥作用的。
5. 结论
综上,本研究验证了鱼鳞胶能够显著促进MC3T3-E1细胞的成骨分化,从而加快骨基质矿化过程,促进了骨形成。这些体外数据都证实了鱼鳞胶对骨质疏松症具有潜在的治疗作用,为全面评价其在临床上防治骨质疏松症的药效作用提供理论依据,但因对鱼鳞胶成分研究较少,其药效药理机制尚不明确,可能涉及多条信号通路及其关键信号分子,这些仍需进一步探讨,广泛地展开相关体内、体外研究进一步证实。
文章引用
王亚辉,董冰子,乔梦菲,王 芳. 鱼鳞胶对MC3T3-E1细胞成骨性能的影响
The Effect of Fish Scale Glue on the Osteogenic Performance of MC3T3-E1 Cells[J]. 临床医学进展, 2023, 13(04): 6798-6806. https://doi.org/10.12677/ACM.2023.134951
参考文献
- 1. Rachner, T.D., Khosla, S. and Hofbauer, L.C. (2011) Osteoporosis: Now and the Future. The Lancet, 377, 1276-1287. https://doi.org/10.1016/S0140-6736(10)62349-5
- 2. 杨婷, 岳月仪, 范雨佳, 等. WISP1与骨质疏松症关系的研究进展[J]. 国际内分泌代谢杂志, 2021, 41(3): 224-228.
- 3. 原发性骨质疏松症诊疗指南(2022) [J]. 中华骨质疏松和骨矿盐疾病杂志, 2022, 15(6): 573-611.
- 4. Marie, P.J. and Kassem, M. (2011) Osteoblasts in Osteoporosis: Past, Emerging, and Future Anabolic Targets. European Journal of Endocrinology, 165, 1-10. https://doi.org/10.1530/EJE-11-0132
- 5. Lin, Y.S., et al. (2011) Mechanical Properties and the Laminate Structure of Arapaima gigas Scales. Journal of the Mechanical Behavior of Biomedical Materials, 4, 1145-1156. https://doi.org/10.1016/j.jmbbm.2011.03.024
- 6. Ravneet, M.L., Sharma and Kang, H.P.S. (2009) High Resolu-tion Scanning Electron Microscope Examination of the Fish Scale: Inspiration for Novel Biomaterials. Journal of Biomi-metics, Biomaterials, and Tissue Engineering, 4, 13-20. https://doi.org/10.4028/www.scientific.net/JBBTE.4.13
- 7. Krishnan, S., et al. (2012) Fish Scale Collagen—A Novel Material for Corneal Tissue Engineering. Artificial Organs, 36, 829-835. https://doi.org/10.1111/j.1525-1594.2012.01452.x
- 8. Gelse, K. (2003) Collagens—Structure, Function, and Bi-osynthesis. Advanced Drug Delivery Reviews, 55, 1531-1546. https://doi.org/10.1016/j.addr.2003.08.002
- 9. Hayashi, Y., Yamada, S., Guchi, K.Y., et al. (2012) Chitosan and Fish Collagen as Biomaterials for Regenerative Medicine. Advances in Food and Nutrition Research, 65, 107-120.
