Advances in Clinical Medicine
Vol.
14
No.
01
(
2024
), Article ID:
80154
,
10
pages
10.12677/ACM.2024.141267
PD-1/PD-L1抑制剂对接受手术、放疗或化疗后晚期食管癌患者的疗效和安全性的Meta分析
张伊莎1,李晓雅2,单晓宇2*
1山东省荣军总医院消化内科,山东 济南
2山东省康复医院内科,山东 济南
收稿日期:2023年12月27日;录用日期:2024年1月21日;发布日期:2024年1月30日

摘要
目的:系统评价PD-1/PD-L1抑制剂对接受手术、放疗或化疗后食管癌患者的疗效和安全性。方法:计算机对PubMed、EMbase、Web of Science、The Cochrane Library、Clinical Trials等多个数据库进行了搜索与筛选工作,以寻找关于手术、放疗或化疗后晚期食管癌患者免疫治疗的相关研究(2018年1月~2022年12月),应用RevMan5.3软件进行Meta分析。纳入标准为临床II期或III期的双盲随机对照试验(Randomized controlled trials, RCTs)。结果:共纳入9项研究,共5558例晚期食管癌患者。通过数据分析研究发现,免疫抑制剂组和对照组12个月的总体生存率(overall survival, OS) (OR = 1.32, 95% CI: 1.00~1.73, P ≤ 0.05;异质性:I2 = 76%,P < 0.01)、无进展生存期(progression-free survival, PFS) (OR = 1.62, 95% CI: 1.15~2.28, P < 0.01;异质性:I2 = 76%,P < 0.01)、客观缓解率(objective response rate, ORR) (RR = 1.98, 95% CI: 1.69~2.32, P < 0.01;异质性:I2 = 18%,P = 0.28)和毒副反应发生率(OR = 0.36, 95% CI: 0.24~0.54, P < 0.01;异质性:I2 = 91%,P < 0.01)具有统计学意义。结论:应用PD-1/PD-L1抑制剂对接受手术、放化疗的晚期食管癌患者能有效提高食管癌患者的总体生存水平,且安全性高。
关键词
食管癌,PD-1,PD-L1,随机对照试验(RCT),Meta分析

Meta Analysis of the Efficacy and Safety of PD-1/PD-L1 Inhibitors in Patients with Advanced Esophageal Cancer after Surgery, Radiotherapy, or Chemotherapy
Yisha Zhang1, Xiaoya Li2, Xiaoyu Shan2*
1Department of Gastroenterology, Shandong Rongjun General Hospital, Jinan Shandong
2Internal Medicine Department of Shandong Rehabilitation Hospital, Jinan Shandong
Received: Dec. 27th, 2023; accepted: Jan. 21st, 2024; published: Jan. 30th, 2024

ABSTRACT
Object: Systematically evaluate the efficacy and safety of PD-1/PD-L1 inhibitors in patients with esophageal cancer after surgery, radiotherapy, or chemotherapy. Method: The computer searched and screened multiple databases such as PubMed, EMbase, Web of Science, The Cochrane Library, Clinical Trials, etc. to search for relevant research on immunotherapy for advanced esophageal cancer patients after surgery, radiotherapy, or chemotherapy (January 2018 to December 2022), applying RevMan5.3 software for meta-analysis. The inclusion criteria were double blind randomized controlled trials for clinical phase II or III (Randomized controlled trials, RCTs). Results: A total of 9 studies were included, with a total of 5558 patients with advanced esophageal cancer. Through data analysis, it was found that the overall survival rates of the immunosuppressive group and the control group at 12 months (overall survival, OS) (OR = 1.32, 95% CI: 1.00~1.73, P ≤ 0.05; heterogeneity: I2 = 76%, P < 0.01), progression-free survival (OR = 1.62, 95% CI: 1.15~2.28, P < 0.01; heterogeneity: I2 = 76%, P < 0.01), objective response rate (RR = 1.98, 95% CI: 1.69~2.32, P < 0.01; heterogeneity: I2 = 18%, P = 0.28) and the incidence of toxic side effects (OR = 0.36, 95% CI: 0.24~0.54, P < 0.01; heterogeneity: I2 = 91%, P < 0.01) have statistically significant. Conclusion: The application of PD-1/PD-L1 inhibitors can effectively improve the overall survival level of advanced esophageal cancer patients undergoing surgery, radiotherapy, and chemotherapy, with high safety.
