Advances in Clinical Medicine
Vol.
10
No.
12
(
2020
), Article ID:
39226
,
6
pages
10.12677/ACM.2020.1012456
硫化氢经AMPK通路调节自噬保护脓毒症相关性肠黏膜损伤的机制研究
赫曼1,聂静云2,康富贵2,柴琛1*
1苏州高新区人民医院,江苏 苏州
2兰州大学第一临床医学院,甘肃 兰州

收稿日期:2020年11月21日;录用日期:2020年12月11日;发布日期:2020年12月18日

摘要
总结硫化氢经AMPK通路调节自噬保护脓毒症相关性肠黏膜损伤的机制。系统检索近年来关于硫化氢经AMPK通路调节自噬保护脓毒症相关性肠黏膜损伤的机制研究的相关文献并进行综述。硫化氢水平与脓毒症相关,可以保护肠黏膜损伤,其机制可能是通过AMPK信号通路介导自噬作用减轻了炎症反应。硫化氢通过AMPK信号通路调控自噬保护肠黏膜,有望成为脓毒症治疗的新靶点。
关键词
硫化氢,脓毒症,自噬,AMPK信号通路,肠黏膜损伤

Mechanism Research of Hydrogen Sulfide via AMPK Pathway Mediated Autophagy to Protect Intestinal Mucosal Injury in Sepsis
Man He1, Jingyun Nie2, Fugui Kang2, Chen Chai1*
1People’s Hospital of Suzhou New District, Suzhou Jiangsu
2The First Clinical Medical College of Lanzhou University, Lanzhou Gansu

Received: Nov. 21st, 2020; accepted: Dec. 11th, 2020; published: Dec. 18th, 2020

ABSTRACT
To summarize the mechanism of hydrogen sulfide (H2S) via AMPK pathway mediated autophagy to protect intestinal mucosal injury in sepsis. The relevant literatures in recent years were systematically searched to review the mechanism of H2S via AMPK pathway mediated autophagy in sepsis. The level of H2S is related to sepsis with the protected function of the intestinal mucosal injury. The mechanism may be that H2S mediates autophagy by AMPK signaling to reduce the inflammatory response. H2S mediates autophagy to protect intestinal mucosal injury via AMPK pathway in sepsis, which is expected to become an effective therapeutic target for sepsis.
Keywords:Hydrogen Sulfide, Sepsis, Autophagy, AMPK Signal Pathway, Intestinal Mucosal Injury

Copyright © 2020 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
脓毒症是外科和重症监护病房(ICU)危重患者死亡的重要原因之一,在ICU中脓毒症死亡率高达36% [1]。2018年脓毒症定义和治疗指南被再次更新,脓毒症被新定义为因宿主对感染的反应失调而导致的危及生命的器官功能障碍 [2]。肠道是脓毒症发展为多器官功能衰竭的始动器官,因感染、氧化应激、循环障碍等机制使肠道上皮细胞损伤,肠黏膜及免疫屏障完整性遭到破坏导致肠功能衰竭 [3] [4]。肠粘膜上皮的自噬机制在维持上皮细胞的正常功能方面扮演了重要角色 [5]。硫化氢(H2S)是近年来发现的新型气体信号分子,可调节多重信号通路通过自噬减轻炎症,保护肠道功能,可望成为治疗脓毒症的新靶点。
2. H2S对肠黏膜损伤的保护作用
在哺乳动物体内中H2S由L-半胱氨酸和高半胱氨酸内源性产生,主要由两种吡哆醛-5’-磷酸(PLP)依赖性酶组成,称为胱硫醚β-合酶(cystathionine β synthase, CBS)和胱硫醚γ-裂解酶(cystathionine-γ-lyase, CSE)机体内源性生成的H2S可以立即释放到血液循环中,同时也以结合的硫烷硫和酸性不稳定硫的形式储存在细胞中。H2S在线粒体氧化成硫代硫酸盐或硫酸盐,然后被硫代血红蛋白清除,通过肺或肾排泄,以及硫酸和硫醇甲基转移酶等甲基化形成二甲基硫醚和甲硫醇 [6]。研究发现脓毒症大鼠模型(CLP法)血浆及肝脏、肺脏等多组织中H2S浓度升高,其合成关键酶CSE的表达水平和活性也明显增加。另外用H2S生成抑制剂DL-炔丙基甘氨酸(PAG)治疗,不仅降低了组织中H2S含量,而且产生了显著抗炎效应 [7]。因此,H2S与脓毒症的发生和发展密切相关。
