Hans Journal of Chemical Engineering and Technology
Vol.
11
No.
02
(
2021
), Article ID:
40804
,
10
pages
10.12677/HJCET.2021.112009
直接甲醇燃料电池阳极催化剂研究现状及展望
李贵贤,祁建军,王东亮,周怀荣,王靖靖,李红伟*
兰州理工大学石油化工学院,甘肃 兰州

收稿日期:2021年1月8日;录用日期:2021年2月27日;发布日期:2021年3月5日

摘要
直接甲醇燃料电池(DMFC)因其具有能量密度高、绿色环保和体积轻便等优势得到广泛关注,其中阳极催化剂活性是决定燃料电池性能、寿命的关键因素。近年来,研究者围绕提高阳极催化剂性能和降低催化剂成本这两个方面展开研究,推动了DMFC的蓬勃发展。本文介绍了电催化剂的催化机理及其分类,详细综述了贵金属催化剂和非贵金属催化剂的合成方法,结合当前研究进展对甲醇电催化剂未来的发展趋势进行展望。
关键词
直接甲醇燃料电池,贵金属催化剂,非贵金属催化剂
Research Progress and Prospect of Anode Catalysts for Direct Methanol Fuel Cells
Guixian Li, Jianjun Qi, Dongliang Wang, Huairong Zhou, Jingjing Wang, Hongwei Li*
School of Petrochemical Engineering, Lanzhou University of Technology, Lanzhou Gansu

Received: Jan. 8th, 2021; accepted: Feb. 27th, 2021; published: Mar. 5th, 2021

ABSTRACT
Direct methanol fuel cell (DMFC) has attracted wide attention due to its advantages of high energy density, environmental protection, and lightness. Among them, the methanol oxidation electrocatalyst is a key factor that determined the performance, life and cost of fuel cells. In recent years, researchers have carried out a lot of researches on improving the activity of anode catalysts and reducing catalyst cost, which has promoted the vigorous development of DMFC. In this paper, the catalytic mechanism and classification of electrocatalysts are introduced, the synthesis methods of noble metal catalysts and non-noble metal catalysts are reviewed in detail, and the research progress of methanol electrocatalyst based on the current research is prospected.
Keywords:Direct Methanol Fuel Cell, Noble Metal Catalyst, Non-Noble Metal Catalyst

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
众所周知,我国的能源结构是“贫油少气,相对富煤”,据报道,截止2019年我国石油资源对外依存度高达73%,天然气对外的依存度也高达40%。然而与石油、天然气资源相比,煤炭资源的储量相对丰富,占我国三大化石能源已探明储量的66.7%左右。按照国际上列出的国家能源安全警戒线,超过51%就会危及能源安全,因此,能源短缺形势日益严峻。我们难以完全依赖石油化工主体能源,因此,煤炭才是我国能源安全的“压舱石”。
在我国所储的煤炭中,高硫和高灰煤占有较大的比重,但这类煤炭难以直接利用。可将这些煤炭用于生产甲醇,并且煤炭中的硫脱后可加以利用 [1]。其次,我国炼钢用焦炭也来自煤炭,每吨煤炭可以获得约0.7吨焦炭,产出大量的焦炉气,焦炉气成分主要是氢气和一氧化碳,这也是生产甲醇的良好原料 [2]。因此,大力发展现代煤化工,将煤炭制备成甲醇是符合国情的现实之选,目前,甲醇是人类已知的最易生产的气体燃料,如何开拓合理利用甲醇的新途径显得十分重要。2019年3月,我国工信部、国家发改委等8部委联合发布了《关于在部分地区开展甲醇汽车应用的指导意见》。《指导意见》指出,为推动传统产业转型升级,加快能源多元化和满足清洁新能源汽车发展要求,对于保障能源安全和推动传统产业升级尤为关键。化石燃料的不可再生性和环境污染使其使用受到限制。清洁能源的开发正受到世界各国科学家的广泛研究。
