Advances in Clinical Medicine
Vol.
13
No.
05
(
2023
), Article ID:
66228
,
8
pages
10.12677/ACM.2023.1351221
Erastin联合Tubacin促进乳腺癌HCC38细胞 死亡
吴灿章*,敬波,郑武平,吴煌福#
海南医学院第二附属医院甲乳外科,海南 海口
收稿日期:2023年4月28日;录用日期:2023年5月21日;发布日期:2023年5月31日

摘要
目的:根据表观遗传特征,可以对恶性肿瘤进行分型,并针对分子异常代谢给予精准打击,实现抗肿瘤目的,这是一种新兴治疗策略。在多数细胞恶变过程中,胱氨酸的成瘾是常见的异常代谢。随后,Erastin被研究者发现,它能抑制胱氨酸谷氨酸转运体(Xc-system),从而诱导细胞死亡。对大多数乳腺癌效果显著。然而,三阴型乳腺癌细胞是非间充质的,对Erastin治疗无反应。为了克服这种耐药性,我们筛选并鉴定出Tubacin,它可以转变恶性肿瘤的胱氨酸不敏感的状态。为了验证Tubacin实际效果,我们尝试在Tubacin、Erastin条件下处理三阴型乳腺癌细胞,对比观察细胞死亡实际情况。方法:本研究的三阴型乳腺癌细胞株HCC38购自ATCC公司,在DMSO、Tubacin、Erastin、及Erastin联合Tubacin条件下进行处理,以流式细胞学等方式检测细胞死亡。结果:1) 单独使用Tubaicn、Erastin处理HCC38细胞,细胞死亡幅度无明显变化。2) Erastin联合Tubacin处理癌细胞可以显著诱导死亡。3) 与DMSO、Tub、Era相比,Era + Tub组别细胞死亡明显增多。4) 与DMSO相比,Tub、Era、Era + Tub组别细胞的活性氧均不同程度增高,后两者显著增高。结论:Erastin联合Tubacin可促进乳腺癌HCC38细胞死亡。
关键词
三阴型乳腺癌,细胞死亡,Tubacin,Erastin

Erastin plus Tubacin Treatment Promotes Breast Cancer Cell Death of HCC38
Canzhang Wu*, Bo Jing, Wuping Zheng, Huangfu Wu#
Department of Breast and Thyroid Surgery, Second Affiliated Hospital of Hainan Medical University, Haikou Hainan
Received: Apr. 28th, 2023; accepted: May 21st, 2023; published: May 31st, 2023

ABSTRACT
Purpose: According to epigenetic features, malignant tumors can be typed and given a precise blow against the molecular aberrant metabolism, achieving antitumor purpose, which is an emerging therapeutic strategy. Addiction to cystine is the more common aberrant metabolism involved in most cellular malignant transformation. Subsequently, the cystine blocker erastin was discovered to inhibit the cystine glutamate transporter (XC-System), which induces cell death and has produced significant effect against most breast cancers. However, TNBC cells are non mesenchymal and do not respond to cystine deprivation treatment. To overcome this resistance, we screened and identified tubacin, which can transform the cystine insensitive state of malignancies. To verify the actual effect of tubacin, we tried to treat triple negative breast cancer cells under tubacin, erastin conditions and observed the cell death reality comparatively. Methods: The triple negative breast cancer cell line HCC38 from this study was purchased from ATCC and treated in DMSO, Tubacin, Erastin, and Erastin plus Tubacin to detect cell death by flow cytometry. Results: 1) In HCC38 cells treated with tubaicn or erastin alone, the magnitude of cell death was unchanged. 2) Treatment of cancer cells with erastin in combination with tubacin significantly induced death. 3) Compared with DMSO, Tub, or Era, cell death was significantly increased in the Erastin plus Tubacin Group. 4) Compared with DMSO, ROS increased in all cell lines in the Tub, Era, and Erastin plus Tubacin groups, and the latter two were significantly higher. Conclusions: Erastin, in combination with tubacin, confers breast cancer cells death of HCC38.