研究表明,H2S可以增加胃黏膜血流,减轻由于非甾体类抗炎药引起胃黏膜损伤。其机制可能是通过降低TNF-α、细胞间粘附分子-1 (ICAM-1)、前列腺素E2的表达发挥抗炎作用 [8]。动物实验证实 [9],结肠炎大鼠模型在造模初期肠组织内中性粒细胞浸润增多,同时H2S的浓度也明显升高;给予H2S抑制剂肠黏膜萎缩,组织内MPO活性明显增加。给予外源性H2S后,结肠的炎症程度明显减轻,TNF-α、IL-1β、IFN-γ等炎性因子的表达下调。这些研究提示H2S与肠道炎症相关,在炎症早期内源性H2S升高可能是为保护肠黏膜机体的防御反应;外源性H2S对肠道炎症有治疗作用。
3. 自噬作用保护脓毒症相关器官功能损伤
脓毒症的本质是机体对感染的免疫失衡。在脓毒症的亚急性期,细胞凋亡和自噬是疾病进展的关键事件 [10],机体通过自噬作用维持细胞稳态及细胞自我更新 [11]。自噬与炎症密切相关,可以调节炎症相关转录因子核因子活化B细胞κ轻链增强子(NF-κB)、核因子红细胞2相关因子2 (NFE2L2)的表达,促进死亡细胞的清除,调节吞噬作用等 [12]。
自噬在脓毒症中被激活,负性调节炎症反应,减轻各种组织和器官的炎症损伤。有研究表明,亚铁血红素加氧酶-1介导的自噬可以保护小鼠免受脓毒症造成的肝损伤 [13];调节自噬和凋亡相关基因表达以增强肝脏自噬活性可以提高脓毒症小鼠的存活率 [14];肾近端小管自噬相关基因7 (Atg7)特异性消融可加重脂多糖(LPS)诱导的急性肾损伤(AKI) [15],而自噬激活在内毒素诱导的肾损伤中具有肾保护作用 [16];在脓毒症小鼠模型中,自噬过程减弱导致心功能障碍,通过mTOR激活自噬可恢复心脏功能,减轻对心肌的损伤 [17]。研究还发现,敲除肺自噬相关基因4B (Atg4B)可加重脓毒症相关性肺损伤 [6],增强Atg12依赖性自噬对脓毒症肺损伤具有保护作用 [18]。此外,自噬缺陷小鼠更容易受内毒素的主要成分LPS的攻击 [19],T细胞自噬缺失抑制脓毒症患者的免疫反应,从而增加死亡率 [20]。
因此,自噬是一个进化保守的过程,通过对受损或未使用的蛋白质和细胞器的再循环来促进细胞在应激条件下的适应能力 [21]。越来越多的研究提示自噬在炎症过程中经多种通路对机体起到一定的保护作用,H2S可以通过多个信号通路激活自噬,如AMPK/mTOR,PI3K/Akt/mTOR和miR-30c等信号传导途径 [11]。
4. H2S经AMPK通路调节自噬
现有证据表明,H2S可经AMPK通路介导自噬作用 [22]。AMPK通过对激素和营养信号做出反应参与调节全身能量代谢平衡,一旦被激活,AMPK磷酸化下游底物,抑制合成过程,促进分解代谢,引起腺苷5’-三磷酸生成和能量恢复。H2S经AMPK通路调节自噬有以下三种机制:
1) 硫氢化钠(NaHS)在氧化应激损伤中可以通过激活CaMKKβ/AMPK和PI3K/AKT信号通路,上调线粒体中碱基切除修复酶DNA POLG和OGG-1,下调OXPHOS活性,恢复线粒体膜电位,保护线粒体功能 [23]。
线粒体损伤会导致氧化应激和代谢障碍,活性氧(ROS)是有氧代谢氧化磷酸化过程中不可避免的副产物,起源于线粒体的电子传递链。ROS生成与消除的不平衡导致氧化应激,引起氧化损伤。线粒体不仅在ROS的产生过程中起着重要作用,而且成为氧化应激的敏感靶点。H2S可以通过内源性合成,调控机体自噬、细胞代谢、炎症、细胞周期和氧化应激等多种病理生理过程。外源性H2S通过激活CaMKKβ/ AMPK和PI3K/AKT通路减轻细胞凋亡、增强了机体抗氧化活性、保护线粒体、增加了mtDNA修复酶的表达、改善分子伴侣蛋白的表达、增加了机体抗氧化剂的生成 [23]。
2) 外源性H2S能通过Nrf2-ROS-AMPK信号通路抑制高血糖时氧化应激诱导的过度自噬,从而保护内皮细胞 [24]。高血糖诱导血管内皮细胞ROS产生增加,增加的ROS导致严重的氧化应激使ATP的产生减少,它可以激活AMPK信号通路。持续和过度激活的AMPK信号通路会导致过度自噬,使动脉血管内皮细胞凋亡。外源性H2S不仅减少了内源性H2S的产生,而且保护了动脉内皮细胞的死亡。因此,H2S通过Nrf2-ROS-AMPK信号通路能调整血管内皮细胞的稳态并增加细胞的成活率。
AMPK是体内细胞能量状态的关键调节因子,在维持葡萄糖稳态方面有着重要的作用 [25]。最近的研究表明,在高血糖时外源性H2S诱导AMPK激活进而调节炎性反应,降低了氧化应激和血管炎症 [26] [27]。另外,在高血糖时给予NaHS治疗可以增强AMPK的磷酸化,稳定糖代谢 [28]。
3) H2S可以通过激活AMPK/mTOR通路激活肝脏自噬,降低血清甘油三酯(TG)。自噬在肝甘油三酯代谢中起重要作用,抑制自噬可以降低TG在肝脏中的清除 [29]。
H2S是自噬的有力促进剂,对高甘油三酯血症(HTG)和非酒精性脂肪肝(NAFLD)具有保护作用。自噬通过噬脂作用调节肝细胞脂质代谢,即将双膜自噬体内的脂质滴固存,与溶酶体融合形成自溶酶体,然后由自溶酶体内的脂肪酶降解TG。肝细胞自噬活性降低导致脂质分解减少,游离脂肪酸(FFA)氧化,导致肝脂肪变性而发展为NAFLD和HTG。相反,抑制肝脏AMPK活化抑制肝脏自噬能力,导致肝脏脂肪变性。H2S通过AMPK/mTOR通路刺激肝脏自噬通量,降低血清TG水平,进而改善NAFLD [29]。
