Advances in Environmental Protection
Vol.
09
No.
03
(
2019
), Article ID:
30984
,
10
pages
10.12677/AEP.2019.93061
Influence of Bioturbation by Benthic Organisms on the Distribution and Bioavailability of Methylmercury in Estuary Sediment
Chi Zhang, Rui Wang*, Jiaoyang Yin
Key Laboratory of Yangtze River Water Environment, Ministry of Education, College of Environmental Science and Engineering, Tongji University, Shanghai

Received: June 5th, 2019; accepted: June 20th, 2019; published: June 27th, 2019

ABSTRACT
Mercury (Hg) is a toxic heavy metal which widely distributed globally. In aquatic ecosystems, sediment acts as important sink and source for Hg, and its distribution and bioavailability can be affected by many factors, including bioturbation by benthic organisms which can modify the physicochemical properties of sediment. In the present study, we chose nereid (Neanthes japonica) as the experimental animal to investigate the effects of bioturbation on methylmercury distribution and bioavailability in estuary sediment, and explored the underlying mechanism. We found that 1) Methylmercury was significantly bioaccumulated by Nereid during exposure. 2) Methylmercury in bioturbated sediment increased gradually during exposure and finally exceeded the methylmercury concentration in non-bioturbated sediment, however, the uptake rate decreased with exposure time. 3) Bioturbated sediment had higher level of organic matter, which might be related with the living activities of benthic organisms, including mucus secretion, burrowing and feeding activities. 4) The bioavailability assessment using Bovine Serum Albumin (BSA) extraction method revealed that the bioavailability of methylmercury in sediment decreased with exposure time, which was consistent with the decreased uptake rate by Nereid. We suggest that bioturbation by Nereid increased the organic content in sediment, which enhanced the binding capacity with methylmercury in the surrounding sediment, thus reducing the bioavailability of methylmercury.
Keywords:Bioturbation, Methylmercury, Sediment, Bioavailability
底栖生物扰动对河口沉积物中甲基汞分布及生物可利用性的影响
张弛,王锐*,阴皎阳
同济大学环境科学与工程学院,长江水环境教育部重点实验室,上海

收稿日期:2019年6月5日;录用日期:2019年6月20日;发布日期:2019年6月27日

摘 要