Keywords:Esophageal Cancer, PD-1, PD-L1, Randomized Controlled Trial (RCT), Meta-Analysis

Copyright © 2024 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
全球范围内,食管癌(Esophageal cancer, EC)作为一种常见病症,其患病人数正在不断增加,已成为世界排名前七的癌症之一 [1] 。由于该病的初期表现并不显著,因此大约有三分之二的患者在被确诊时已经处于中晚期 [2] ,这使得他们不能接受彻底性的外科手术,并且常规的放、化学疗及靶向治疗的效果也相当有限,导致了较高的复发率与转移率,五年存活率只有不到20% [3] [4] [5] 。
随着癌症免疫学领域不断发展壮大,给食管癌患者带来了全新的医疗方案并取得了显著成果。研究表明,食管癌患者进展速度及其生存率与其周边侵入的淋巴细胞密度有着直接关系 [6] [7] [8] 。免疫治疗的热门话题是基于程序性死亡受体1/程序性死亡配体1 (PD-1/PD-L1)信号通路开发出的新型免疫抑制剂,其被广泛应用于黑色素瘤、非小细胞肺癌等疾病的诊疗中,同时也在胃肠道恶性肿瘤方面展现出巨大的潜力。我们的目的在于评估PD-1/PD-L1抑制剂疗法对接受手术、放疗或化疗的晚期食管癌患者的疗效及安全性,为晚期食管癌的免疫治疗提供可能依据,更好地指导临床。
2. 资料与方法
2.1. 文献检索
我们利用了如PubMed、EMbase、Web of Science、The Cochrane Library及Clinical Trials等多种在线资源对相关研究进行了全面的查找与分析;时间范围设定自2018年初至2022年底。通过使用主导词配合关键字来完成检索,同时还借助人工方式从已纳入的研究中提取出相关的引用研究作为补充。英文检索词包括advanced esophageal cancer、Immune checkpoint inhibitors、PD-1/PD-L1 inhibitors和immunotherapy。
2.2. 纳入、排除标准
2.2.1. 纳入标准
1) 对照临床试验进行随机分析的研究;2) 有关晚期食管癌的研究;3) 在一线治疗后的研究;4) 可以获取到总生存期(OS)、无发展生存期(PFS),客观减轻率(ORR)和毒副反应等相关数据的研究。
2.2.2. 排除标准
1) 仅包含摘要而未提供正文或重复报告的研究;2) 针对一线治疗的研究;3) 免疫治疗无法提供所需信息的研究;4) 与书籍、病例报告、动物实验等相关的文献以及非英文的文章。
2.3. 结局指标
包含治疗效果和安全性两个方面的评估。治疗效果指标涵盖了客观缓解率以及12个月OS和PFS;而安全性的指标则是关于免疫治疗相关的副作用的发生频率等。
2.4. 资料提取
两位单独的研究者按照统一的程序进行文献筛选和数据收集,如果存在分歧,则请求第三位研究者提供帮助。所收集到的数据包括:研究主题、首位作者、发布日期、样本数量、性别、年龄以及质量评估指标和结果指标等信息。
2.5. 文献质量评价
采用Cochrane手册5.10针对RCT的偏倚风险进行评估,并我们利用Cochrane手册5.10对RCT的偏倚风险做出了评估,并使用改良版的Jadad量表 [9] 来衡量其质量,评估内容主要包含随机化、生成随机排序、盲法操作、退出以及失访等,1~3分视为低质量,4~7分视为高质量。
2.6. 统计学分析
利用RevMan 5.3工具进行了Meta分析。对纳入研究异质性的评估依据是I2 ≥ 或 < 50%,如果I2 < 50%,说明数据并没有明显的异质性,则选择固定效应模型(Fixed Effect Model, FEM);如果I2 ≥ 50%,表明具有明显异质性,需选择随机效应模型(Random Effect Model, REM)。通过OR、95% CI和P值对各项研究指标进行评估,P ≤ 0.05认为具有统计学意义。
3. 结果
3.1. 文献筛选结果
经过数据库的检索,共320个相关研究。通过进一步筛查,68项研究因存在重复的研究而被剔除,另有233项研究由于标题、摘要不符合或是非随机对照试验等原因被剔除。最后,根据纳入、排除标准,有9项随机对照研究符合条件,总计5558 [10] - [18] 例患者。
3.2. 纳入研究的基本特征和质量评价
所纳入的临床研究均为随机对照临床试验研究(表1)。这些纳入的研究都详细地阐述了如何生成并隐藏随机分配,并且都实行双盲设计,没有出现退出的或患者失访情况。至于其他偏倚情况均不清楚(图1)。依据修改后的Jadad评级标准,其中5项临床研究获得7分,另外4项则达到了5分。
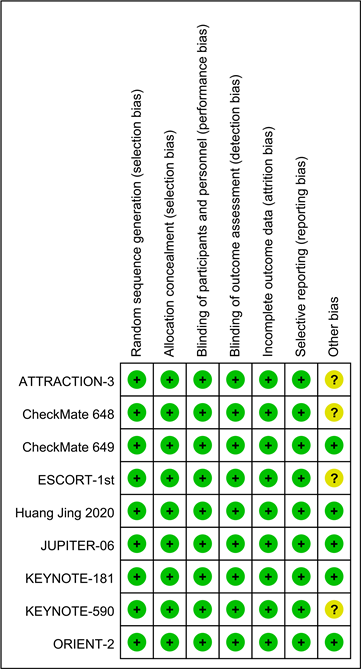
Figure 1. Summary of risk bias in inclusion studies
图1. 纳入研究风险偏倚总结