综上所述,AMPK能够感知细胞ATP的变化,是自噬的重要调控因子。其下游靶点包括负向调节物mTOR,抑制其生成可以促进自噬体的形成 [30]。因此,AMPK介导的对mTOR磷酸化和活化的抑制可诱导多种不同细胞类型的自噬反应。自噬通过多种信号途径进行调节,AMPK/mTOR是其关键的信号途径。H2S是脓毒症发生发展的重要调节因子,外源性H2S通过调节AMPK/mTOR通路诱导自噬,除了可维持肠黏膜正常功能外,还对心功能、血脂、糖尿病并发症等具有重要作用(图1)。今后可进一步研究H2S 激活AMPK/mTOR的上游机制,深入了解AMPK/mTOR通路活化后的多种生理学机制,为将来H2S 供体应用于临床提供理论依据。
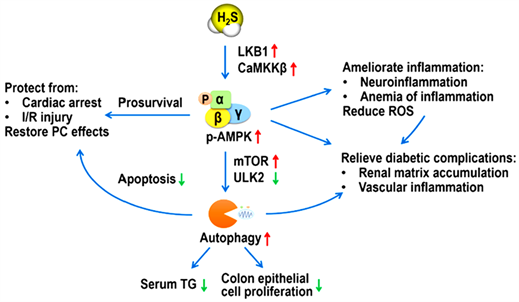 LKB1:肝激酶B1;CaMKKb:钙调素激活蛋白激酶b;ULK2:类UNC-51激酶2;TG:甘油三酯;I/R:缺血再灌注;PC:后处理;ROS:活性氧。
LKB1:肝激酶B1;CaMKKb:钙调素激活蛋白激酶b;ULK2:类UNC-51激酶2;TG:甘油三酯;I/R:缺血再灌注;PC:后处理;ROS:活性氧。
Figure 1. Biological function of hydrogen sulfide mediates autophagy by regulating
AMPK/mTOR pathway [22]
图1. H2S通过调节AMPK/mTOR通路介导自噬的生物学作用 [22]
基金项目
本文受苏州高新区医疗卫生科技计划重点项目(2019Z003)和甘肃省自然科学基金项目(17JR5RA263)资助。
文章引用
赫 曼,聂静云,康富贵,柴 琛. 硫化氢经AMPK通路调节自噬保护脓毒症相关性肠黏膜损伤的机制研究
Mechanism Research of Hydrogen Sulfide via AMPK Pathway Mediated Autophagy to Protect Intestinal Mucosal Injury in Sepsis[J]. 临床医学进展, 2020, 10(12): 3033-3038. https://doi.org/10.12677/ACM.2020.1012456
参考文献
- 1. Zuber, B., Tran, T.C., Aegerter, P., Grimaldi, D., Charpentier, J., Guidet, B., Mira, J.P. and Pene, F. (2012) Impact of Case Volume on Survival of Septic Shock in Patients with Malignancies. Critical Care Medicine, 40, 55-62. https://doi.org/10.1097/CCM.0b013e31822d74ba
- 2. Napolitano, L.M. (2018) Sepsis 2018: Definitions and Guideline Changes. Surgical Infections (Larchmt), 19, 117-125. https://doi.org/10.1089/sur.2017.278
- 3. Jiang, L.Y., Zhang, M., Zhou, T.E., Yang, Z.F., Wen, L.Q. and Chang, J.X. (2010) Changes of the Immunological Barrier of Intestinal Mucosa in Rats with Sepsis. World Journal of Emergency Medicine, 1, 138-143.
- 4. Chang, R.M., Wen, L.Q., Chang, J.X., Fu, Y.R., Jiang, Z.P. and Chen, S. (2013) Repair of Damaged Intestinal Mucosa in a Mouse Model of Sepsis. World Journal of Emergency Medicine, 4, 223-228. https://doi.org/10.5847/wjem.j.issn.1920-8642.2013.03.012
- 5. Hu, J., Nie, Y. and Yan, X. (2015) The Role of Autophagy in the Gut Pathogens Clearance and Evasion. Current Protein & Peptide Science, 16, 632-645. https://doi.org/10.2174/1389203716666150630134205
