Hans Journal of Biomedicine
Vol.08 No.02(2018), Article ID:24435,7
pages
10.12677/HJBM.2018.82004
Mr Imaging of Superparamagnetic Iron Oxide Nanoparticles Coated L-Ascorbic Acid Stearic Acid Palmitate and in Vitro Labeling of RAW264.7 Cells
Ruilan Zhao1, Zhiyong Wang2, Yuqing Liu2, Junfeng Li1*
1College of Materials, Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu Sichuan
2Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen Guangdong
Received: Mar. 30th, 2018; accepted: Apr. 13th, 2018; published: Apr. 20th, 2018

ABSTRACT
Objective: The L-ascorbic acid stearic acid palmitate (Vc) had been used to coat superparamagnetic iron oxide nanoparticles (SPIO) to build nano probes. The imaging effects of magnetic resonance imaging agents of different materials were investigated. This contrast probe was applied to label mouse mononuclear macrophage cells-RAW264.7 in vitro. The cellular activity was measured in this study. Methods: The quality ratio of Vc and SPIO were adjusted (2:1 and 5:1) to construct the Vc-SPIO nano probes. And then magnetic resonance imaging was completed. Mice RAW264.7 macrophages were cultured routinely. Using the Vc-SPIO nanometer probe (iron concentration 0~25 ug/ml) to complete the cell marking, the cytotoxicity of cell was detected by CCK-8 test. Results: When the molecular ratio of Vc:SPIO was about 2:1, the cells remained normal activity under the concentration of iron in the experiment. However, if the molecular ratio of Vc:SPIO was about 5:1, cell activity will decrease with the increase of iron concentration. When iron concentration was greater than 15 ug/ml, it exhibited toxicity. Conclusion: Vc-SPIO had no obvious effect on the biological activity of RAW264.7 with the concentration below 15 ug/ml. It could be used to label RAW264.7, which lay the foundation for the next animal experiment.
Keywords:Superparamagnetic Iron Oxide Nanoparticles, RAW264.7, Cell Viability, Magnetic Resonance Imaging (MRI)