汞是全球广泛分布的有毒重金属。沉积物是汞在水生生态系统中的重要“源”和“汇”,但生物扰动等因素可能通过改变沉积物的理化性质,影响汞的分布和生物可利用性。本研究选取了底栖生物沙蚕作为研究对象,探究了生物扰动对于甲基汞在沉积物中的分布和生物可利用性的影响,并探讨了该影响的机理。研究发现,1) 沙蚕对甲基汞有较强的吸收能力;2) 暴露过程中生物周边沉积物汞含量逐渐升高,并最终高于非周边沉积物,但沙蚕对汞的吸收速率放缓;3) 生物周边沉积物中,有机质含量显著提高,这可能与沙蚕分泌物、掘穴与摄食等活动有关;4) 利用牛血清蛋白对沉积物中汞的生物可利用性评估发现,沉积物生物可利用性随暴露时间降低,这与沙蚕对汞吸收速率的放缓一致。我们推测沙蚕扰动提高了沉积物中有机质含量,增加了周边沉积物与甲基汞结合的能力,从而使得汞的生物可利用性有所下降。
关键词 :生物扰动,甲基汞,沉积物,生物可利用性

Copyright © 2019 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
汞是一种常见的重金属污染物,以甲基汞为代表的有机汞因其毒性强、易吸收富集、难排出的特点而受到广泛关注。经由自然途径和人为途径排放进入环境的汞,经过复杂的迁移和转化,最终有98%以上存留在沉积物中 [1] 。沉积物在汞的生物地球化学循环中,起着“源”和“汇”的重要作用。受到外界因素影响时,沉积物中大量汇聚的汞可能被重新释放,对生态环境造成污染 [2] [3] [4] 。生物扰动 [5] (bioturbation)也是影响沉积物汞的生物地球化学过程的主要因素之一。研究发现,沉积物底栖生物通过分泌粘液、掘穴、摄食、生物淋洗等行为可以对其周边的沉积物产生影响,包括增加有机质含量 [6] 、促进沉积物内的氧化作用 [7] 、改变沉积物微环境 [8] 、影响微生物活动 [9] 等,从而影响汞的分布。随着对汞污染研究的深入,人们发现不能用汞浓度作为评价污染状况的唯一标准 [10] [11] [12] ,汞的形态、环境理化性质和其他物质(例如硒)等都会影响汞的吸收和累积,而汞的生物可利用性是表征汞的生态风险的重要指标。
目前,针对底栖生物活动在沉积物汞分布中的作用,已有较多研究进行了探究和阐述。一方面,底栖生物的活动在宏观上影响了沉积物中甲基汞含量 [13] [14] 和沉积物–水之间的物质交换通量 [15] [16] ;另一方面,从微观角度出发,沉积物的有机质、铁锰氧化物、酸可挥发性硫化物等理化性质 [6] [8] [15] [17] [18] ,与汞的分布存在直接联系,这些研究也进一步揭示了底栖生物活动对这些理化性质可能存在影响。然而,少有研究直接验证过底栖生物对沉积物结合汞能力的影响,本研究沿图1所示的技术流程,利用河口沉积物与底栖生物搭建了实验室暴露体系,研究了底栖生物沙蚕的扰动对沉积物中甲基汞分布状况及沉积物与汞结合能力的影响,利用BSA (牛血清蛋白)萃取法对汞生物可利用性进行了评估,并结合沙蚕体内汞的生物累积结果对生物扰动的影响机制进行了初步探索。

Figure 1. Technical flow chart of this study
图1. 本研究的技术流程图
2. 材料与方法
2.1. 受试生物、试剂及实验仪器
本次研究所用的沙蚕(Neanthes japonica)购自上海亿鑫水产品有限公司,沉积物采集自黄浦江入长江口表层(31.3˚N, 121.5˚E)。选取体型适中且相近的沙蚕在沉积物中饲养,饲养条件如表1。
Table 1. Nereid culturing condition in the laboratory
表1. 沙蚕的实验室饲养条件
主要试剂和仪器:氯化甲基汞(MeHgCl, 96%, Dr. Ehrenstorfer GmbH),牛血清蛋白,河口沉积物标准品GBW-07334 (中国标准物质中心),沉积物标准品ERM-CC580 (National Institutes Standard and Technology),硝酸(HNO3,65%, MERK),过氧化氢(H2O2,30%,国药集团化学试剂有限公司);微波消解仪(CEM,MARS,USA),ICP-MS系统(7700x, Agilent,USA)。
2.2. 有无生物扰动下沉积物中汞分布的探究
沙蚕经驯养一个月之后,在实验室构建的“沉积物–沙蚕–水”微暴露体系中进行试验,如图2。取500 mL烧杯若干,每只烧杯加入湿重300 g沉积物,搅拌均匀后静置3 d;再加入50 mL含0.2% (v/v) 7 mmol/L MeHgCl的人工海水(人工海水按表2所示配制),搅拌均匀后静置3 d;最后加入100 mL人工海水并曝气。曝气3 d后开始放入沙蚕,记为暴露第0天;分别在暴露第0、2、7、14天采集沙蚕、沙蚕周边沉积物(明显受到沙蚕活动影响的沉积物)和非周边沉积物(取出周边沉积物后,利用四分法对剩余沉积物取样所得)。

Figure 2. “Sediment-benthic organism-water” exposure system
图2. “沉积物–底栖生物–水”暴露系统
Table 2. Chemical composition of artificial sea water
表2. 人工海水的化学成分
为研究无生物扰动条件下该暴露体系中沉积物汞浓度的稳定性,我们按照上述步骤设置无生物对照组,与有生物实验组类似,在系统稳定9天后,于第2、7、14天取沉积物样,取样时采用四分法收集沉积物。