近年来,在我国能源结构禀赋下和政策引领下,直接甲醇燃料电池(DMFC)成为甲醇开发利用的一个重要方向,并在近年来取得了较快发展 [3] [4]。由于其燃料简单、便于输送、产品无污染,具有较高的能量转换效率,是一种环境友好的绿色能源 [5] [6] [7]。这也促使DMFC具有更高的研究热度。直接甲醇燃料电池工作原理如图1所示。在阳极,甲醇在催化剂的作用下失去电子生成氢质子,氢质子透过质子交换膜迁移并在阴极与氧反应;在阴极上,氧气与从阳极扩散过来的氢离子反应生成水。
但是,也存在一些限制DMFC商业化应用的因素:1) 甲醇在低温下的电催化氧化活性低,反应速率慢 [8] [9] [10];2) 甲醇在质子交换膜处的渗透现象严重,直接导致甲醇燃料的利用效率大幅降低,并且渗透甲醇在阴极处引发混合电位,进一步与氧气发生燃烧反应,降低电池的工作电压 [9] [11] [12]。因此,解决上述瓶颈问题的关键,是提高催化剂电催化活性,使甲醇在较低温度下快速转化,提高甲醇利用效率,减小质子交换膜的渗透压,从而抑制甲醇渗透,提升电池的电化学性能。文章从DMFC的工作原理及甲醇的氧化机理出发,对几种不同元甲醇氧化催化剂的性能进行了罗列比较(见表1),并从贵金属和非贵金属两个方面对催化剂的种类与特点加以综述。

Figure 1. Working principle of direct methanol fuel cell
图1. 直接甲醇燃料电池的工作原理
Table 1. Performance comparison of several catalysts
表1. 几种催化剂的性能比较
2. 催化机理
甲醇的电催化氧化(MOR)过程主要分为两步(如图2所示),首先,甲醇吸附在阳极催化剂上,在催化剂作用下脱质子形成中间产物COads和氢质子,同时释放6个电子;接着碳中间体与含氧物种反应氧化生成CO2,甲醇氧化反应的中间产物COads极易吸附在催化剂表面活性位点上致使催化剂中毒失活 [20],造成催化剂的使用寿命和稳定性降低,严重影响电池的使用 [21]。阳极释放的电子沿着外电路到达阴极,H+穿过质子交换膜到达阴极,与氧气反应生成H2O [7] [9] [22]。电极表面的电化学反应如下 [23]:
阳极反应:CH3OH + H2O → CO2 + 6H+ + 6e− E0 = 0.016 V/SHE (1)
阴极反应:3/2O2 + 6H+ + 6e− → 3H2O E0 = 1.229 V/SHE (2)
总反应:CH3OH + 3/2O2 → CO2 + 2 H2O E0 = 1.21 V/SHE (3)
迄今为止,Pt基催化剂仍是低温条件下电催化氧化甲醇的最佳选择。以Pt基催化剂为例,阳极上MOR反应机理 [24] 如图3所示:
CH3OH + Pt → Pt-CH2OHads + H+ + e− (4)
Pt-CH2OH + Pt → Pt2-CHOHads + H+ + e− (5)
Pt2-CHOH + Pt → Pt3-COHads + H+ + e− (6)
Pt3-COHads + Pt → Pt-COads + H+ + e− (7)
Pt + H2O → Pt-OHads + H+ + e− (8)
Pt-COads + Pt-OHads → 2Pt + CO2 + H+ + e− (9)
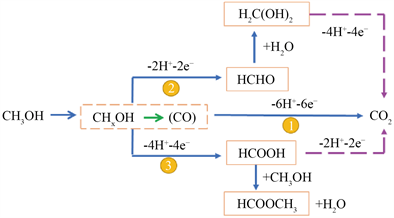
Figure 2. Schematic diagram of oxidation mechanism of methanol
图2. 甲醇氧化机理示意图
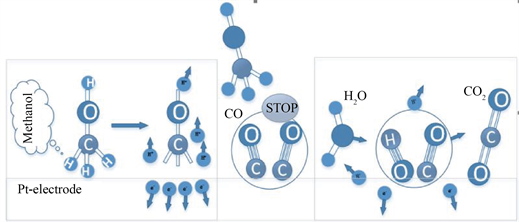
Figure 3. Schematic diagram of different reaction steps of methanol oxidation on platinum catalyst surface
图3. 铂催化剂表面甲醇氧化的不同反应步骤示意图
在氧化过程中步骤(7)占主要部分,生成的COads等中间产物会在Pt表面不断积累,致使催化剂中毒,导致MOR反应电流迅速衰减。步骤(8)和(9)显示,吸附在Pt表面的水解离生成的含氧物种OHads可与中间产物COads结合生成CO2。然而对于纯Pt催化剂而言,吸附在Pt上的水分子只有在较高的电位下才能解离出含氧物种,而在此电位区域内又极易生成COads中间产物,导致催化剂中毒。
3. 甲醇氧化电催化剂的种类和特点