L-抗坏血酸硬脂酸棕榈酸酯包裹氧化铁纳米粒子的磁共振成像以及RAW264.7细胞体外标记检测
赵瑞兰1,王志勇2,刘雨晴2,李峻峰1*
1成都理工大学,材料与化学化工学院,四川 成都
2中国科学院,深圳先进技术研究院,广东 深圳
收稿日期:2018年3月30日;录用日期:2018年4月13日;发布日期:2018年4月20日

摘 要
目的:应用L-抗坏血酸硬脂酸棕榈酸酯(Vc)包裹的超顺磁性氧化铁纳米粒子(SPIO),构建纳米探针,考察不同材料组分的磁共振造影剂成像效果,实现RAW264.7小鼠单核巨噬细胞的体外标记并探究其生物活性影响。方法:调节Vc与SPIO质量比例(2:1和5:1)构建纳米Vc-SPIO探针;并完成磁共振成像检测;常规培养RAW264.7巨噬细胞,应用Vc-SPIO纳米探针(铁浓度0~25 ug/ml)完成细胞标记,进行细胞毒性CCK-8检测研究。结果:当Vc:SPIO为2:1时,在实验的铁浓度范围内,细胞依然保持正常活性;当Vc:SPIO为5:1时,随着铁浓度的增大细胞活性降低,当铁浓度大于15 ug/ml时有一定的细胞毒性。结论:Vc-SPIO在低于15 ug/ml浓度时对RAW264.7的生物活性没有明显影响,可以实现RAW264.7细胞标记,为下一步动物实验奠定基础。
关键词 :超顺磁性氧化铁,RAW264.7,细胞活性,磁共振成像

Copyright © 2018 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
随着生命科学领域的发展,人们对疾病的发生发展过程有了更多的认识 [1] 。而分子影像学技术的出现,能够实现对细胞水平早期诊断并满足个性化治疗需求 [2] 。现阶段临床常用的显像技术包括放射性核素显像、计算机断层扫描成像、磁共振成像、光学成像和超声成像。相比而言,磁共振成像具有高的空间分辨率,广阔的成像深度、无辐射性损伤 [3] 。因此,磁共振成像在分子影像领域备受重视 [4] 。
超顺磁性氧化铁(supermagneticironoxide, SPIO)纳米粒子具备良好的生物可降解性能,在细胞内,能够通过正常的生化代谢途径完整降解并进入体内铁循环参与生物利用。作为一种新型磁共振造影剂,超顺磁性氧化铁纳米粒子已经成功的应用于细胞标记领域 [5] [6] 。但由于现阶段超顺磁性氧化铁造影剂的灵敏度仍显不足,针对细胞示踪往往需要高浓度纳米粒子完成标记,这会造成一定的细胞毒性 [7] [8] 。如能将单一的氧化铁纳米颗粒构建成致密的团簇结构,不但能够维持原有超顺磁特性,并且其成像性能也能够大大提高 [9] 。因此,如何调控纳米氧化铁团簇粒子水中的分散性,以及细胞毒性至关重要。因此,本论文提出采用L-抗坏血酸硬脂酸棕榈酸酯(Vc)来包裹超顺磁性氧化铁纳米粒子,通过亲疏水自组装作用实现团簇结构的制备。得到细胞毒性小且在水中的悬浮分散稳定性好的Vc-SPIO纳米簇,并用所合成的Vc-SPIO对小鼠单核巨噬细胞(RAW264.7)进行标记,探讨该材料的细胞标记适宜浓度,以及不同标记浓度对细胞的生物活性影响,为下一步的活体实验奠定基础。
2. 材料与方法
2.1. 材料
实验所采用材料有:乙酰丙酮铁、1,2-二羟基十六烷(97%)、油酸(90%)、油胺(>70%)、二苄醚(99%)、多聚甲醛、盐酸、氯化铁标准液、氢氧化钠(Aladdin Reagent),L-抗坏血酸硬脂酸棕榈酸酯(Vc)、四氢呋喃(Alfa Aesar),磷酸盐缓冲液、高糖培养基、琼脂糖粉(Gibco USA),巨噬细胞(中国科学院基因与细胞工程实验室),菲啰嗪、新亚铜试剂、醋酸铵、抗坏血酸、普鲁士蓝、高锰酸钾、乙醇、三氯甲烷(Sigmaaldrich),CCK-8(碧云天)。
2.2. SPIO与Vc-SPIO的合成与表征
SPIO是由高温热解法制备 [10] ,具体过程为:将1 mmol乙酰丙酮铁、5 mmol 1,2-二羟基十六烷、3 mmol油酸、3 mmol油胺和10 mmol的二苄醚混合,在搅拌下加热回流200℃保温2 h,再将混合体系匀速升温到300℃保温1 h。
取SPIO 5 mg,Vc 10 mg,分别溶解于250 ul的四氢呋喃中,充分溶解后将两者混合均匀,超声分散于5 ml水中。匀速率滴加,然后静置两小时,摇床摇晃12 h。旋转蒸发掉混合体系中的四氢呋喃,得到Vc:SPIO为2:1的样品。同样方法可制备Vc:SPIO为5:1的样品。