Keywords:Triple Negative Breast Neoplasms, Cell Death, Tubacin, Erastin

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
三阴型乳腺癌(Triple Negative Breast Cancer, TNBC)在各类乳腺癌类型的占比约为20% [1] ,概念为雌孕激素受体表达缺乏,且没有HER-2扩增。因此,TNBC的治疗方式有限。与其他类型乳腺癌相比,TNBC具有较高侵袭性,患者预后较差,术后5年内容易早期复发,复发转移患者的总体生存期仅约为18个月 [1] 。手术和化疗仍是最主要的治疗方案,其中化疗是系统性抗肿瘤的治疗方式 [2] [3] 。但是,化疗所致呕吐、脱发及骨髓抑制等化疗不良反应是不可避免 [4] ,且容易出现药物抗性,这导致患者在治疗过程中需要承担更多风险及痛苦。然而,由于生物基因工程技术日益成熟和生物分子学机制架构日渐清晰,有部分科研学者达成共识,他们认为“三阴型乳腺癌可以根据异常代谢、分子表达等特征进一步分出TNBC亚型”,并针对TNBC亚型,提出可研发出精准打击TNBC的分子代谢异常特征的靶向药物 [5] 。比如,程序性死亡受体-1 (Programmed Cell Death 1 Receptor, PD-1)是免疫T细胞的表面蛋白,正常情况表达很少,但是在三阴型乳腺癌细胞中就可以见到PD-1的高表达并促进肿瘤生长,因此,PD-1抗体(Opdivo和Keytruda)应运而生,这种抗体药物可以与PD-1结合激活淋巴T细胞,实现抑制肿瘤增殖的目的,而PD-1的这一特性让其被归属到TNBC 6种亚型之一的免疫调节型 [5] [6] 。
随着基因学、分子生物学、药物等学科的协同发展,研究者们在不断地提出前沿理论和创新技术,对肿瘤内部分子和架构,以及相互之间的联系有了更多的了解 [7] 。目前肿瘤分子代谢的研究中,多数正常人体细胞在向恶性肿瘤过程过渡时,都存在着代谢异常,这一肿瘤特征也受到学界普遍认可 [7] [8] 。随着恶性肿瘤细胞的代谢改变,也往往会引起细胞内外环境的变化,这包括内部分子的异常表达,即原本高表达且具有抗肿瘤的分子表达显著减少,或者原本低表达并具有促癌作用的分子则异常升高;有的分子则是正常情况下,促进正常细胞增殖,异常表达,则促进肿瘤复发转移 [8] ;除了细胞内部代谢改变之外,还有可能出现细胞外环境改变,比如细胞基质,有些恶性肿瘤,会通过改变细胞表面黏附蛋白,逐渐向间充质状态过渡,而这一过程中,会不断向外环境分泌刺激因子,降低细胞周围基质的致密性,使得原本固定在位的恶性肿瘤细胞,获得向周围浸润或者远处转移的能力 [9] 。总而言之,细胞恶变引起的内外环境改变,赋予了恶性肿瘤增殖的生命力、侵袭性的特征。然而,细胞恶变引起的异常代谢过程往往是比较脆弱的,一旦外界干预破坏恶性肿瘤的异常代谢,往往就能有效阻断癌细胞的活力和增殖 [10] 。因此,针对肿瘤细胞的代谢脆弱性,目前研究者普遍认为,这是治疗肿瘤的具有前景的靶向方案。胱氨酸成瘾的代谢改变也是肿瘤常见的一类表观遗传代谢改变,尤其是在乳腺癌当中,常常为研究者关注。恶变的细胞中,细胞外二硫化物胱氨酸通过胱氨酸谷氨酸转运体持续转入细胞内,这是细胞内胱氨酸主要来源,随后转化成半胱氨酸,与其他氨基酸反应共同组合成谷胱甘肽(glutathione, r-glutamyl cysteingl + glycine, GSH),而GSH还会进一步参与磷脂过氧化氢谷胱甘肽过氧化物酶4 (Phospholipid Hydroperoxide Glutathione Peroxidase 4, GPX4)的合成 [11] 。细胞内,GPX4具有对抗活性氧、羟基自由基等超氧化物的功能,而这些强氧化性物质可以破坏细胞内部细胞器、细胞膜,诱导细胞的死亡 [11] 。只要减弱抗氧化系统或者增强氧化反应过程,就可以使得更多氧化性物质产生,达到抑制肿瘤的目的。恶变细胞的胱氨酸代谢途径同样是脆弱的 [12] 。由于恶性肿瘤肿瘤细胞的胱氨酸依赖性的特点,Erastin作为胱氨酸谷氨酸交换体抑制剂被研究者发现,并且研究证实,Erastin抑制胱谷交换体,细胞中GSH、GPX4的合成显著减少,而活性氧等强氧化性物质显著增加,脂质过氧化反应迅速增加 [12] ,并且这一过程与铁死亡密切关联 [13] [14] [15] 。针对胱氨酸代谢异常的癌细胞,Erastin的治疗效果已被证明是一种抑制癌细胞的可靠方法,并且这种方法仅限制某一种氨基酸,这种药物在人体应用可行性来说是比较高的 [16] ,但Erastin在临床研究还是相对较少。然而,令人费解的是,三阴性乳腺恶性肿瘤对胱氨酸并没有成瘾性,并且对Erastin的治疗没有明显的效果 [17] ,存在药物抗性。所以,有学者提出,通过药物的干预使得TNBC细胞表观遗传特征发生变化,也许可以作为一种改善其胱氨酸代谢和降低癌细胞药物抗性的有效方式 [18] 。