- 6. Aguirre, A., Lopez-Alonso, I., Gonzalez-Lopez, A., Amado-Rodriguez, L., Batalla-Solis, E., Astudillo, A., Blazquez-Prieto, J., Fernandez, A.F., Galvan, J.A., dos Santos, C.C. and Albaiceta, G.M. (2014) Defective Autophagy Impairs ATF3 Activity and Worsens Lung Injury during Endotoxemia. Journal of Molecular Medicine (Berl), 92, 665-676. https://doi.org/10.1007/s00109-014-1132-7
- 7. Zhang, H., Moochhala, S.M. and Bhatia, M. (2008) Endogenous Hydrogen Sulfide Regulates Inflammatory Response by Activating the ERK Pathway in Polymicrobial Sepsis. The Journal of Immunology, 181, 4320-4331. https://doi.org/10.4049/jimmunol.181.6.4320
- 8. Brunkhorst, F.M., Oppert, M., Marx, G., Bloos, F., Ludewig, K., Putensen, C., Nierhaus, A., Jaschinski, U., Meier-Hellmann, A., Weyland, A., Grundling, M., Moerer, O., Riessen, R., Seibel, A., Ragaller, M., Buchler, M.W., John, S., Bach, F., Spies, C., Reill, L., Fritz, H., Kiehntopf, M., Kuhnt, E., Bogatsch, H., Engel, C., Loeffler, M., Kollef, M.H., Reinhart, K. and Welte, T. (2012) Effect of Empirical Treatment with Moxifloxacin and Meropenem vs Meropenem on Sepsis-Related Organ Dysfunction in Patients with Severe Sepsis: A Randomized Trial. JAMA, 307, 2390-2399. https://doi.org/10.1001/jama.2012.5833
- 9. Arts, R.J., Gresnigt, M.S., Joosten, L.A. and Netea, M.G. (2017) Cellular Metabolism of Myeloid Cells in Sepsis. Journal of Leukocyte Biology, 101, 151-164. https://doi.org/10.1189/jlb.4MR0216-066R
- 10. Watanabe, E., Thampy, L.K. and Hotchkiss, R.S. (2018) Immunoadjuvant Therapy in Sepsis: Novel Strategies for Immunosuppressive Sepsis Coming down the Pike. Acute Medicine & Surgery, 5, 309-315. https://doi.org/10.1002/ams2.363
- 11. Wu, D., Wang, H., Teng, T., Duan, S., Ji, A. and Li, Y. (2018) Hydrogen Sulfide and Autophagy: A Double Edged Sword. Pharmacological Research, 131, 120-127. https://doi.org/10.1016/j.phrs.2018.03.002
- 12. Qiu, P., Liu, Y. and Zhang, J. (2019) Review: The Role and Mechanisms of Macrophage Autophagy in Sepsis. Inflammation, 42, 6-19. https://doi.org/10.1007/s10753-018-0890-8
- 13. Carchman, E.H., Rao, J., Loughran, P.A., Rosengart, M.R. and Zuckerbraun, B.S. (2011) Heme Oxygenase-1-Mediated Autophagy Protects against Hepatocyte Cell Death and Hepatic Injury from Infection/Sepsis in Mice. Hepatology, 53, 2053-2062. https://doi.org/10.1002/hep.24324