采用动态光散射仪(DLS Malvern, USA)用来表征SPIO与Vc-SPIO的粒子特性,Vc-SPIO中的铁含量是由酶标仪在550 nm波长测定。Vc-SPIO的弛豫性能 [11] 由核磁共振扫描仪(Magnetom Trio Tim, Siemens Healthcare, Germany)在室温下测定,扫描重复时间TR = 5000 ms,回波时间TE从10到150 ms;TR = 10,000 ms时,TI从100到4500 ms,5:1和2:1的Vc-SPIO样品中铁浓度均为0.025,0.05,0.1,0.15,0.2,0.3,0.4,0.5 mmoL/L。
2.3. 细胞毒性试验
采用CCK-8实验 [12] 检测Vc-SPIO纳米粒子的细胞毒性。具体方法是:将细胞接种到96孔板中(1.6 × 104/孔),培养细胞贴壁后,加入铁浓度为0,1,2,6,10,15,20,25 ug/ml的样品,分别培养6h和24 h,然后用PBS洗两次,换新培养基200 ul培养24 h。之后加入CCK-8每孔10 ul,用酶标仪(AAS; 700P, Analytik Jena, Germany)在450 nm波长测吸光度从而计算细胞活性。
2.4. 细胞铁含量分析
将小鼠RAW264.7细胞接种到24孔板(3 × 105/孔),培养24 h后用2 ml DMEM冲洗两次,然后加入Vc-SPIO(2/1)样品铁浓度为0,1,2,6,10,15,20 ug/ml的500 ul DMEM,培养24 h。用2 ml冰PBS洗两次,完全除去PBS后加200 ul胰酶消化,加400 ul DMEM终止消化,1000 r/min离心4 min。在潮湿环境下,细胞于200 ul 50 mM的NaOH中裂解2 h,得到细胞裂解液。分别取100 ul的细胞裂解液,100 ul 10 mM HCl 和100 ul铁释放试剂(1 mol/l的HCl与4.5%的KMnO4等体积混合)混合加入离心管,烘箱中孵育2 h,冷却到室温。30 ul铁检测试剂(一定质量的菲啰嗪、新亚铜试剂、醋酸铵、抗坏血酸与水混合)加入到孵育后的离心管中,每管取280 ul加到96孔板中 [13] 。另设一组铁浓度为15 ug/ml培养时间为0,1,2,4,6,12,24 h,实验过程同上。在550 nm波长下测定吸光度,求出铁浓度。
3. 结果与讨论
3.1. SPIO与Vc-SPIO纳米粒子的表征
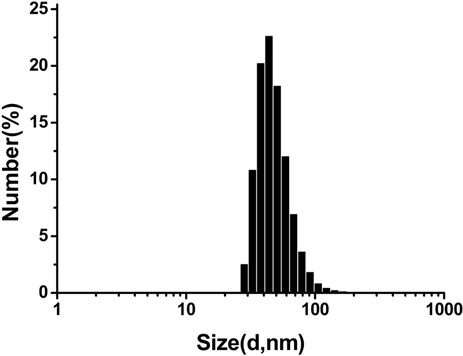
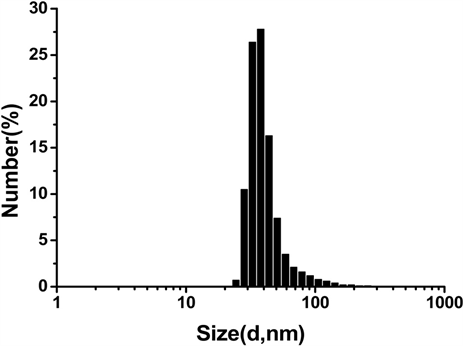
将所合成的SPIO悬浮分散于CHCl3中,Vc-SPIO分散于水中,用动力学光散射仪(DLS)对其粒度大小及分步和zeta电势进行表征,得到结果如图1。图1(a)结果显示SPIO纳米颗粒直径在20~100 nm之间,
 (a)
(a)
 (b)
(b)
 (c)
(c)
 (d)
(d)
 (e)
(e)
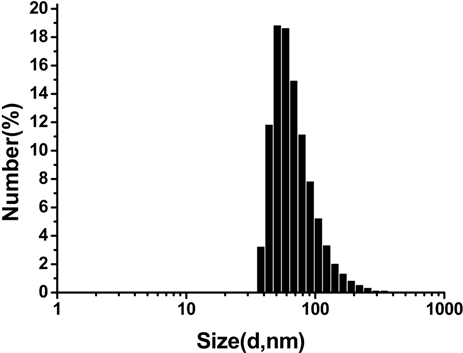
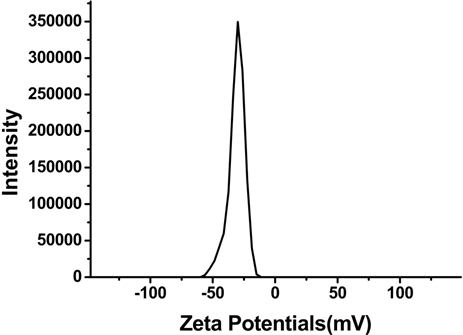
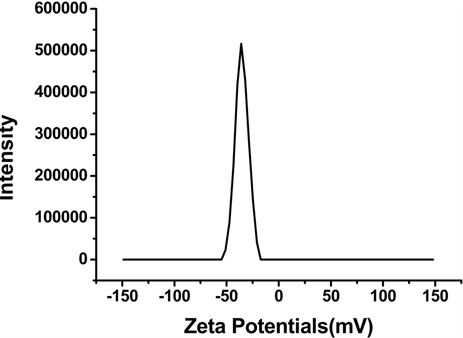
Figure 1. Characterization of SPIO and Vc-SPIO nanoparticles: (a) DLS analysis of SPIO nanocrystals dispersed in chloroform; (b) DLS analysis of Vc-SPIO nanoparticles(2/1) dispersed in water; (c) DLS analysis of Vc-SPIO nanoparticles(5/1) dispersed in water; (d) Zeta potential of Vc-SPIO(2/1); (e) Zeta potential of Vc-SPIO(5/1)
图1. SPIO与Vc-SPIO纳米粒子的表征:(a)油相(三氯甲烷)中SPIO的粒径分布图;(b)水相中Vc-SPIO(2/1)的粒径分布图;(c)水相中Vc-SPIO(5/1)的粒径分布图;(d)水相中Vc-SPIO(2/1)的电势分布图;(e)水相中Vc-SPIO(5/1)的电势分布图