比如,这其中就包括了组蛋白去乙酰化酶6 (Histone Deacetylase 6, HDAC6)抑制剂,在传统治疗方案当中,HDAC6抑制剂,通过促使α-微管蛋白高乙酰化,诱导恶性肿瘤的死亡 [19] 。近年来,常常作为佐剂,增强其他化疗药物或者靶向药物致死癌细胞的效果,尤其是产生药物抗性的恶性肿瘤,它可以通过直接损伤癌细胞DNA来转变癌细胞对抗肿瘤药物敏感性 [20] [21] 。有学者发现,在胱氨酸依赖性的管腔亚型或者具有较高侵袭性、转移性类型恶性肿瘤当中,出现了DNA高甲基化。这种表观遗传差异引起不同胱氨酸代谢的恶性肿瘤,对胱氨酸依赖性的代谢特征起决定作用 [22] [23] 。因此,我们尝试为非间充质TNBC寻找死亡增敏剂,将胱氨酸不敏感的TNBC细胞转变为胱氨酸敏感的TNBC,增强Erastin对TNBC死亡诱导。通过查阅文献及表观遗传化合物库 [24] [25] [26] 筛选,我们发现HDAC6抑制剂Tubaicn似乎是一种理想的佐剂,为了验证Erastin联合Tubacin对TNBC细胞致死效果,所以我们展开了进一步研究。
2. 材料及方法
2.1. 细胞来源及主要试剂
三阴型乳腺癌细胞HCC38细胞购自ATCC;Erastin、Tubacin购自Cayman Chemicals;7-Aminoactinomycin D (7-AAD)、DMSO购自ThermoFisher Scientific;DMEM培养基购自Hyclone;EDTA胰酶消化液购自Biosharp;PBS购自Boster、ROS探针均购自南京建成生物研究所。
2.2. 细胞活力实验
严格按照说明书,通过7-AAD染色HCC38细胞,对细胞活力进行测定。将7-AAD原液5 μl加入到在缓冲液中配成100 μl的7-AAD工作液,在培养基的96孔板中培养的HCC38细胞中,37℃孵育细胞5分钟,并使用流式细胞仪检测发光强度。
2.3. 活性氧的测定
按照ROS测定试剂盒的说明,将细胞与2¢,7¢-二氯二氢荧光素二乙酸酯(DCFH-DA)探针在黑暗中孵育30分钟。然后,在荧光微孔板下观察荧光强度。受激光波长为485 nm,发射光波长为525 nm。ROS水平(%) = 干预组/对照组的荧光值100%。
3. 结果
3.1. Erastin以浓度梯度的方式培养HCC38,细胞死亡无明显变化
正常细胞恶变过程中,大多数都会出现氨基酸代谢异常,其中癌细胞增殖过程中的胱氨酸成瘾性的特征较为常见,因此,针对癌细胞胱氨酸代谢脆性,研制出各种有效的靶向药物,其中,Erastin作为胱氨酸谷氨酸交换体抑制剂被研究者发现。然而,三阴型乳腺恶性肿瘤细胞生长和增殖过程中,并不过分依赖胱氨酸。因此,为了验证单用Erastin处理三阴型乳腺癌细胞的实际效果。我们将HCC38细胞以Erastin浓度倍数增加的条件下进行培养,并用7-AAD试剂及流式检测细胞死亡情况。结果显示,随着Erastin药物浓度增加,HCC38细胞死亡无明显变化,见图1。
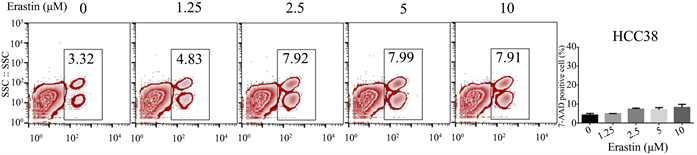
Figure 1. Cell death of triple negative breast cancer treated by Erastin
图1. Erastin处理的三阴型乳腺癌细胞死亡情况
3.2. Tubacin以浓度梯度的方式培养HCC38,死亡没有明显变化
Tubacin是细胞表观遗传特征的抑制剂,在各类研究当中,常常作为佐剂,影响癌细胞的分子代谢,与其他化疗或者靶向药物联合杀死肿瘤细胞 [27] [28] 。并且,我们发现Tubaicn也可以用来改变癌细胞胱氨酸不敏感的代谢特点,这为Erastin对TNBC治疗提供了新思路。因此,为了验证Tubacin处理三阴型乳腺癌细胞的实际效果。我们将HCC38细胞以Tubacin浓度倍数增加的条件下进行培养,并用7-AAD试剂染色细胞,流式检测细胞死亡情况。结果显示,随着Tubacin药物浓度增加,三阴型乳腺癌细胞死亡仅少量增加,但细胞死亡幅度变化不明显,见图2。
3.3. Erastin + Tubacin处理HCC38,细胞死亡显著增加
根据上述数据,我们证实Tubacin、Erastin单独使用时并不能有效抑制TNBC细胞,接下来,我们将联合二者处理细胞,观察细胞死亡。我们先将Erastin浓度取固定的观察值5 μM/L,同时加入浓度以倍数增加的Tubacin,对HCC38进行处理。我们观察到,随着Tuabcin浓度增加,7-AAD阳性细胞比率越大,细胞死亡越多,见图3。
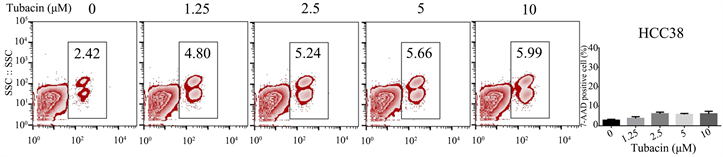
Figure 2. Cell death of triple negative breast cancer treated by Tubain
图2. Tubacin处理的三阴型乳腺癌细胞死亡情况
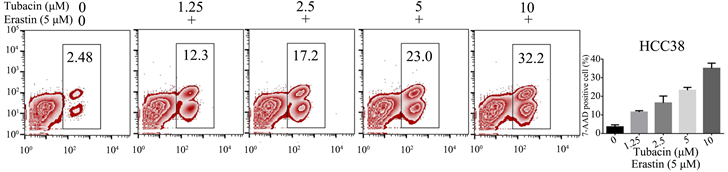