目前,限制DMFC阳极催化剂商业化应用的主要因素有:1) 催化剂活性差,甲醇氧化反应缓慢;2) 作为MOR最常用的催化剂,Pt存储量低,负载率高,成本昂贵;3) 催化剂稳定性差,易中毒。因此,研究一种催化活性好,稳定性高,抗CO中毒性能好的催化剂迫在眉睫 [3] [25] [26]。目前,阳极催化剂领域的研究发展已成为众多科研工作者的关注热点 [21] [27] [28] [29]。
3.1. 贵金属催化剂
3.1.1. 一元催化剂
一元催化剂主要以Pt基催化剂为主,但是纯Pt催化剂价格过于昂贵,且在甲醇电催化氧化过程中极易被中间产物CO毒化,导致其催化性能大幅下降 [21] [22] [30]。因此,寻求一种导电性能良好、比表面积大、抗腐蚀性良好和孔结构适宜的负载材料是提升催化剂性能的一种重要途径 [26]。Zhang等 [31] 报道了一种性能良好的电催化剂Pd/Ti3C2Tx-rGO (1:1),其对MOR的质量比活性为753 mA∙mg−1,是传统Pd/C催化剂(446 mA∙mg−1)的1.7倍。此外,其电流密度也高达212 mA∙mg−1,在7200 s后其电流密度是Pd/C催化剂(23 mA∙mg−1)的9倍。优异的循环稳定性,主要归功于Ti3C2Tx和还原氧化石墨烯在Pd和Ti3C2Tx/rGO的协同作用下,增大了表面积,提高了催化活性和质量/电荷转移能力。此外,通过控制载体形貌进而增加催化剂活性位点的方法,也可有效提高催化活性 [32]。Qiao等 [33] 提出了一种通过瞬态热辐射法合成Pt NC/CB的方法,并利用Pt NC/CB负载超细铂纳米团簇(0.68 ± 0.13 nm),通过测试证明材料具有良好的抗毒性能和甲醇电催化氧化活性。研究发现,超细铂纳米团簇在0.4 V的电位下可以促进CO的氧化,具有高的峰值电流密度(0.57~1.06 mA∙cm−2)。同时,与商用Pt/C和Pt NP/CB相比,催化活性也得到了显著提高,主要原因是纳米团簇结构中具有不同配位数的Pt活性位点。
3.1.2. 二元催化剂
一元Pt基催化剂的中毒失活问题,主要是由于在催化过程与CO结合形成了强Pt=CO键 [34] [35],从而使催化剂表面损失大量活性位点,进而严重影响了其催化活性。为了克服这一问题,通常在催化剂中引入另一种金属元素,如Ru [28]、Sn [36]、Co [37]、Pd [27]、Ni [38] 等,通过双功能机制合理调控催化活性位点 [39]。与纯铂相比,多相双金属纳米催化剂具有良好的抗中毒性能和电催化活性 [40] [41],这是由于复合第二种金属可明显改善催化剂的表面应变能力、几何调控作用(Pt-Pt距离)以及电子转移能力(Pt的d带空位) [42]。二元催化剂由于金属间的协同作用,其抗CO中毒性能够得到大幅提高 [43] [44]。此外,选择不同的负载材料,催化剂性能也会有得到不同程度的改变 [37]。Aramesh等 [45] 发现Pt-Sn薄膜在MOR中具有高效的电催化性能,研究结果表明,Pt-Sn/RGO薄膜比Pt-Sn薄膜具有更大的比表面积和更优异的导电性,这也是Pt-Sn/RGO薄膜具有更高电催化活性的主要原因。Khotseng等 [46] 分别研究了多壁碳纳米管(MWCNTs)、TiO2、MoO2和炭黑Vulcan XC-72等不同载体对Pt-Ru和Pt-Sn双金属催化剂性能的影响,结果表明,Pt-Sn/C具有最佳的活性,活性高低顺序为Pt-Sn/C > Pt/C > Pt-Ru/MWCNT > Pt-Ru/C > Pt-Sn/MWCNT > Pt-Sn/MoO2 > Pt-Ru/MoO2 > Pt-Sn/TiO2 > Pt-Ru/TiO2。Shi等 [35] 采用简单的一氧化硅还原法制备了低Pt的纳米线网状结构的Pt-Au催化剂,网状结构有利于催化剂的电子迁移,进而有效提升催化效率。此外,XRD结果证实Pt-Au复合材料可有效抑制压缩晶格应变,与其他不同Pt含量的Pt-Au催化剂相比,Pt含量较小的催化剂发生更大的应变程度,这种晶格应变使Pt的d轨道中心发生位移。Wang等 [47] 采用原位生长的方法,在石墨烯薄片(GNs)上成功合成了一种棒状的CeO2 (NRCeO2)催化剂。GNs用作防止石墨烯堆积的隔离剂,当生长出的杆状CeO2与点缀有铂纳米颗粒的石墨烯薄片连续堆叠时,则形成三维Pt-NRCeO2/GNs复合结构材料,与其他催化剂相比,具有更高的ECSA值(72.6 m2∙g−1)和更大的催化电流密度(498 mA∙mg−1)。
研究发现,通过控制催化剂表面形态来调整电子结构也可以提高电催化性能。Quyang等 [40] 指出电子结构的改变对电催化活性有显著影响,Pt-Ag双金属纳米晶催化剂和商用Pt/C催化剂相比,具有更高的催化活性和更好的稳定性。理论研究表明,Pt-Ag双金属纳米管表面的Pt外壳显著优化了Pt的电子结构,改善了甲醇的解离吸附特性,同时提高了抗中毒性能,使电极动力学速率加快,催化电流密度提高,稳定性也得到明显提升。Pt-Ag双金属纳米晶具有良好催化性能主要归因于Pt薄外壳的电子结构的优化,提高了甲醇分子的解离速度,减少了CO分子的吸附积累。因此,通过调控材料的形貌使其暴露更多的活性位点,是提高催化性能的有效方法。