- 14. Lin, C.W., Lo, S., Perng, D.S., Wu, D.B., Lee, P.H., Chang, Y.F., Kuo, P.L., Yu, M.L., Yuan, S.S. and Hsieh, Y.C. (2014) Complete Activation of Autophagic Process Attenuates Liver Injury and Improves Survival in Septic Mice. Shock, 41, 241-249. https://doi.org/10.1097/SHK.0000000000000111
- 15. Mei, S., Livingston, M., Hao, J., Li, L., Mei, C. and Dong, Z. (2016) Autophagy Is Activated to Protect against Endotoxic Acute Kidney Injury. Scientific Reports, 6, 22171-22181. https://doi.org/10.1038/srep22171
- 16. Livingston, M.J. and Dong, Z. (2014) Autophagy in Acute Kidney Injury. Seminars in Nephrology, 34, 17-26. https://doi.org/10.1016/j.semnephrol.2013.11.004
- 17. Hsieh, C.H., Pai, P.Y., Hsueh, H.W., Yuan, S.S. and Hsieh, Y.C. (2011) Complete Induction of Autophagy Is Essential for Cardioprotection in Sepsis. Annals of Surgery, 253, 1190-1200. https://doi.org/10.1097/SLA.0b013e318214b67e
- 18. Takaoka, Y., Goto, S., Nakano, T., Tseng, H.P., Yang, S.M., Kawamoto, S., Ono, K. and Chen, C.L. (2014) Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) Prevents Lipopolysaccharide (LPS)-Induced, Sepsis-Related Severe Acute Lung Injury in Mice. Scientific Reports, 4, 5204-5212. https://doi.org/10.1038/srep05204
- 19. Nakahira, K., Haspel, J.A., Rathinam, V.A., Lee, S.J., Dolinay, T., Lam, H.C., Englert, J.A., Rabinovitch, M., Cernadas, M., Kim, H.P., Fitzgerald, K.A., Ryter, S.W. and Choi, A.M. (2011) Autophagy Proteins Regulate Innate Immune Responses by Inhibiting the Release of Mitochondrial DNA Mediated by the NALP3 Inflammasome. Nature Immunology, 12, 222-230. https://doi.org/10.1038/ni.1980
- 20. Lin, C.W., Lo, S., Hsu, C., Hsieh, C.H., Chang, Y.F., Hou, B.S., Kao, Y.H., Lin, C.C., Yu, M.L., Yuan, S.S. and Hsieh, Y.C. (2014) T-Cell Autophagy Deficiency Increases Mortality and Suppresses Immune Responses after Sepsis. PLoS ONE, 9, e102066. https://doi.org/10.1371/journal.pone.0102066
- 21. Mizushima, N. (2009) Physiological Functions of Autophagy. Current Topics in Microbiology and Immunology, 335, 71-84. https://doi.org/10.1007/978-3-642-00302-8_3
- 22. Wang, M., Tang, W. and Zhu, Y.Z. (2017) An Update on AMPK in Hydrogen Sulfide Pharmacology. Frontiers in Pharmacology, 8, 810. https://doi.org/10.3389/fphar.2017.00810