2.3. 生物扰动下沉积物甲基汞结合能力评估
在清洁沉积物中饲养沙蚕14天后,取沙蚕周边沉积物和非周边沉积物各50 g (湿重),加入50 mL含0.2% (v/v) 7 mmol/L MeHgCl的人工海水搅匀,在150 rpm、20℃下振荡,分别在振荡6 h、12 h、24 h时取出,以3000 rpm离心20 min,取下层沉淀待处理。
2.4. 生物扰动下甲基汞的生物可利用性评估
体外消化液萃取法可以在体外利用生物提取或人工配制的模拟消化液,从食物中提取污染物,以此模拟污染物在生物消化道内的生化过程,估测其生物可利用性,该方法已经有广泛的使用 [19] [20] [21] 。BSA因含有6%的-SH位点,可以很好地模拟消化液中蛋白质对汞的萃取 [20] [22] 。从2.2中每次获得的沙蚕周边沉积物取1 g,加入10 mL 5 g/L BSA溶液,在150 rpm、20℃下振荡5 h,取出后1600 rpm离心10 min取上清液待测。
2.5. 样品的预处理和测定
在测定总汞的过程中,对取得的沉积物和沙蚕样品进行冷冻干燥和充分研磨(沉积物样品需要再过200目筛)后取0.05 g左右,利用混酸体系(6 mL HNO3 + 1 mL H2O2)和微波消解仪在180℃下消解30 min;对取得的水样和萃取液,取4 mL水样或5 mL萃取液以与上述相同的消解手段处理。消解液使用0.45 μm滤膜过滤,并以2% (v/v) HNO3稀释后,采用ICP-MS系统进行总汞含量的测定。
2.6. 沙蚕对甲基汞吸收速率常数的计算
在暴露过程中,沙蚕和沉积物内的汞含量始终处于动态变化的状态,为了评估沙蚕对汞的实际吸收速率,我们利用以下公式对沙蚕的汞吸收速率常数,即单位时间内从周边沉积物吸收汞的量进行了计算。
(1)
:吸收速率常数,d−1,
:沙蚕体内汞浓度随时间的变化率,μg∙g−1∙d−1,
:两次取样间隔中,沙蚕周边沉积物汞含量的平均值,μg∙g−1。
3. 结果与讨论
3.1. 无生物条件下汞在沉积物中的分布
我们发现,沉积物经过9天的稳定后,在后续的14天内,汞含量始终稳定保持在0.55 μg/g左右,未发生显著波动(图3)。因此可以认为,在本次研究所设置的暴露条件下,汞可以稳定地存在于沉积物中,为沙蚕提供较稳定的暴露环境,外部条件施加的干扰可以忽略。

Figure 3. Mercury distribution in sediment without benthic organisms
图3. 无生物条件下沉积物中汞的分布
3.2. 沙蚕扰动对沉积物汞分布的影响
通过对沉积物汞含量变化的观察,发现在暴露初期,沙蚕周边和非周边沉积物的汞含量均显著下降(图4),可能是由于沙蚕从沉积物中吸收并累积汞所致。在暴露初期,沙蚕周边沉积物中汞含量比非周边沉积物更高,而在暴露第7天和第14天,生物周边沉积物汞含量显著上升,并超过非生物周边沉积物中汞的含量。而在暴露过程中,沙蚕体内的汞含量是持续上升的(图5)。我们推测这一系列现象可能是因为沙蚕的活动改变了其周边沉积物的理化性质,提高了周边沉积物的汞结合能力,使甲基汞向周边沉积物中转移。

Figure 4. Mercury concentration in bioturbated and non-bioturbated sediment during exposure
图4. 沙蚕周边及非周边沉积物汞含量随暴露时间的变化

Figure 5. Mercury concentration in nereids during exposure
图5. 沙蚕体内汞含量随暴露时间的变化
为了进一步探究这一现象,我们以三次取样间隔为界线,计算了沙蚕对周边沉积物甲基汞的平均吸收速率常数 ,结果发现随着暴露时间的延长,沙蚕对甲基汞的吸收速率常数有了明显的降低(表3),说明尽管生物周边的沉积物中甲基汞含量增加了,但沉积物中甲基汞的生物可利用性却在降低。
Table 3. Mercury in nereids and bioaccumulating rate constant during exposure
表3. 暴露过程中沙蚕体内汞含量及吸收速率常数的变化
此外,实验2.3的结果显示(图6),经过14天饲养沙蚕的周边沉积物,在与含汞溶液混合振荡6h后明显较非周边沉积物结合了更高含量的汞,说明在暴露过程中,沙蚕周边沉积物的理化性质可能发生了改变,使之具有更强的甲基汞结合能力。根据过往研究,有机质是体现甲基汞结合能力的重要组分之一,其既可以成为有效的汞吸附剂 [23] ,本身又可以作为甲基化微生物的营养物质,促进汞的甲基化 [24] [25] ;而底栖生物的掘穴、生物淋洗、分泌、排泄等活动,也为沉积物引入了新的有机质,进一步促成了上述过程。

Figure 6. Concentration of mercury binding to both bioturbated and non-bioturbated sediments during incubation
图6. 沙蚕周边及非周边沉积物与汞结合量的变化