Lei等 [48] 采用简单高温液相合成法制备了多晶Pt-M (Fe, Co, Ni)双金属纳米粒子(NBs),其表面具有丰富的台阶和扭结,使其在酸性电解质中具有良好的甲醇氧化(MOR)性能。结果证明,金属形态和组成对其活性具有重要影响,活性排序为Pt-Co/C > Pt-Fe/C > Pt-Ni/C > 商业Pt/C (或Pt-Ru/C)。Guo等 [49] 采用微波辅助乙二醇还原氯铂酸法制备了利用CeO2掺杂的Pt催化剂,研究了不同水热条件和不同CeO2的加入量对Pt催化剂性能的影响。结果表明,CeO2的添加使得催化性能明显提高,且水热反应时间为39 h制备的催化剂,其比表面积为120.15 m2/g,同时电催化性能、稳定性和抗中毒性最佳。Guo等 [50] 提出了一种简便合成法,即在分布均匀的Te纳米上,通过电偶取代和NaClO蚀刻,合成具有极大活性面积的Pd-Pt多孔纳米材料,这是由于非均质组分、OHads吸收能量和Pt活性位点之间的协同效应,增强了其催化性能。Sahoo等 [51] 制备了Pt/ZrC材料,并用于MOR反应,经DFT计算表明,Pt纳米粒子与ZrC和XC具有协同作用,证明Pt/ZrC-XC是一种很有前途的甲醇电氧化催化剂。
3.1.3. 三元催化剂
除上述研究外,近年来三元催化剂也吸引了诸多研究者的目光。Wang等 [52] 采用一锅两步法制备了石墨烯负载的Pt-Fe-Co三元合金纳米复合催化剂,该方法不仅降低了Pt含量,而且有效促进了Fe和Co的协同效应,提高了贵金属的利用率,提高了抗毒性和稳定性,这得益于石墨烯网状结构、表面应变及金属间的电子效应。Zhao等 [53] 采用晶相转变法在Pt-Bi合金相中掺杂高分散铜得到了Pt-Bi-Cu纳米晶体,该催化剂具有丰富的表面缺陷、Pt的d带中心的下降、Pt-Bi和Pt-Cu的双重活性位点,加快甲醇的氧化速率,从而提高了活性和耐久性。Zheng等 [54] 制备了一种Pd-偶联Ag和ZnO的Pd@Ag/ZnO复合电化学催化剂,实验表明,其活性分别是Pd/ZnO和20% Pd/C催化剂的1.8倍和2.8倍。此外,由于催化剂的强双金属相互作用,合成的Pd@Ag/ZnO催化剂对MOR表现出优异的电催化活性,根据密度泛函理论计算结果可知,Pd与Ag之间的强电子效应降低了负载Pd的电子密度,从而消除了d轨道电子对CO反键的反向作用,d轨道电子到CO反键轨道以及Pd和ZnO上存在-OH基团,从而削弱和消除了催化剂对CO的吸附作用。Yuan等 [55] 合成了一种Pt-Bi2WO6/Cu2S复合催化剂材料,通过循环伏安法、CO溶出伏安法、计时安培法评价了该催化剂的电催化性能,结果发现,引入Cu2S显著提高了Pt-Bi2WO6对MOR的电催化活性和稳定性,此外,Cu2S不仅为沉积Pt纳米粒子和捕获活化分子提供了更多的活化位点,而且作为空穴传输通道,可以有效分离Bi2WO6激发的光生电子-空穴对,从而提高光辅助电催化性能。
根据最新报道,通过引入其他金属氧化物,利用金属之间的相互作用也可有效改善催化剂性能。Baneshi等 [21] 通过掺杂CuO、CeO2、ZrO2、Al2O3等金属氧化物提升催化剂性能。Ren等 [56] 以铌掺杂的纳米半球形壳(HSS)为载体,定向沉积少量铂纳米粒子,这种特殊的HSS纳米结构显著增强了铂纳米颗粒在其表面的沉积效果,以此获得了高效的Pt/Nb-TiO2 HSS催化剂,该催化剂的甲醇氧化活性非常高(0.21 V/RHE),比Pt-Ru/C催化剂低0.05 V。这主要是由于底物具有较强的金属载体相互作用效应,在微型DMFC中,这些HSS纳米结构加快了甲醇在阳极内的扩散,使阴极极化的最大输出功率达到0.0931 W;其次,它还充当保护壳,可以最大限度地减少铂金属纳米颗粒的溶解,防止了其通过膜的扩散现象,有效抑制了催化反应中Pt的损失。此外,Basumatary等 [57] 制备了一种具有杂化特性的Pt-Sc-Ni/MoS2@石墨烯催化剂,首先将MoS2沉积在石墨烯薄片上,然后将Pt-Sc-Ni纳米颗粒接枝到MoS2@石墨烯载体上,制备了不同掺杂比(x = 0.2、0.5和1.0)的Sc催化剂,与商用Pt/C催化剂相比,其功率密度要低3.7倍。
3.2. 非贵金属催化剂
由于贵金属成本高昂,因此非贵金属催化剂已经成为当下的研究热点。Guo等 [58] 制备了系列含氧MoS2超薄纳米薄片,由于适量的氧气融入到MoS2中,创造了大量的边缘位点,进一步提高了电导率,An等 [38] 报道了一种用油胺合成Ni-Cu纳米粒子的简单方法,并评价了它们在电催化MOR反应中的活性,结果表明,NiCu-220在质量电流密度为1028 mA∙mg−1时(相对于RHE)性能最佳,其原因在于:1) 合金结构促进了电荷在原子水平上的再分配;2) 与单金属催化剂相比,合金化诱导的电子效应是提高双金属催化剂MOR活性的重要因素;3) Ni-Cu合金催化剂中的Cu有助于抑制低活性副产物-γ-NiOOH的生成。