Table 1. Basic characteristics of included literature research
表1. 所纳入文献研究的基本特征
3.3. Meta分析结果
3.3.1. 长期生存情况
Meta分析纳入研究患者12个月OS和PFS发生率。12个月OS (OR = 1.32, 95% CI: 1.00~1.73, P ≤ 0.05),具有统计学意义(图2)。12个月PFS (OR = 1.62, 95% CI: 1.15~2.28, P < 0.01)差异明显,具有统计学意义(图3)。

Figure 2. Meta analysis of overall survival (OS) at 12 months in immunotherapy group and control group
图2. 免疫治疗组和对照组12个月总生存期(OS) Meta分析
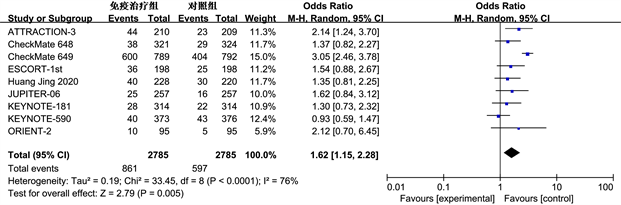
Figure 3. Meta analysis of 12 month progression free survival (PFS) in immunotherapy group and control group
图3. 免疫治疗组和对照组12个月无进展生存期(PFS) Meta分析
3.3.2. 客观缓解率和亚组分析
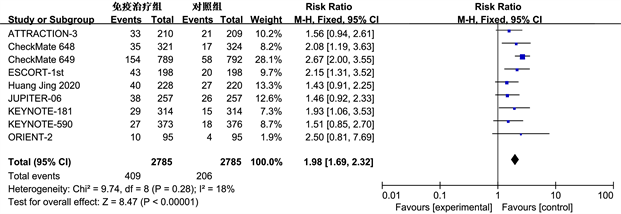 (a)
(a)
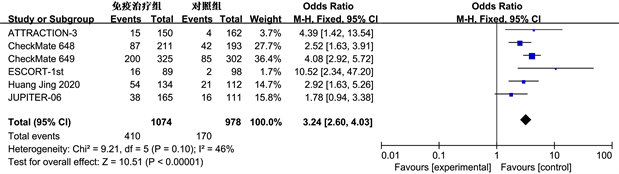 (b)
(b)
Figure 4. Meta analysis of 12 month OS (b) in immunotherapy group and control group with ORR (a) and PD-L1 expression CPS > 1 subgroup patients
图4. 免疫治疗组和对照组ORR (a)和PD-L1表达CPS > 1亚组患者12个月OS (b)的Meta分析
客观缓解率(ORR)是指肿瘤患者治疗后肿瘤缩小并且保持一定时间所占的比例,包括完全缓解和部分缓解。纳入的9项研究都记录了ORR的数据。ORR的Meta分析结果如图4(a)所示,免疫治疗组与对照组的ORR差异显著(RR = 1.98, 95% CI: 1.69~2.32, P < 0.01)。同时亚组分析评估联合阳性分数(Combined Positive Score, CPS) > 1的人群(图4(b)),有6项研究 [9] 报告了相关数据,在PD-L1 CPS > 1的亚组人群中,免疫组和对照组12个月OS发生率差异明显(OR = 3.24, 95% CI: 2.60~4.03, P < 0.01),免疫治疗占据优势。但是亚组分析缺乏PD-L1 CPS < 1的相关数据,无法进行对比。
3.3.3. 安全性分析
根据世界卫生组织对癌症治疗中常见的副作用等级划分(从0到IV级别),我们将低于III级的副作用定义为轻度,而高于或等于III级的则被视为重度。通过对9项研究中的毒副作用发生情况进行meta分析,我们发现两组毒副反应总的发生率(OR = 0.36, 95% CI: 0.24~0.54, P < 0.01)和重度毒副反应的发生率(OR = 0.48, 95% CI: 0.34~0.67, P < 0.01)差异都具统计学意义,这表明免疫疗法相关毒副作用具有较高的安全性(图5)。
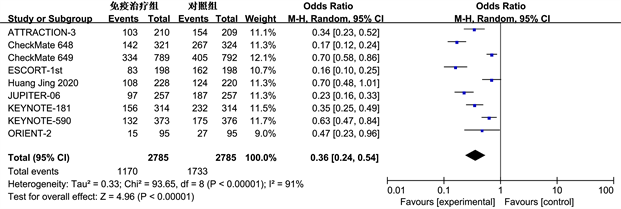 (a)
(a)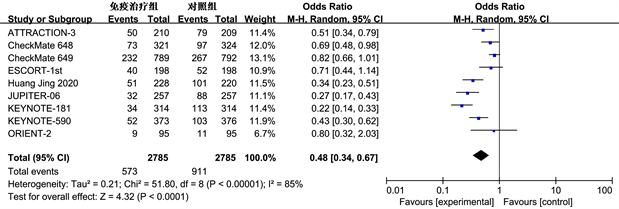 (b)
(b)
Figure 5. Meta analysis of the overall incidence of toxic side effects (a) and severe toxic side effects (b) in the immunotherapy group and control group
图5. 免疫治疗组和对照组总体毒副反应(a)和重度毒副反应(b)发生率的Meta分析
4. 讨论
食管癌是我国最常见的消化道恶性肿瘤之一,确诊时约80%患者已是中晚期 [19] ,治疗只能局限在放疗、化疗,其效果不理想,毒副反应发生率高 [20] 。相对而言,免疫治疗对晚期食管癌患者疗效显著,安全性强 [21] 。
PD-1是存在于活跃状态下的T细胞表面的一种主要抗原,其能与位于癌细胞上的PD-L1和PD-L2配体相结合并降低T细胞引发的免疫应答,进而减缓T淋巴细胞及肿瘤侵入淋巴细胞(tumor infiltrating lymphocyte, TIL)的功能发挥 [22] 。PD-1抑制剂目的是通过阻断这一通路,达到恢复部分T细胞的功能的作用,增强机体自身对肿瘤的免疫反应 [23] [24] [25] 。当前,包括Pembrolizumab和Nivolumab在内的多款基于食管癌PD-1/PD-L1通路开发的免疫抑制剂已成功应用于临床 [26] ,并在消化道恶性肿瘤领域取得满意的临床效果 [27] 。我们根据对多项随机对照实验的研究进行了meta分析,结果显示PD-1/PD-L1免疫抑制剂能显著延长患者的生命周期,同时其副作用相对较小,对于食管癌的治疗有重要的参考意义。