为验证沙蚕扰动是否带来了沉积物有机质含量的变化,我们将经沙蚕暴露14天扰动后的生物周边及非生物周边沉积物经冷冻干燥后,置于400℃下灼烧8小时,以LOI (烧失比,灼烧前后质量差与灼烧前质量之比)来表征有机质含量。结果可以发现(图7)周边沉积物的LOI显著高于非周边沉积物,即沙蚕的扰动确实导致了有机质的增加。生物周边沉积物有机质的增加可能来自于:1) 沙蚕分泌物本身 [26] 或分泌物从水中吸附的有机碎片 [27] 含有汞;2) 沙蚕分泌物及其吸附碎片含有易结合汞的官能团;3) 以沙蚕分泌物吸附的有机碎片为食的微生物在周边沉积物内繁殖,对汞具有更强富集能力 [6] 。
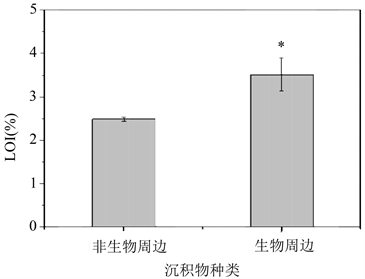
Figure 7. LOI in both bioturbated and non-bioturbated sediments; *refers to significant difference (p < 0.05)
图7. 沙蚕周边及非周边沉积物的烧失比;*代表具有显著差异(p < 0.05)
此外,沙蚕活动可能导致了沉积物中氧化还原电位的改变,掘穴和生物淋洗可引入氧气及含氧水体,改变沉积物的氧化还原电位 [7] [28] ,同时促进相关铁氧化微生物的活动 [18] ,增加沉积物中铁锰氧化物的含量,而铁锰氧化物具有表面积大、吸附能力强的特点 [29] ,可以进一步增加汞的聚集。在取样过程中也观察到了周边沉积物和非周边沉积物之间颜色的差异,可能预示着沙蚕活动增强了周边沉积物中的氧化活动,但具体理化性质的变化还需进一步研究。
3.3. 沙蚕扰动对其周边沉积物汞生物可利用性的影响
我们进一步利用BSA对沉积物中汞的生物可利用性进行评估,发现可萃取汞百分比在暴露过程中逐渐降低(图8),表明其生物可利用性降低,这与沙蚕在暴露后吸收速率常数的降低相一致。结合沉积物中有机质,我们认为沙蚕的分泌物和掘穴、摄食等过程提高了沉积物中有机质的含量,富含有机质的沉积物颗粒和甲基汞紧密结合,可能会阻止汞在底栖动物消化道内溶出及生物吸收 [30] ,从而减缓汞在沙蚕体内的生物累积速率。

Figure 8. BSA extractable mercury in bioturbated sediment
图8. 沙蚕周边沉积物中可被BSA萃取的汞含量变化
4. 结论
本研究通过构建沉积物-沙蚕-水体的微暴露系统,对比分析了沙蚕扰动下位于生物周边与非周边沉积物中汞的分布情况,并通过对沉积物与甲基汞结合能力评估及利用BSA萃取方法评估汞的生物可利用性等,对底栖生物活动对于沉积物中汞分布及生物可利用性的影响机理进行了初步探究。研究发现:
1) 沙蚕对甲基汞有较强的吸收能力,使其在沉积物中的含量短期内显著下降;
2) 经沙蚕暴露扰动后,生物周边沉积物汞含量显著高于非生物周边沉积物,但沙蚕对甲基汞的平均吸收速率常数随暴露时间延长有明显下降;
3) 生物周边沉积物中,有机质含量显著提高,沉积物结合甲基汞的能力也有所增强,这可能与沙蚕分泌物、掘穴与摄食等活动有关,有待开展进一步的机理探究;
4) 沙蚕扰动导致的生物周边沉积物中有机质的提高,使得其对汞的结合能力提升,但却降低了汞的生物可利用性,从而使得沙蚕在暴露后期对甲基汞的吸收速率放缓。
基金项目
本研究受到上海市教育委员会和上海市教育发展基金会“晨光计划”项目(14CG17)支持。
文章引用
张 弛,王 锐,阴皎阳. 底栖生物扰动对河口沉积物中甲基汞分布及生物可利用性的影响
Influence of Bioturbation by Benthic Organisms on the Distribution and Bioavailability of Methylmercury in Estuary Sediment[J]. 环境保护前沿, 2019, 09(03): 439-448. https://doi.org/10.12677/AEP.2019.93061
参考文献
- 1. Clarkson, T.W., Hamada, R. and Amin-Zaki, L. (1984) Mercury. In: Nriagu, J.O., Ed., Changing Metal Cycles and Human Health, Springer-Verlag, Berlin, 285-309. https://doi.org/10.1007/978-3-642-69314-4_17
- 2. Balcom, P.H., Hammerschmidt, C.R., Fitzgerald, W.F., Lamborg, C.H. and O’Connor, J.S. (2008) Seasonal Distributions and Cycling of Mercury and Methylmercury in the Waters of New York/New Jersey Harbor Estuary. Marine Chemistry, 109, 1-17. https://doi.org/10.1016/j.marchem.2007.09.005
- 3. Kim, D., Wang, Q., Sorial, G.A., Dionysiou, D.D. and Timberlake, D. (2004) A Model Approach for Evaluating Effects of Remedial Actions on Mercury Speciation and Transport in a Lake System. Science of the Total Environment, 327, 1-15. https://doi.org/10.1016/j.scitotenv.2003.09.011