Pitussi等 [36] 将负载锡(Sn)的薄铂层作为具有更大CO耐受性的催化剂,通过密度泛函理论计算可知,一个CO分子在Pt单层上的吸附能量为1.04 eV,与在2、3、5单层和金属Pt (111)上的吸附能量(1.82~1.92 eV)相比,得到了显著提高。因此,Pt/Sn催化剂对CO的毒害表现出较高的耐受性,成为燃料电池中最具研究前景的低Pt催化剂。Javan等 [59] 报道了一种简便、绿色的电化学合成碳量子点(CQDs)的方法,并用镍纳米粒子进行电化学还原改性,改性后的Ni-NPs/RCQD催化剂对MOR表现出良好的催化活性和耐久性。并采用SEM、EDX和XRD等技术对电化学制备的电极进行了形貌和结构分析,验证了该合成方法的准确性、有效性和可控性,这与CQDs提供的大比表面积以及Ni和CQDs之间的良性相互作用有关,使得甲醇更快更易氧化,并在Ni-NPs/RCQD/GCE催化剂上有快的电子转移速率,碳量子点的约束效应可有效抑制Ni纳米粒子的聚集,使其在催化剂催化过程中形态均匀性增强,稳定性更好。
4. 结论与展望
本文就近年来甲醇氧化电催化剂的研究现状进行系统地综述,主要讲述了催化剂形貌对其性能的影响机制,载体与催化剂性能间的内在关系,以及不同制备方法与催化剂构效关系的本质联系。甲醇氧化电催化剂的研究将主要集中在以下几个方面:
1) 开展非贵金属催化剂的研发,如开发与铂催化性能相媲美的碳化钨、碳化钼替代贵金属等,以降低催化剂的高昂成本。
2) 开展甲醇电催化氧化的反应机理研究,弄清甲醇转化路线,合理设计催化剂结构,为降低CO产率和抑制催化剂中毒提供理论指导。
3) 合成导电性好、活性比表面积大、利于甲醇扩散的多级孔催化剂载体,进而制备高活性、低成本、长寿命的可回收金属基催化剂。
总之,研究高活性和优良抗毒化性催化剂的构效关系对直接甲醇燃料电池的商业化应用具有重要的意义。
文章引用
李贵贤,祁建军,王东亮,周怀荣,王靖靖,李红伟. 直接甲醇燃料电池阳极催化剂研究现状及展望
Research Progress and Prospect of Anode Catalysts for Direct Methanol Fuel Cells[J]. 化学工程与技术, 2021, 11(02): 66-75. https://doi.org/10.12677/HJCET.2021.112009
参考文献
- 1. 周鸿宇, 张甜甜, 芦娅妮. 煤化工技术的发展与新型煤化工技术[J]. 化工设计通讯, 2018, 44(12): 15. http://dx.chinadoi.cn/10.3969/j.issn.1003-6490.2018.12.014
- 2. 赵俊学, 马成, 胡冰, 邹冲, 刘军利, 刘启航. 以低温干馏为基础的低阶煤分质高效利用技术分析[J]. 煤炭加工与综合利用, 2017(6): 1-6. http://dx.chinadoi.cn/10.16200/j.cnki.11-2627/td.2017.06.001
- 3. 刘洋洋, 孙燕芳, 靳文, 李奎. 直接甲醇燃料电池阳极催化剂的研究进展[J]. 电源技术, 2019, 43(8): 1397-1402. http://dx.chinadoi.cn/10.3969/j.issn.1002-087X.2019.08.044
- 4. Tong, Y., Yan, X., Liang, J. and Dou, S.X. (2019) Metal-Based Electrocatalysts for Methanol Electro-Oxidation: Progress, Opportunities, and Challenges. Small, 2019, Article ID: 1904126. https://doi.org/10.1002/smll.201904126
- 5. Araujo, R.B., Daniei, M.Y., Santos, E., Cornell, A. and Pettersson, L.G.M. (2020) Elucidating the Role of Ni to Enhance the Methanol Oxidation Reaction on Pd Electrocatalysts. Electrochemical Acta, 360, Article ID: 136954. https://doi.org/10.1016/j.electacta.2020.136954