在各类临床试验中,对于晚期食管癌,免疫抑制剂的安全性有着不同的观点。我们在这次研究中还探讨了免疫治疗组和对照组之间的副作用。mate分析结果表明,免疫治疗具备较高的安全性。目前关于免疫疗法治疗晚期食管癌的安全性的文献研究较少,Wang等 [28] 的研究得出PD-1/PD-L1抑制剂在晚期胃食管患者中具有可控的安全性的结论;而在Yang等 [29] 的与单纯化疗相比的研究中,免疫疗法表现出更高的毒副反应。经过我们对免疫抑制剂的毒副反应分析进行的补充和完善,发现无论是重度还是总体的毒副反应发生率,免疫治疗组都优于对照组。
此次研究的优势在于纳入了国际上完善的RCTs,并对晚期食管癌免疫抑制剂疗法的疗效及安全性进行了分析,并在亚组分析中考虑到CPS表达情况等影响疗效的有关因素。但本研究的局限性在于:1) 各个研究化疗方案不统一,结果的一致性受影响;2) 影响疗效的因素,如年龄、免疫治疗频次及周期等未纳入分析;3) 可能存在选择性偏倚。因此,我们期待更深入的分层分析研究,得出更有说服力的研究结论,以确定免疫治疗的适用群体,确保疗效和安全性。
总之,对于晚期食管癌的治疗,PD-1/PD-L1抑制剂疗法的疗效是肯定的,效果优于化疗及姑息治疗,且毒副反应发生率相对较低。此外,PD-L1表达CPS > 1的人群似乎比总体人群受益疗效更显著。故PD-1/PD-L1抑制剂是晚期食管癌患者二线或晚期治疗的新选择。
文章引用
张伊莎,李晓雅,单晓宇. PD-1/PD-L1抑制剂对接受手术、放疗或化疗后晚期食管癌患者的疗效和安全性的Meta分析
Meta Analysis of the Efficacy and Safety of PD-1/PD-L1 Inhibitors in Patients with Advanced Esophageal Cancer after Surgery, Radiotherapy, or Chemotherapy[J]. 临床医学进展, 2024, 14(01): 1888-1897. https://doi.org/10.12677/ACM.2024.141267
参考文献
- 1. Cai, Z. and Liu, Q. (2021) Understanding the Global Cancer Statistics 2018: Implications for Cancer Control. Science China Life Sciences, 64, 1017-1020. https://doi.org/10.1007/s11427-019-9816-1
- 2. Hoefnagel, S., Boonstra, J.J., Russchen, M., et al. (2021) Towards Personalized Treatment Strategies for Esophageal Adenocarcinoma; A Review on the Molecular Characterization of Esophageal Adenocarcinoma and Current Research Efforts on Individualized Curative Treatment Regimens. Cancers, 13, Article 4881. https://doi.org/10.3390/cancers13194881
- 3. Li, Q., Liu, T. and Ding, Z. (2022) Neoadjuvant Immunotherapy for Resectable Esophageal Cancer: A Review. Frontiers in Immunology, 13, Article 1051841. https://doi.org/10.3389/fimmu.2022.1051841
- 4. Han, D., Li, B., Zhao, Q., et al. (2022) The Key Clinical Ques-tions of Neoadjuvant Chemoradiotherapy for Resectable Esophageal Cancer-A Review. Frontiers in Oncology, 12, Arti-cle ID: 890688. https://doi.org/10.3389/fonc.2022.890688
- 5. Yan, Y., Feng, X., Li, C., et al. (2022) Treatments for Resectable Esophageal Cancer: From Traditional Systemic Therapy to Immunotherapy. Chinese Medical Journal, 135, 2143-2156. https://doi.org/10.1097/CM9.0000000000002371