Figure 3. Cell death in triple negative breast cancer treated with Tubacin in combination with Erastin
图3. Tubacin联合Erastin处理的三阴型乳腺癌细胞死亡情况
3.4. 与DMSO、Tub、Era相比,Era + Tub组别中死亡显著增加
为了清晰观察Tuabcin、Erastin与细胞死亡的关系,在上述实验基础上,我们选择合适浓度为5 μmol/L,归纳成DMSO、Tubacin、Erastin、Tubacin + Erastin四个条件的组别,对四种条件下细胞死亡情况,进行组内比较,Era + Tub组别当中诱导TNBC细胞死亡效果明显优于相同浓度条件下单独使用Tubacin、Erastin以及空白对照组DMSO。见图4。
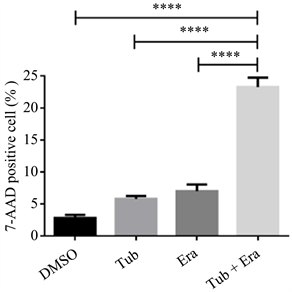
Figure 4. Cell death in TNBC treated with DMSO, Tubacin, Erastin or Erastin in combination with Tubacin
图4. DMSO、Tubacin、Erastin以及Erastin联合Tubacin处理的TNBC细胞死亡情况
3.5. 与DMSO、Tub、Era相比,Era + Tub组别中ROS显著增加
我们使用活性氧(Reactive Oxygen Species, ROS)探针分别检测DMSO、Tub (5 μM/L)、Era (5 μM/L)、Era + Tub。我们观察到在Era、Era + Tub组中活性氧均不同程度升高,其中后者显著增高。见图5。
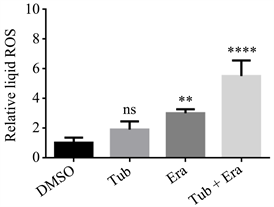
Figure 5. ROS production in TNBC cells treated with DMSO, Tubacin, Erastin or Erastin in combination with Tubacin
图5. DMSO、Tubacin、Erastin、Erastin联合Tubacin处理的TNBC细胞活性氧生成情况
4. 讨论
三阴型乳腺癌是乳腺肿瘤中最具挑战性的亚型,患者的预后差,生存率低,容易出现药物抗性 [29] 。而开发新的靶向药物在治疗上可以提供新的方案和策略。肿瘤的靶向治疗是有前景的治疗策略,可以通过调节肿瘤代谢或者阻断某些分子来治疗肿瘤。尤其是,消耗胱氨酸可以杀死具有胱氨酸依赖的乳腺肿瘤类型,这主要包括高度侵袭性乳腺癌、复发性乳腺癌以及部分luminal型乳腺癌等 [30] [31] 。然而,三阴型乳腺癌增殖和生长并不过分依赖胱氨酸,对Erastin的治疗没有明显反应。因此,Xc-system抑制剂Erastin在治疗TNBC中效果平平,我们的实验中,即便Erastin剂量浓度以倍数增加时细胞死亡幅度仍不明显,这证实三阴型乳腺癌细胞对Erastin治疗不敏感,这种情况也称之为药物抗性。在基础研究中,肿瘤表观遗传的改变往往是自身发展和促进药物抗性的重要因素 [32] [33] 。因此,许多表观遗传激活剂或抑制剂被用作佐剂,以克服肿瘤细胞的耐药性,增强药物治疗效果。在前人研究基础上及表观遗传学库筛选,我们发现Tubacin可以作为佐剂,转变恶性肿瘤的胱氨酸不敏感情况,并协助化疗药物或者靶向药物抗肿瘤治疗。因此,我们尝试将Tubacin加入Erastin中,验证二者联合用药,是否能增强TNBC细胞的死亡。在实验中,我们观察到仅仅单独使用Tubaicn或者Erastin处理HCC38细胞,即便药物浓度以倍数增长,细胞死亡情况并不明显。随后,我们将Erastin取5 μmol/L作为观察浓度,再次以浓度倍数递增方式加入Tubacin,观察到细胞死亡情况显著增加。为了清晰阐述Tubacin、Erastin与TNBC细胞死亡之间的联系,基于已获取的数据,我们归纳DMSO、Tub (5 μM/L)、Era (5 μM/L)、Era + Tub四个组别。与DMSO、Tub、Era组相比较,Era + Tub组细胞死亡情况显著增高。因此,我们可以确定Erastin联合Tubacin可以显著增强三阴型乳腺癌细胞死亡。此外,在前人研究中,Erastin是一种谷氨酸胱氨酸转运体抑制剂,也是铁死亡诱导剂,可以通过抑制谷氨酸胱氨酸转运体阻止GSH/GPX-4的合成,使得强氧化性物质相对增多,这其中就包括活性氧,最终诱导细胞死亡 [34] 。因此,对ROS的检测也常常视为铁死亡的非特异性检测指标。在我们的实验中,我们通过活性氧(ROS)探针 [35] ,初步检测出各组ROS产生情况,我们观察到,与DMSO相比,Tub、Era、Era + Tub均不同程度的增高,后两者显著增高。然而,由于ROS并不是细胞铁死亡特异性标志物 [36] ,并不能为鉴定细胞铁死亡提供直接的证据。因此,对于细胞死亡类型我们尚不能明确,但是联合用药过程中包括活性氧在内的强氧化物质增多是可以确定的,而这或许是细胞致死主要因素之一。并且,在接下来的实验中,我们也将继续沿着细胞死亡类型进行研究。无论如何,Tubacin的发现,让我们为TNBC的靶向治疗提供新的思路,我们期待今后有更多类似Tubacin的佐剂,能够协助Erastin,增强对TNBC细胞的打击。