- 6. Jo, W.K., Moru, S., Lee, D.E. and Tonda, S. (2020) Cobalt- and Iron-Coordinated Graphitic Carbon Nitride on Reduced Grapheme Oxide: A Nonprecious Bimetallic M-Nx-C Ana-logue Electrocatalyst for Efficient Oxygen Reduction Reaction in Acidic Media. Applied Surface Science, 531, Article ID: 147367. https://doi.org/10.1016/j.apsusc.2020.147367
- 7. Fard, H.F., Khodaverdi, M., Pourfayaz, F. and Ahmadi, M.H. (2020) Application of N-Doped Carbon Nanotube-Supported Pt-Ru as Electrocatalyst Layer in Passive Direct Methanol Fuel Cell. International Journal of Hydrogen Energy, 45, 25307-25316. https://doi.org/10.1016/j.ijhydene.2020.06.254
- 8. Zhang, T., Sun, Y., Li, X., Li, X.Y., Liu, D.L., Liu, G.Q., et al. (2020) PtPdAg Hollow Nanodendrites: Template-Free Synthesis and High Electrocatalytic Activity for Methanol Oxidation Reaction. Small Methods, 4, Article ID: 1900709. https://doi.org/10.1002/smtd.201900709
- 9. Xiao, F., Xu, G.L., Sun, C.J., Xu, M.J., Wen, W., Wang, Q., et al. (2019) Nitrogen-Coordinated Single Iron Atom Catalysts Derived from Metal Organic Frameworks for Oxygen Reduc-tion Reaction. Nano Energy, 61, 60-68. https://doi.org/10.1016/j.nanoen.2019.04.033
- 10. Sheng, H.Y., Xu, J.H., Liang, X.D., Pan, Z.-W., Tong, Y.-X., Wu, M.M., et al. (2014) Correction: Cu2O Template Synthesis of High-Performance PtCu Alloy Yolk-Shell Cube Catalysts for Direct Methanol Fuel Cells. Chemical Communications, 50, 12337-12340. https://dx.doi.org/10.1039/C4CC04108A
- 11. Zeng, Y.F., Wang, Y.Y., Huang, G., Chen, C., Huang, L.L., Chen, R., et al. (2018) Porous CoP Nanosheets Converted from Layered Double Hydroxides with Superior Electrochemical Activity for Hydrogen Evolution Reactions at Wide pH Ranges. Chemical Communications, 54, 1465-1468.
- 12. Ramli, Z., Kamarudin, S.K., Basri, S. and Zainoodin, A.M. (2020) The Potential of Novel Carbon Nanocages as a Carbon Support for an Enhanced Methanol Electro, Oxidation Reaction in a Direct Methanol Fuel Cell. International Journal of Energy Research, 44, 10071-10086. https://doi.org/10.1002/er.5621
- 13. Li, H., Kang, D., Wang, H. and Wang, R. (2011) Carbon-Supported PtRuCo Nanoparticles with Low-Noble-Metal Content and Superior Catalysis for Ethanol Oxidization. International Journal of Electrochemical Science, 6, 1058-1065.