- 4. Fairbrother, A., Wenstel, R., Sappington, K. and Wood, W. (2007) Framework for Metals Risk Assessment. Ecotoxicology and Environmental Safety, 68, 145-227. https://doi.org/10.1016/j.ecoenv.2007.03.015
- 5. François, F., Gerino, M., Stora, G., Durbec, J.P. and Poggiale, J.C. (2002) Functional Approach to Sediment Reworking by Gallery-Forming Macrobenthic Organisms: Modeling and Application with the Polychaete Nereis diversicolor. Marine Ecology Progress Series, 229, 127-136. https://doi.org/10.3354/meps229127
- 6. Sizmur, T., Canário, J., Edmonds, S., Godfrey, A. and O’Driscoll, N.J. (2013) The Polychaete Worm Nereis diversicolor Increases Mercury Lability and Methylation in Intertidal Mudflats. Environmental Toxicology and Chemistry, 32, 1888-1895. https://doi.org/10.1002/etc.2264
- 7. Gilbert, F., Stora, G. and Bonin, P. (1998) Influence of Bioturbation on Denitrification Activity in Mediterranean Coastal Sediments: An in Situ Experimental Approach. Marine Ecology Progress Series, 163, 99-107.https://doi.org/10.3354/meps163099
- 8. Caetano, M., Madureira, M.J. and Vale, C. (2003) Metal Remobilisation during Resuspension of Anoxic Contaminated Sediment: Short-Term Laboratory Study. Water, Air, and Soil Pollution, 143, 23-40. https://doi.org/10.1023/A:1022877120813
- 9. Papaspyrou, S., Gregersen, T., Kristensen, E., Christensen, B. and Cox, R.P. (2006) Microbial Reaction Rates and Bacterial Communities in Sediment Surrounding Burrows of Two Nereidid Polychaetes (Nereisdiversicolor and N. virens). Marine Biology, 148, 541-550. https://doi.org/10.1007/s00227-005-0105-3
- 10. Muhaya, B.B.M., Leermakers, M. and Baeyens, W. (1997) Total Mercury and Methylmercury in Sediments and in the Polychaete Nereis diversicolor at Groot Buitenschoor (Scheldt Estuary, Belgium). Water, Air, and Soil Pollution, 94, 109-123. https://doi.org/10.1007/BF02407097
- 11. Mason, R.P. and Lawrence, A.L. (1999) Concentration, Distribution, and Bioavailability of Mercury and Methylmercury in Sediments of Baltimore Harbor and Chesapeake Bay, Maryland, USA. Environmental Toxicology and Chemistry, 18, 2438-2447. https://doi.org/10.1002/etc.5620181109