- 6. Wang, Z., Shao, C., Wang, Y., et al. (2022) Efficacy and Safety of Neoadjuvant Immunotherapy in Surgically Resectable Esophageal Cancer: A Systematic Review and Me-ta-Analysis. International Journal of Surgery, 104, Article ID: 106767. https://doi.org/10.1016/j.ijsu.2022.106767
- 7. Fang, P., Zhou, J., Liang, Z., et al. (2022) Immunotherapy Re-sistance in Esophageal Cancer: Possible Mechanisms and Clinical Implications. Frontiers in Immunology, 13, Article 975986. https://doi.org/10.3389/fimmu.2022.975986
- 8. Waters, J.K. and Reznik, S.I. (2022) Update on Man-agement of Squamous Cell Esophageal Cancer. Current Oncology Reports, 24, 375-385. https://doi.org/10.1007/s11912-021-01153-4
- 9. Olivo, S.A., Macedo, L.G., Gadotti, I.C., et al. (2008) Scales to Assess the Quality of Randomized Controlled Trials: A Systematic Review. Physical Therapy, 88, 156-175. https://doi.org/10.2522/ptj.20070147
- 10. Sun, J.M., Shen, L., Shah, M.A., et al. (2021) Pembrolizumab plus Chemotherapy versus Chemotherapy Alone for First-Line Treatment of Advanced Oesophageal Cancer (KEYNOTE-590): A Randomised, Placebo-Controlled, Phase 3 Study. Lancet, 398, 759-771. https://doi.org/10.1016/S0140-6736(21)01234-4
- 11. Kato, K., Cho, B.C., Takahashi, M., et al. (2019) Nivolumab versus Chemotherapy in Patients with Advanced Oesophageal Squamous Cell Carcinoma Refractory or Intolerant to Pre-vious Chemotherapy (ATTRACTION-3): A Multicentre, Randomised, Open-Label, Phase 3 Trial. The Lancet Oncology, 20, 1506-1517. https://doi.org/10.1016/S1470-2045(19)30626-6
- 12. Kojima, T., Shah, M.A., Muro, K., et al. (2020) Randomized Phase III KEYNOTE-181 Study of Pembrolizumab versus Chemotherapy in Advanced Esophageal Cancer. Journal of Clinical Oncology, 38, 4138-4148. https://doi.org/10.1200/JCO.20.01888
- 13. Wang, Z.X., Cui, C., Yao, J., et al. (2022) Toripalimab plus Chemo-therapy in Treatment-Naive, Advanced Esophageal Squamous Cell Carcinoma (JUPITER-06): A Multi-Center Phase 3 Trial. Cancer Cell, 40, 277-288. https://doi.org/10.1016/j.ccell.2022.02.007