- 14. Lim, D.H., Choi, D.H., Lee, W.D. and Lee, H. I. (2009) A New Synthesis of a Highly Dispersed and CO Tolerant PtSn/C Electrocatalyst for Low-Temperature Fuel Cell; Its Electrocatalytic Activity and Long-Term Durability. Applied Catalysis B: Environmental, 89, 484-493. https://doi.org/10.1016/j.apcatb.2009.01.011
- 15. Wang, S., Yang, G. and Yang, S. (2015) Pt-frame@Ni quasi Core-Shell Concave Octahedral PtNi3 Bimetallic Nanocrystals for Electrocatalytic Methanol Oxidation and Hydrogen Evolution. The Journal of Physical Chemistry C, 119, 27938-27945. https://doi.org/10.1021/acs.jpcc.5b10083
- 16. Hoseini, S.J., Bahrami, M. and Dehghani, M. (2014) Formation of Snowman-Like Pt/Pd Thin Film and Pt/Pd/Reduced-Graphene Oxide Thin Film at Liquid-Liquid Interface by Use of Organometallic Complexes, Suitable for Methanol Fuel Cells. RSC Advances, 4, 13796-13804. https://doi.org/10.1039/C4RA01625D
- 17. Ding, L.X., Li, G.R., Wang, Z.L., Liu, Z.Q., Liu, H. and Tong, Y.X. (2012) Porous Ni@Pt Core-Shell Nanotube Array Electrocatalyst with High Activity and Stability for Methanol Oxida-tion. Chemistry-A European Journal, 18, 8386-8391. https://doi.org/10.1002/chem.201200009
- 18. Wu, S., Liu, J., Liang, D., Sun, H., Ye, Y., Tian, Z. and Liang, C. (2016) Photo-Excited in Situ Loading of Pt Clusters onto rGO Immobilized SnO2 with Excellent Catalytic Performance toward Methanol Oxidation. Nano Energy, 26, 699-707. https://doi.org/10.1016/j.nanoen.2016.06.038
- 19. Yang, L., Ge, J., Liu, C., Wang, G. and Xing, W. (2017) Approaches to Improve the Performance of Anode Methanol Oxida-tion Reaction—A Short Review. Current Opinion in Electrochemistry, 4, 83-88. https://doi.org/10.1016/j.coelec.2017.10.018
- 20. Ren, X., Wang, Y., Liu, A., Zhang, Z.H., Lv, Q.Y. and Liu, B.H. (2020) Current Progress and Performance Improvement of Pt/C Catalysts for Fuel Cells. Journal of Materials Chemistry A, 8, 24284-24306. https://doi.org/10.1039/D0TA08312G
- 21. Baneshi, J., Haghighi, M., Jodeiri, N., et al. (2013) Homogeneous Precipitation and Urea-Nitrate Combustion Preparation of Nanostructured CuO/CeO2/ZrO2/Al2O3 Oxides used in Hy-drogen Production from Methanol for Fuel Cells. Particulate Science and Technology, 2018, Article ID: 1455778.
- 22. Zhang, F.F., Wang, Z.Y., Xu, K., Xia, J.F., Liu, Q.Y. and Wang, Z.H. (2018) Highly Dispersed Ul-trafine Pt Nanoparticles on Nickel-Cobalt Layered Double Hydroxide Nanoarray for Enhanced Electrocatalytic Methanol Oxidation. International Journal of Hydrogen Energy, 43, 16302-16310. https://doi.org/10.1016/j.ijhydene.2018.07.059
- 23. 王萌. 质子交换膜燃料电池设计与综合优化研究[D]: [博士学位论文]. 北京: 北京科技大学, 2019.
- 24. 王子豪. CeO2/G复合物为载体的直接甲醇燃料电池阳极催化剂性能研究[D]: [硕士学位论文]. 包头: 内蒙古科技大学, 2019.
- 25. 苏敏, 贺春林, 马国峰, 鲁志颖. 直接甲醇燃料电池阳极催化剂性能[J]. 沈阳大学学报(自然科学版), 2019, 31(3): 178-183.
- 26. 罗远来, 梁振兴, 廖世军. 直接甲醇燃料电池阳极催化剂研究进展[J]. 催化学报, 2010, 31(2): 141-149. http://dx.chinadoi.cn/10.3724/SP.J.1088.2010.90740
- 27. Dobrovetska, O., Saldan, I., Lubomir, O., Karlsson, D., Sahlberg, M.H., Semenyuk, Y., et al. (2020) Electrocatalytic Activity of Pd-Au Nanoalloys during Methanol Oxidation Reaction. International Journal of Hydrogen Energy, 45, 4444-4456. https://doi.org/10.1016/j.ijhydene.2019.12.029
- 28. Chen, W., Li, D., Peng, C., Qian, G., Duan, X.Z., Chen, D., et al. (2017) Mechanistic and Kinetic Insights into the Pt-Ru Synergy during Hydrogen Generation from Ammonia Borane over PtRu/CNT Nanocatalysts. Journal of Catalysis, 356, 186-196. https://doi.org/10.1016/j.jcat.2017.10.016