平均粒径为43.8 nm,多分散性指数PDI = 0.181。图1(b)的Vc-SPIO(2/1)纳米簇直径在40~200 nm之间,平均粒径为50.8 nm,多分散性指数PDI = 0.382。图1(c)的Vc-SPIO(5/1)纳米簇直径在30~100 nm之间,平均粒径为37.84 nm,多分散性指数PDI = 0.633。图1(d)(e)显示Vc-SPIO(2/1)纳米簇zeta电势为−29.9 mv,Vc-SPIO(5/1)的zeta电势为−39.7 mv,均为电负性颗粒,在水中能稳定分散存在。粒度与zeta电势结果显示Vc-SPIO纳米簇具有适宜的尺寸和良好的水溶液稳定性。
分别在TR = 5000ms,TE:10~150 ms和TR = 10000 ms,TI:100~4500 ms参数下核磁共振扫描测定Vc:SPIO为2:1与5:1,铁浓度0.025~0.5 mmol/l的MR图,结果如图2所示。由图2可以看出,随着样品铁浓度的增大样品磁共振扫描图像逐渐变暗。
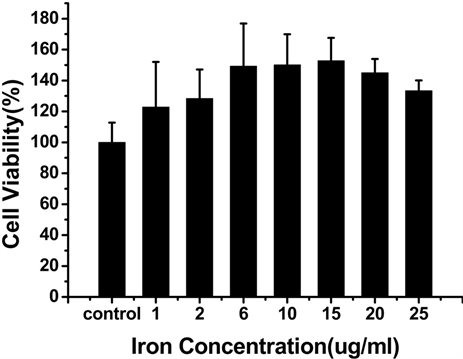
3.2. Vc-SPIO纳米粒子的细胞毒性
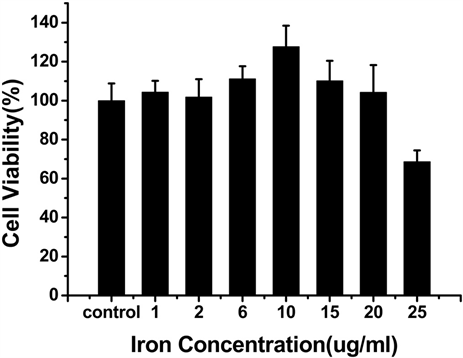
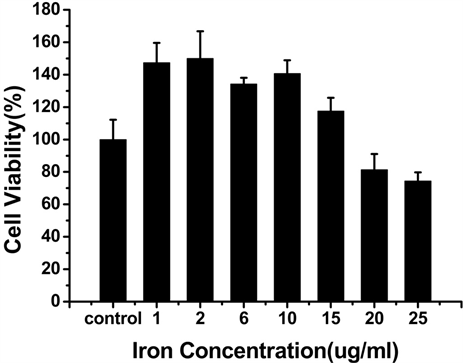
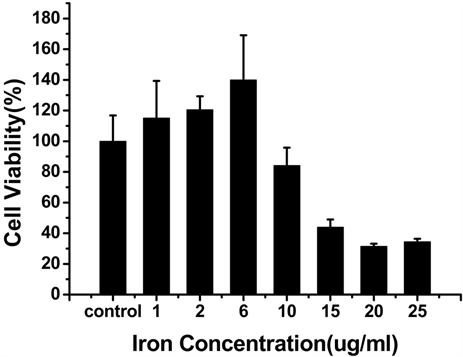
为了检测Vc-SPIO纳米粒子的细胞毒性,进行了CCK-8实验 [12] ,结果如图3所示,当Vc:SPIO为2:1 时,铁浓度在0~15 ug/ml范围内,培养6 h和24 h,细胞活性基本不受影响;当Vc:SPIO为5:1,铁浓度超过15 ug/ml时,细胞活性明显受到影响。
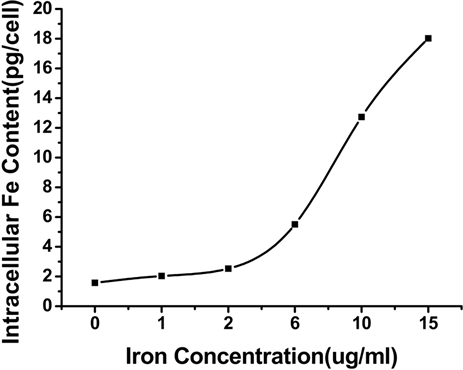
3.3. 细胞内的铁含量
对细胞铁含量进行分析,实验结果如图4。图4(a)是Vc:SPIO为2:1,铁浓度为0~20 ug/ml时细胞内
 (a)
(a) (b)
(b)
Figure 2. (a) MR images of Vc-SPIO(2/1) nanoparticles at different iron concentrations (mM); (b) MR images of Vc-SPIO(5/1) nanoparticles at different iron concentrations (mM). MR images were obtained using a 3T clinical MRI scanner
图2. (a) Vc-SPIO(2/1)不同铁浓度样品磁共振成像图;(b) Vc-SPIO(5/1)不同铁浓度样品磁共振成像图
 (a)
(a)
 (b)
(b)
 (c)
(c)
 (d)
(d)
Figure 3. The viability of mouse mononuclear macmphage cells-RAW264.7 labeling with Vc-SPIO nanoparticles at different iron concentrations. (a and b) The incubation time of Vc-SPIO(2/1) are 6 h and 2 4h, the same as (c and d) Vc-SPIO(5/1)
图3. (a)和(b)分别是Vc-SPIO(2/1)培养时间为6 h和24 h的细胞活性与铁浓度的关系图(c)和(d)分别是Vc-SPIO(5/1)培养时间为6 h和24 h的细胞活性与铁浓度的关系图
 (a)
(a)
 (b)
(b)
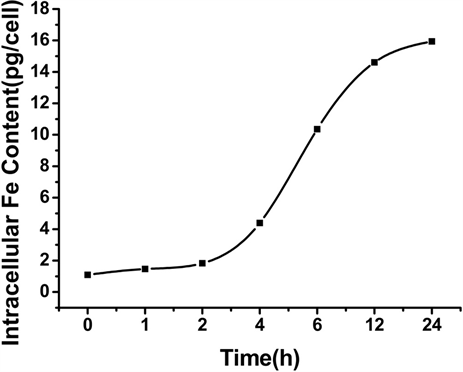
Figure 4. The average iron uptake per cell at different sample concentration (a) and time (b)
图4. (a)和(b)分别是Vc-SPIO(2/1)培养时间为6 h和24 h的细胞活性与铁浓度的关系图
的铁含量与铁浓度之间的关系,可以看出,随着铁浓度的增加,细胞内的铁含量也随之增大。图4(b)是Vc:SPIO为2:1,铁浓度为15 ug/ml,培养时间为0,1,2,4,6,12,24 h时,细胞内的铁含量与培养时间的关系。细胞平均吞铁量关系图结果显示,随着时间的延长,细胞内的铁含量也随之增多,最后趋于稳定,说明12 h后细胞吞吐的铁趋于达到一定的平衡。