基金项目
海南省重点研发计划(ZDYF2021SHFZ248)。
文章引用
吴灿章,敬 波,郑武平,吴煌福. Erastin联合Tubacin促进乳腺癌HCC38细胞死亡
Erastin plus Tubacin Treatment Promotes Breast Cancer Cell Death of HCC38[J]. 临床医学进展, 2023, 13(05): 8733-8740. https://doi.org/10.12677/ACM.2023.1351221
参考文献
- 1. Garrido-Castro, A.C., Lin, N.U. and Polyak, K. (2019) Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discovery, 9, 176-198. https://doi.org/10.1158/2159-8290.CD-18-1177
- 2. Jiang, Y.Z., Ma, D., Suo, C., et al. (2019) Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell, 35, 428-440. https://doi.org/10.1016/j.ccell.2019.02.001
- 3. Cho, B., Han, Y., Lian, M., et al. (2019) Evaluation of Racial/Ethnic Differences in Treatment and Mortality among Women with Triple-Negative Breast Cancer. JAMA Oncolo-gy, 7, 1016-1023.
- 4. Poggio, F., Bruzzone, M., Ceppi, M., et al. (2018) Platinum-Based Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis. Annals of Oncology, 29, 1497-1508. https://doi.org/10.1093/annonc/mdy127
- 5. Lyons, T.G. (2019) Targeted Therapies for Triple-Negative Breast Cancer. Current Treatment Options in Oncology, 20, Article No. 82. https://doi.org/10.1007/s11864-019-0682-x
- 6. Wu, S.Y., Xu, Y., Chen, L., et al. (2022) Combined Angiogenesis and PD-1 Inhibition for Immunomodulatory TNBC: Concept Exploration and Biomarker Analysis in the FUTURE-C-Plus Trial. Molecular Cancer, 21, Article No. 84. https://doi.org/10.1186/s12943-022-01536-6
- 7. Hanahan, D. and Weinberg, R.A. (2011) Hallmarks of Cancer: The Next Generation. Cell, 144, 646-674. https://doi.org/10.1016/j.cell.2011.02.013
- 8. Hainaut, P. and Plymoth, A. (2013) Targeting the Hallmarks of Cancer: Towards a Rational Approach to Next- Generation Cancer Therapy. Current Opinion in Oncology, 25, 50-51. https://doi.org/10.1097/CCO.0b013e32835b651e
- 9. Sui, S., Xu, S. and Pang, D. (2022) Emerging Role of Fer-roptosis in Breast Cancer: New Dawn for Overcoming Tumor Progression. Pharmacology & Therapeutics, 232, Article ID: 107992. https://doi.org/10.1016/j.pharmthera.2021.107992