- 14. Huang, J., Xu, J., Chen, Y., et al. (2020) Camrelizumab versus In-vestigator’s Choice of Chemotherapy as Second-Line Therapy for Advanced or Metastatic Oesophageal Squamous Cell Carcinoma (ESCORT): A Multicentre, Randomised, Open-Label, Phase 3 Study. The Lancet Oncology, 21, 832-842. https://doi.org/10.1016/S1470-2045(20)30110-8
- 15. Luo, H., Lu, J., Bai, Y., et al. (2021) Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients with Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA, 326, 916-925. https://doi.org/10.1001/jama.2021.12836
- 16. Xu, J., Li, Y., Fan, Q., et al. (2022) Clinical and Biomarker Analyses of Sintilimab versus Chemotherapy as Second-Line Therapy for Advanced or Metastatic Esophageal Squamous Cell Car-cinoma: A Randomized, Open-Label Phase 2 Study (ORIENT-2). Nature Communications, 13, Article No. 857. https://doi.org/10.1038/s41467-022-28408-3
- 17. Janjigian, Y.Y., Shitara, K., Moehler, M., et al. (2021) First-Line Nivolumab plus Chemotherapy versus Chemotherapy Alone for Advanced Gastric, Gastro-Oesophageal Junction, and Oesophageal Adenocarcinoma (CheckMate 649): A Randomised, Open-Label, Phase 3 Trial. Lancet, 398, 27-40. https://doi.org/10.1016/S0140-6736(21)00797-2
- 18. Doki, Y., Ajani, J.A., Kato, K., et al. (2022) Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. The New England Journal of Medicine, 386, 449-462. https://doi.org/10.1056/NEJMoa2111380
- 19. Cao, W., Chen, H.D., Yu, Y.W., et al. (2020) Changing Profiles of Cancer Burden Worldwide and in China: A Secondary Analysis of the Global Cancer Statistics. Chinese Medical Journal, 134, 783-791. https://doi.org/10.1097/CM9.0000000000001474
- 20. Attia, H. and Smyth, E. (2021) Evolving Therapies in Ad-vanced Oesophago-Gastric Cancers and the Increasing Role of Immunotherapy. Expert Review of Anticancer Therapy, 21, 535-546. https://doi.org/10.1080/14737140.2021.1866548
- 21. Freeman, G.J., Long, A.J., Iwai, Y., et al. (2000) Engage-ment of the PD-1 Immunoinhibitory Receptor by A Novel B7 Family Member Leads to Negative Regulation of Lym-phocyte Activation. Journal of Experimental Medicine, 192, 1027-1034. https://doi.org/10.1084/jem.192.7.1027