- 10. Saravanan, S., Leena, R.S. and Selvamurugan, N. (2016) Chitosan Based Biocomposite Scaffolds for Bone Tissue Engineering. International Journal of Biological Macromolecules, 93, 1354-1365. https://doi.org/10.1016/j.ijbiomac.2016.01.112
- 11. Feng, H., et al. (2020) The Lamellar Structure and Biomimetic Properties of a Fish Scale Matrix. RSC Advances, 10, 875-885. https://doi.org/10.1039/C9RA08189E
- 12. Wu, W., et al. (2021) Construction and Characterization of Degradable Fish Scales for Enhancing Cellular Adhesion and Potential Using as Tissue Engineering Scaffolds. Materials Science and Engineering: C, 122, Article ID: 111919. https://doi.org/10.1016/j.msec.2021.111919
- 13. McBeath, R., et al. (2004) Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Developmental Cell, 6, 483-495. https://doi.org/10.1016/S1534-5807(04)00075-9
- 14. Selhuber-Unkel, C., et al. (2010) Cell Adhesion Strength Is Controlled by Intermolecular Spacing of Adhesion Receptors. Biophysical Journal, 98, 543-551. https://doi.org/10.1016/j.bpj.2009.11.001
- 15. Matsumoto, R., et al. (2015) Rapid Oriented Fibril Formation of Fish Scale Collagen Facilitates Early Osteoblastic Differentiation of Human Mesenchymal Stem Cells. Journal of Bio-medical Materials Research Part A, 103, 2531-2539. https://doi.org/10.1002/jbm.a.35387
- 16. Khan, A.A., et al. (2015) Diagnosis and Management of Osteonecrosis of the Jaw: A Systematic Review and International Consensus. Journal of Bone and Mineral Research, 30, 3-23. https://doi.org/10.1002/jbmr.2405
- 17. Ruggiero, S.L., et al. (2009) American Association of Oral and Maxillofa-cial Surgeons Position Paper on Bisphosphonate-Related Osteonecrosis of the Jaws—2009 Update. Journal of Oral and Maxillofacial Surgery, 67, 2-12. https://doi.org/10.1016/j.joms.2009.01.009
- 18. Zanchetta, M.B., et al. (2014) Assessment of Bone Microarchitec-ture in Postmenopausal Women on Long-Term Bisphosphonate Therapy with Atypical Fractures of the Femur. Journal of Bone and Mineral Research, 29, 999-1004. https://doi.org/10.1002/jbmr.2107
- 19. Cosman, F., et al. (2014) Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporosis International, 25, 2359-2381. https://doi.org/10.1007/s00198-014-2794-2
- 20. Cauley, J.A., et al. (2003) Effects of Estrogen plus Progestin on Risk of Fracture and Bone Mineral Density: The Women’s Health Initiative Randomized Trial. JAMA, 290, 1729-1738.
- 21. An, J., Yang, H., Zhang, Q., et al. (2016) Natural Products for Treatment of Osteoporosis: The Effects and Mechanisms on Promoting Osteoblast-Mediated Bone For-mation. Life Sciences, 147, 46-58.
- 22. Vahle, J.L., et al. (2004) Bone Neoplasms in F344 Rats Given Teriparatide [rhPTH(1-34)] Are Dependent on Duration of Treatment and Dose. Toxicologic Pathology, 32, 426-438. https://doi.org/10.1080/01926230490462138
- 23. Kraenzlin, M.E. and Meier, C. (2011) Parathyroid Hormone An-alogues in the Treatment of Osteoporosis. Nature Reviews Endocrinology, 7, 647-656. https://doi.org/10.1038/nrendo.2011.108
- 24. Chen, L., et al. (2019) Biomineralized Hydrogel with Enhanced Toughness by Chemical Bonding of Alkaline Phosphatase and Vinylphosphonic Acid in Collagen Framework. ACS Bi-omaterials Science & Engineering, 5, 1405-1415. https://doi.org/10.1021/acsbiomaterials.8b01197
- 25. Park, K., et al. (2021) Calycosin-7-O-β-Glucoside Isolated from Astragalus membranaceus Promotes Osteogenesis and Mineralization in Human Mesenchymal Stem Cells. Inter-national Journal of Molecular Sciences, 22, 11362. https://doi.org/10.3390/ijms222111362
- 26. Stein, G.S., Lian, J.B. and Owen, T.A. (1990) Relationship of Cell Growth to the Regulation of Tissue-Specific Gene Expression during Osteoblast Differentiation. The FASEB Journal, 4, 3111-3123. https://doi.org/10.1096/fasebj.4.13.2210157
- 27. Komori, T. (2020) Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. International Journal of Molecular Sciences, 21, 7513. https://doi.org/10.3390/ijms21207513
- 28. Franceschi, R.T., et al. (2007) Transcriptional Regulation of Osteoblasts. Annals of the New York Academy of Sciences, 1116, 196-207. https://doi.org/10.1196/annals.1402.081