- 10. Song, C.W., Clement, J.J. and Levitt, S.H. (1976) Preferen-tial Cytotoxicity of 5-Thio-D-glucose against Hypoxic Tumor Cells. JNCI: Journal of the National Cancer Institute, 57, 603-605. https://doi.org/10.1093/jnci/57.3.603
- 11. Badgley, M.A., Kremer, D.M., Maurer, H.C., et al. (2020) Cysteine Depletion Induces Pancreatic Tumor Ferroptosis in Mice. Science, 368, 85-89. https://doi.org/10.1126/science.aaw9872
- 12. Xu, T., Ding, W., Ji, X., et al. (2019) Molecular Mechanisms of Ferroptosis and Its Role in Cancer Therapy. Journal of Cellular and Molecular Medicine, 23, 4900-4912. https://doi.org/10.1111/jcmm.14511
- 13. Schonberg, D.L., Miller, T.E., Wu, Q., et al. (2015) Preferential Iron Trafficking Characterizes Glioblastoma Stem- Like Cells. Cancer Cell, 28, 441-455. https://doi.org/10.1016/j.ccell.2015.09.002
- 14. Chen, M.S., Wang, S.F., Hsu, C.Y., et al. (2017) CHAC1 Degra-dation of Glutathione Enhances Cystine-Starvation- Induced Necroptosis and Ferroptosis in Human Triple Negative Breast Cancer Cells via the GCN2-eIF2α-ATF4 Pathway. Oncotarget, 8, 114588-114602. https://doi.org/10.18632/oncotarget.23055
- 15. Yang, W.S., SriRamaratnam, R., Welsch, M.E., et al. (2014) Regu-lation of Ferroptotic Cancer Cell Death by GPX4. Cell, 156, 317-331. https://doi.org/10.1016/j.cell.2013.12.010
- 16. Bansal, A. and Simon, M.C. (2018) Glutathione Metabolism in Cancer Progression and Treatment Resistance. Journal of Cell Biology, 217, 2291-2298. https://doi.org/10.1083/jcb.201804161
- 17. Tang, X., Ding, C.K., Wu, J., et al. (2017) Cystine Addiction of Tri-ple-Negative Breast Cancer Associated with EMT Augmented Death Signaling. Oncogene, 36, 4235-4242. https://doi.org/10.1038/onc.2016.394
- 18. Rodenhiser, D.I. andrews, J., Kennette, W., et al. (2008) Epigenetic Mapping and Functional Analysis in a Breast Cancer Metastasis Model Using Whole-Genome Promoter Tiling Microar-rays. Breast Cancer Research, 10, R62. https://doi.org/10.1186/bcr2121