- 12. Chen, C.Y., Dionne, M., Mayes, B.M., Ward, D.M., Sturup, S. and Jackson, B.P. (2009) Mercury Bioavailability and Bioaccumulation in Estuarine Food Webs in the Gulf of Maine. Environmental Science & Technology, 43, 1804-1810.https://doi.org/10.1021/es8017122
- 13. Steiner, Z., Lazar, B., Levi, S., Tsroya, S., Pelled, O., Bookman, R. and Erez, J. (2016) The Effect of Bioturbation in Pelagic Sediments: Lessons from Radioactive Tracers and Planktonic Foraminifera in the Gulf of Aqaba, Red Sea. Geochimica et Cosmochimica Acta, 194, 139-152. https://doi.org/10.1016/j.gca.2016.08.037
- 14. Benoit, J.M., Shull, D.H., Harvey, R.M. and Beal, S.A. (2009) Effect of Bioirrigation on Sediment-Water Exchange of Methylmercury in Boston Harbor, Massachusetts. Environmental Science & Technology, 43, 3669-3674.https://doi.org/10.1021/es803552q
- 15. Gagnon, C., Pelletier, É. and Mucci, A. (1997) Behaviour of An-thropogenic Mercury in Coastal Marine Sediments. Marine Chemistry, 59, 159-176. https://doi.org/10.1016/S0304-4203(97)00071-6
- 16. Hammerschmidt, C.R. and Fitzgerald, W.F. (2008) Sediment-Water Ex-change of Methylmercury Determined from Shipboard Benthic Flux Chambers. Marine Chemistry, 109, 86-97. https://doi.org/10.1016/j.marchem.2007.12.006
- 17. Hammerschmidt, C.R., Fitzgerald, W.F., Lamborg, C.H., Balcom, P.H. and Visscher, P.T. (2004) Biogeochemistry of Methylmercury in Sediments of Long Island Sound. Marine Chemistry, 90, 31-52. https://doi.org/10.1016/j.marchem.2004.02.024
- 18. Beam, J.P., Scott, J.J., McAllister, S.M., Chan, C.S., McManus, J., Meysman, F.J. and Emerson, D. (2018) Biological Rejuvenation of Iron Oxides in Bioturbated Marine Sediments. The ISME Journal, 12, 1389-1394. https://doi.org/10.1038/s41396-017-0032-6
- 19. Voparil, I.M. and Mayer, L.M. (2004) Commercially Available Chemicals that Mimic a Deposit Feeder’s (Arenicola marina) Digestive Solubilization of Lipids. Environmental Science & Technology, 38, 4334-4339. https://doi.org/10.1021/es049506y
- 20. Zhong, H. and Wang, W.-X. (2008) Methylmercury Extraction from Artificial Sediments by the Gut Juice of the Sipunculan, Sipunculus nudus. Environmental Toxicology and Chemistry, 27, 138-145. https://doi.org/10.1897/07-049.1