4. 结论
本文通过调节Vc与SPIO质量比例(2:1和5:1)构建了纳米Vc-SPIO探针。动力学光散射仪对其粒度大小及分步和zeta电势表征结果显示Vc-SPIO纳米簇具有适宜的尺寸和良好的水溶液稳定性。磁共振成像结果显示,Vc:SPIO为2:1与5:1的Vc-SPIO探针,随着样品铁浓度由0.025~0.5 mmol/l逐渐增大样品磁共振扫描图像均逐渐变暗。CCK-8实验结果显示,当Vc:SPIO为2:1时,铁浓度在0~15 ug/ml范围内,培养6 h和24 h时,细胞活性基本不受影响;当Vc:SPIO为5:1时,铁浓度超过15 ug/ml,细胞活性明显受到影响。细胞铁含量分析结果表明,当Vc:SPIO为2:1,培养细胞的铁浓度在0~20 ug/ml范围内逐渐增加,细胞内的铁含量也随之增大;培养细胞的铁浓度为15 ug/ml时,随着培养时间由0~24 h逐渐增加,细胞内的铁含量在0~4 h缓慢增加,4~12 h明显增加,12 h后基本趋于稳定,说明12 h后细胞吞吐的铁达到一定的平衡。
综上所述,运用Vc-SPIO标记小鼠RAW264.7巨噬细胞是安全可行的。Vc-SPIO标记细胞的安全浓度为1~15 ug/ml,对于今后的活体试验研究提供监测保障。
基金项目
四川省科技支撑计划项目(2015GZ0054)。
文章引用
赵瑞兰,王志勇,刘雨晴,李峻峰. L-抗坏血酸硬脂酸棕榈酸酯包裹氧化铁纳米粒子的磁共振成像以及RAW264.7细胞体外标记检测
Mr Imaging of Superparamagnetic Iron Oxide Nanoparticles Coated L-Ascorbic Acid Stearic Acid Palmitate and in Vitro Labeling of RAW264.7 Cells[J]. 生物医学, 2018, 08(02): 27-33. https://doi.org/10.12677/HJBM.2018.82004
参考文献
- 1. Walter, G.A., Santra, S., Thattaliyath, B. and Grant, S.C. (2008) (Super) Paramagnetic Nanoparticles: Applications in Noninvasive MR Imaging of Stem Cell Transfer. In: Bulte, J.W.M. and Modo, M.M.J., Eds., Nanoparticles in Biomedical Imaging, Springer, New York, 91-140. https://doi.org/10.1007/978-0-387-72027-2_6
- 2. Mc Kay, R. (2000) Stem Cells-Hype and Hope. Nature, 406, 361-364. https://doi.org/10.1038/35019186
- 3. Fayol, D., Luciani, N., Lartigue, L., Gazeau, F. and Wilhelm, C. (2013) Managing Magnetic Nanoparticle Aggregation and Cellular Uptake: A Precondition for Efficient Stem-Cell Differentiation and MRI Tracking. Advanced Healthcare Materials, 2, 313-325. https://doi.org/10.1002/adhm.201200294
- 4. Rinck, P.A. (2001) Magnetic Resonance in Medicine. 4th Edition, Blackwell Science, Berlin.
- 5. Liang, C., Wang, C. and Liu, Z. (2013) Stem Cell Labeling and Tracking with Nanoparticles. Particle and Particle Systems Characterization, 30, 1006-1017. https://doi.org/10.1002/ppsc.201300199