- 23. Chen, X., Zhao, X., Cai, H., Sun, H., Hu, Y., Huang, X., Kong, W. and Kong, W. (2017) The Role of Sodium Hydrosulfide in Attenuating the Aging Process via PI3K/AKT and CaMKKbeta/AMPK Pathways. Redox Biology, 12, 987-1003. https://doi.org/10.1016/j.redox.2017.04.031
- 24. Liu, J., Wu, J., Sun, A., Sun, Y., Yu, X., Liu, N., Dong, S., Yang, F., Zhang, L., Zhong, X., Xu, C., Lu, F. and Zhang, W. (2016) Hydrogen Sulfide Decreases High Glucose/Palmitate-Induced Autophagy in Endothelial Cells by the Nrf2-ROS-AMPK Signaling Pathway. Cell & Bioscience, 6, 33. https://doi.org/10.1186/s13578-016-0099-1
- 25. Zhang, B.B., Zhou, G. and Li, C. (2009) AMPK: An Emerging Drug Target for Diabetes and the Metabolic Syndrome. Cell Metabolism, 9, 407-416. https://doi.org/10.1016/j.cmet.2009.03.012
- 26. Zhou, X., Cao, Y., Ao, G., Hu, L., Liu, H., Wu, J., Wang, X., Jin, M., Zheng, S., Zhen, X., Alkayed, N.J., Jia, J. and Cheng, J. (2014) CaMKK Beta-Dependent Activation of AMP-Activated Protein Kinase Is Critical to Suppressive Effects of Hydrogen Sulfide on Neuroinflammation. Antioxidants & Redox Signaling, 21, 1741-1758. https://doi.org/10.1089/ars.2013.5587
- 27. Manna, P. and Jain, S.K. (2013) L-Cysteine and Hydrogen Sulfide Increase PIP3 and AMPK/PPARgamma Expression and Decrease ROS and Vascular Inflammation Markers in High Glucose Treated Human U937 Monocytes. Journal of Cellular Biochemistry, 114, 2334-2345. https://doi.org/10.1002/jcb.24578
- 28. Kundu, S., Pushpakumar, S., Khundmiri, S.J. and Sen, U. (2014) Hydrogen Sulfide Mitigates Hyperglycemic Remodeling via Liver Kinase B1-Adenosine Monophosphate-Activated Protein Kinase Signaling. Biochimica et Biophysica Acta, 1843, 2816-2826. https://doi.org/10.1016/j.bbamcr.2014.08.005
- 29. Sun, L., Zhang, S., Yu, C., Pan, Z., Liu, Y., Zhao, J., Wang, X., Yun, F., Zhao, H., Yan, S., Yuan, Y., Wang, D., Ding, X., Liu, G., Li, W., Zhao, X., Liu, Z. and Li, Y. (2015) Hydrogen Sulfide Reduces Serum Triglyceride by Activating Liver Autophagy via the AMPK-mTOR Pathway. American Journal of Physiology-Endocrinology and Metabolism, 309, E925-E935. https://doi.org/10.1152/ajpendo.00294.2015
- 30. Kim, Y.C. and Guan, K.L. (2015) mTOR: A Pharmacologic Target for Autophagy Regulation. Journal of Clinical Investigation, 125, 25-32. https://doi.org/10.1172/JCI73939
NOTES
*通讯作者。