- 22. Cao, S., Li, J., Lu, J., et al. (2019) Mycobacterium Tuberculosis Anti-gens Repress Th1 Immune Response Suppression and Promotes Lung Cancer Metastasis through PD-1/PDl-1 Signaling Pathway. Cell Death & Disease, 10, Article No. 44. https://doi.org/10.1038/s41419-018-1237-y
- 23. Suzuki, K., Tajima, M., Tokumaru, Y., et al. (2023) Anti-PD-1 Antibodies Recognizing the Membrane-Proximal Region Are PD-1 Agonists That Can Down-Regulate Inflammatory Diseases. Science Immunology, 8, eadd4947. https://doi.org/10.1126/sciimmunol.add4947
- 24. Tan, Z., Chiu, M.S., Yang, X., et al. (2023) Isoformic PD-1-Mediated Immunosuppression Underlies Resistance to PD-1 Blockade in Hepatocellular Carcinoma Patients. Gut, 72, 1568-1580. https://doi.org/10.1136/gutjnl-2022-327133
- 25. Rousseau, B., Bieche, I., Pasmant, E., et al. (2022) PD-1 Blockade in Solid Tumors with Defects in Polymerase Epsilon. Cancer Discovery, 12, 1435-1448. https://doi.org/10.1158/2159-8290.CD-21-0521
- 26. Kanie, K., Iguchi, G., Bando, H., et al. (2021) Mechanistic Insights into Immune Checkpoint Inhibitor-Related Hypophysitis: A Form of Paraneoplastic Syndrome. Cancer Immu-nology, Immunotherapy, 70, 3669-3677. https://doi.org/10.1007/s00262-021-02955-y
- 27. Makker, V., Colombo, N., Casado, H.A., et al. (2023) Len-vatinib plus Pembrolizumab in Previously Treated Advanced Endometrial Cancer: Updated Efficacy and Safety from the Randomized Phase III Study 309/KEYNOTE-775. Journal of Clinical Oncology, 41, 2904-2910. https://doi.org/10.1200/JCO.22.02152
- 28. Wang, B.C., Zhang, Z.J., Fu, C., et al. (2019) Efficacy and Safety of Anti-PD-1/PD-L1 Agents vs Chemotherapy in Patients with Gastric or Gastroesophageal Junction Cancer: A Systematic Review and Meta-Analysis. Medicine, 98, e18054. https://doi.org/10.1097/MD.0000000000018054
- 29. Yang, L., Dong, X.Z., Xing, X.X., et al. (2020) Efficacy and Safety of Anti-PD-1/Anti-PD-L1 Antibody Therapy in Treatment of Advanced Gastric Cancer or Gastroesophageal Junction Cancer: A Meta-Analysis. World Journal of Gastrointestinal Oncology, 12, 1346-1363. https://doi.org/10.4251/wjgo.v12.i11.1346
NOTES
*通讯作者。