- 19. Komatsu, S., Moriya, S., Che, X.F., et al. (2013) Combined Treatment with SAHA, Bortezomib, and Clarithromycin for Concomitant Targeting of Aggresome Formation and Intracellular Proteolyt-ic Pathways Enhances ER Stress- Mediated Cell Death in Breast Cancer Cells. Biochemical and Biophysical Research Communications, 437, 41-47. https://doi.org/10.1016/j.bbrc.2013.06.032
- 20. Namdar, M., Perez, G., Ngo, L., et al. (2010) Selective Inhibition of Histone Deacetylase 6 (HDAC6) Induces DNA Damage and Sensitizes Transformed Cells to Anticancer Agents. Proceedings of the National Academy of Sciences of the United States of America, 107, 20003-20008. https://doi.org/10.1073/pnas.1013754107
- 21. Li, T., Zhang, C., Hassan, S., et al. (2018) Histone Deacetylase 6 in Cancer. Journal of Hematology & Oncology, 11, Article No. 111. https://doi.org/10.1186/s13045-018-0654-9
- 22. Feinberg, A.P. (2007) Phenotypic Plasticity and the Epigenetics of Human Disease. Nature, 447, 433-440. https://doi.org/10.1038/nature05919
- 23. Elsheikh, S.E., Green, A.R., Rakha, E.A., et al. (2009) Global Histone Modifications in Breast Cancer Correlate with Tumor Phenotypes, Prognostic Factors, and Patient Outcome. Cancer Re-search, 69, 3802-3809. https://doi.org/10.1158/0008-5472.CAN-08-3907
- 24. Lee, S.W., Yeon, S.K., Kim, G.W., et al. (2021) HDAC6-Selective Inhibitor Overcomes Bortezomib Resistance in Multiple Myeloma. International Journal of Molecular Sciences, 22, Article 1341. https://doi.org/10.3390/ijms22031341
- 25. Yu, S., Cai, X., Wu, C., et al. (2017) Targeting HSP90-HDAC6 Regu-lating Network Implicates Precision Treatment of Breast Cancer. International Journal of Biological Sciences, 13, 505-517. https://doi.org/10.7150/ijbs.18834
- 26. Marcus, A.I., Zhou, J., O’Brate, A., et al. (2005) The Synergistic Combination of the Farnesyl Transferase Inhibitor Lonafarnib and Paclitaxel Enhances Tubulin Acetylation and Requires a Functional Tubulin Deacetylase. Cancer Research, 65, 3883-3893. https://doi.org/10.1158/0008-5472.CAN-04-3757