- 21. Zhong, H. and Wang, W.-X. (2009) Inorganic Mercury Binding with Different Sulfur Species in Anoxic Sediments and Their Gut Juice Extractions. Environmental Toxicology and Chemistry, 28, 1851-1857. https://doi.org/10.1897/08-539.1
- 22. Zhong, H. and Wang, W.-X. (2006) Sediment-Bound Inorganic Hg Extraction Mechanisms in the Gut Fluids of Marine Deposit Feeders. Environmental Science & Technology, 40, 6181-6186. https://doi.org/10.1021/es061195z
- 23. He, T., Lu, J., Yang, F. and Feng, X. (2007) Horizontal and Vertical Variability of Mercury Species in Pore Water and Sediments in Small Lakes in Ontario. Science of the Total Environment, 386, 53-64.https://doi.org/10.1016/j.scitotenv.2007.07.022
- 24. Lambertsson, L. and Nilsson, M. (2006) Organic Material: The Primary Control on Mercury Methylation and Ambient Methyl Mercury Concentrations in Estuarine Sediments. Environmental Science & Technology, 40, 1822-1829. https://doi.org/10.1021/es051785h
- 25. Gray, J.E. and Hines, M.E. (2009) Biogeochemical Mercury Methylation Influenced by Reservoir Eutrophication, Salmon Falls Creek Reservoir, Idaho, USA. Chemical Geology, 258, 157-167. https://doi.org/10.1016/j.chemgeo.2008.09.023
- 26. Duchêne, J.C. and Rosenberg, R. (2001) Marine Benthic Faunal Activity Patterns on a Sediment Surface Assessed by Video Numerical Tracking. Marine Ecology Progress Series, 223, 113-119. https://doi.org/10.3354/meps223113
- 27. Petrash, D.A., Lalonde, S.V., Gingras, M.K. and Konhauser, K.O. (2011) A Surrogate Approach to Studying the Chemical Reactivity of Burrow Mucous Linings in Marine Sediments. Palaios, 26, 594-600. https://doi.org/10.2110/palo.2010.p10-140r
- 28. Ravichandran, M. (2004) Interactions between Mercury and Dissolved Organic Matter—A Review. Chemosphere, 55, 319-331. https://doi.org/10.1016/j.chemosphere.2003.11.011
- 29. Turner, A. (2000) Trace Metal Contamination in Sediments from UK Estuaries: An Empirical Evaluation of the Role of Hydrous Iron and Manganese Oxides. Estuarine, Coastal and Shelf Science, 50, 355-371.https://doi.org/10.1006/ecss.1999.0573
- 30. Lawrence, A.L. and Mason, R.P. (2001) Factors Controlling the Bioaccumulation of Mercury and Methylmercury by the Estuarine Amphipod Leptocheirus plumulosus. Environmental Pollution, 111, 217-231.https://doi.org/10.1016/S0269-7491(00)00072-5
NOTES
*通讯作者。