- 6. Barrow, M., Taylor, A., Murray, P., Rosseinsky, M.J. and Adams, D.J. (2015) Design Considerations for the Synthesis of Polymer Coated Iron Oxide Nanoparticles for Stem Cell Labelling and Tracking Using MRI. Chemical Society Reviews, 44, 6733-6748. https://doi.org/10.1039/C5CS00331H
- 7. Zelivyanskaya, M.L., Nelson, J.A., Poluektova, L., et al. (2003) Tracking Superparamagnetic Iron Oxide Labeled Monocytes in Brain by High-Field Magnetic Resonance Imaging. Journal of Neuroscience Research, 73, 284-295. https://doi.org/10.1002/jnr.10693
- 8. van den Bos, E.J., Wagner, A., Mahrholdt, H., et al. (2003) Improved Efficacy of Stem Cell Labeling for Magnetic Resonance Imaging Studies by the Use of Cationic Liposomes. Cell Transplantation, 12, 743-756. https://doi.org/10.3727/000000003108747352
- 9. 程昌明. 面向磁共振成像应用的Fe3O4纳米颗粒/团簇的可控制备[D]: [博士学位论文]. 上海: 上海交通大学化学化工学院, 2010.
- 10. Sun, S., Zeng, H., Robinson, D.B., Raoux, S., Rice, P.M., Wang, S.X. and Li, G. (2004) Monodisperse MFe2O4 (M = Fe Co, Mn) Nanoparticles. Journal of the American Chemical Society, 126, 273-279. https://doi.org/10.1021/ja0380852
- 11. Wan, Q., Xie, L., Gao, L., Wang, Z., Nan, X., Lei, H., Long, X., Chen, Z.Y., He, C.Y., Liu, G., Liu, X. and Qiu, B. (2013) Self-Assembled Magnetic Theranostic Nanoparticles for Highly Sensitive MRI of Minicircle DNA Delivery. Nanoscale, 5, 744-752. https://doi.org/10.1039/C2NR32438E
- 12. Song, W., Zhang, J., Guo, J., Zhang, J., Ding, F., Li, L. and Sun, Z. (2010) Role of the Dissolved Zinc Ion and Reactive Oxygen Species in Cytotoxicity of ZnO Nanoparticles. Toxicology Letters, 199, 389-397. https://doi.org/10.1016/j.toxlet.2010.10.003
- 13. Riemer, J., Hoepken, H.H., Czerwinska, H., Robinson, S.R. and Dringen, R. (2004) Colorimetric Ferrozine-Based Assay for the Quantitation of Iron in Cultured Cells. Analytical Biochemistry, 331, 370-375. https://doi.org/10.1016/j.ab.2004.03.049