- 27. Aldana-Masangkay, G.I., Rodriguez-Gonzalez, A., Lin, T., et al. (2011) Tubacin Suppresses Proliferation and Induces Apoptosis of Acute Lymphoblastic Leukemia Cells. Leukemia & Lymphoma, 52, 1544-1555. https://doi.org/10.3109/10428194.2011.570821
- 28. Zuo, Q., Wu, W., Li, X., et al. (2012) HDAC6 and SIRT2 Promote Bladder Cancer Cell Migration and Invasion by Targeting Cortactin. Oncology Reports, 27, 819-824.
- 29. Kim, C., Gao, R., Sei, E., et al. (2018) Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell, 173, 879-893.e13. https://doi.org/10.1016/j.cell.2018.03.041
- 30. Romayor, I., García-Vaquero, M.L., Márquez, J., et al. (2022) Discoidin Domain Receptor 2 Expression as Worse Prognostic Marker in Invasive Breast Cancer. The Breast Journal, 2022, Article ID: 5169405. https://doi.org/10.1155/2022/5169405
- 31. Wu, C., Ying, J., Dai, M., Peng, J., et al. (2022) Co-Expression of DDR2 and IFITM1 Promotes Breast Cancer Cell Proliferation, Migration and Invasion and Inhibits Apoptosis. Journal of Cancer Research and Clinical Oncology, 148, 3385-3398. https://doi.org/10.1007/s00432-022-04110-1
- 32. Brown, R., Curry, E., Magnani, L., et al. (2014) Poised Epige-netic States and Acquired Drug Resistance in Cancer. Nature Reviews Cancer, 14, 747-753. https://doi.org/10.1038/nrc3819
- 33. Dworkin, A.M., Huang, T.H. and Toland, A.E. (2009) Epigenetic Alterations in the Breast: Implications for Breast Cancer Detection, Prognosis and Treatment. Seminars in Cancer Biology, 19, 165-171. https://doi.org/10.1016/j.semcancer.2009.02.007
- 34. Zhao, Y., Li, Y., Zhang, R., et al. (2020) The Role of Erastin in Ferroptosis and Its Prospects in Cancer Therapy. OncoTargets and Therapy, 13, 5429-5441. https://doi.org/10.2147/OTT.S254995
- 35. Hao, H., Cao, L., Jiang, C., et al. (2017) Farnesoid X Receptor Regula-tion of the NLRP3 Inflammasome Underlies Cholestasis-Associated Sepsis. Cell Metabolism, 25, 856-867.e5. https://doi.org/10.1016/j.cmet.2017.03.007
- 36. Zheng, D., Liu, J., Piao, H., et al. (2022) ROS-Triggered Endothe-lial Cell Death Mechanisms: Focus on Pyroptosis, Parthanatos, and Ferroptosis. Frontiers in Immunology, 13, Article ID: 1039241. https://doi.org/10.3389/fimmu.2022.1039241
NOTES
*第一作者。
#通讯作者。