- 29. Guo, X., Yang, L., Shen, B., Wei, Y.J., Yang, Y., Yang, C.Z., et al. (2020) Ultrafine Pd Nanocrystals Anchored onto Single-Walled Carbon Nanohorns: A Highly-Efficient Multifunctional Electrocatalyst with Ultra-Low Pd Loading for Formic Acid and Methanol Oxidation. Materials Chemistry and Physics, 250, Article ID: 123167. https://doi.org/10.1016/j.matchemphys.2020.123167
- 30. Mai, L.N.T., Bach, L.G., Bui, Q.B. and Nhac-Vu, H.-T. (2020) Ultra-Small Platinum Nanoparticles Deposited Graphene Supported on 3D Framework as Self-Supported Catalyst for Methanol Oxidation. Synthetic Metals, 263, Article ID: 116355. https://doi.org/10.1016/j.synthmet.2020.116355
- 31. Zhang, P., Fan, C., Wang, R., Xu, C.X., Cheng, J.G., Wang, L.C., et al. (2019) Pd/MXene (Ti3C2Tx)/Reduced Graphene Oxide Hybrid Catalyst for Methanol Electro-Oxidation. Nanotechnology, 31, 09LT01. https://doi.org/10.1088/1361-6528/ab5609
- 32. Li, M., Fang, Y., Zhang, G., Cui, P., Yang, Z.Z. and He, J.B. (2020) CARBON-Supported Pt5P2 Nanoparticles Used as a High-Performance Electrocatalyst for Methanol Oxidation Reaction. Journal of Materials Chemistry A, 8, 10433-10438. https://doi.org/10.1039/D0TA02300K
- 33. Qiao, Y., Liu, Y., Liu, Y., Dong, Q., Zhong, G., Wang, X., et al. (2020) Thermal Radiation Synthesis of Ultrafine Platinum Nanoclusters toward Methanol Oxidation. Small Methods, 4, Article ID: 2000265. https://doi.org/10.1002/smtd.202000265
- 34. Thota, A., Boga, K., Narayan, R., Bojja, S. and Rao, C.R.K. (2019) Synthesis of Star Shaped Electroactive, LEB State Aniline Oligomer and Its High Performing Pt and Pt-Au Nanocatalyst for MOR. International Journal of Hydrogen Energy, 44, 11066-11078. https://doi.org/10.1016/j.ijhydene.2019.02.207
- 35. Shi, H., Liao, F., Zhu, W., Shao, C.R. and Shao, M.W. (2020) Effective PtAu Nanowire Network Catalysts with Ultralow Pt Content for Formic Acid Oxidation and Methanol Oxidation. International Journal of Hydrogen Energy, 45, 16071-16079. https://doi.org/10.1016/j.ijhydene.2020.04.003
- 36. Pitussi, I., Schechter, A., Teller, H., Natan, A. and Kornweitz, H. (2020) Tailored Pt Coatings on Metallic Tin-An Effective Catalyst for Fuel Cells Anodes. Journal of the Electro-chemical Society, 167, Article ID: 044512. https://doi.org/10.1149/1945-7111/ab754b
- 37. Burhan, H., Ay, H., Kuyuldar, E. and Sen, F. (2020) Monodis-perse Pt-Co/GO Anodes with Varying Pt: Co Ratios as Highly Active and Stable Electrocatalysts for Methanol Elec-trooxidation Reaction. Scientific Reports, 10, Article No. 6114. https://doi.org/10.1038/s41598-020-63247-6
- 38. An, Y., Ijaz, H., Huang, M., Qu, J.Q. and Hu, S. (2020) The One-Pot Synthesis of CuNi Nanoparticles with Ni-Rich Surface for Electrocatalytic Methanol Oxidation Reaction. Dalton Transactions, 49, 1646-1651. https://doi.org/10.1039/C9DT04661E
- 39. Song, T., Gao, F., Zhang, Y., Chen, C.Y., Wang, C., Li, S.J., et al. (2020) Efficient Polyalcohol Oxidation Electrocatalysts Enabled by PtM (M=Fe, Co, Ni) Nanocubes Surrounded by (200) Crystal Facets. Nanoscale, 12, 9842-9848. https://doi.org/10.1039/D0NR00163E
- 40. Ouyang, Y., Cao, H., Wu, H., Wu, D.B., Wang, F.Q., Fan, X.J., et al. (2020) Tuning Pt-Skinned PtAg Nanotubes in Nanoscales to Efficiently Modify Electronic Structure for Boosting Performance of Methanol Electrooxidation. Applied Catalysis B: Environmental, 265, Article ID: 118606. https://doi.org/10.1016/j.apcatb.2020.118606
- 41. Liang, Z., Song, L., Elnabawy, A.O., Marinkovic, N., Mav-rikakis, M. and Adzic, R.R. (2020) Platinum and Palladium Monolayer Electrocatalysts for Formic Acid Oxidation. Top-ics in Catalysis, 63, 742-749. https://doi.org/10.1007/s11244-020-01264-5
- 42. Khan, I.A., Khan, L., Khan, S.I. and Badshah, A. (2020) Shape-Control Synthesis of PdCu Nanoparticles with Excellent Catalytic Activities for Direct Alcohol Fuel Cells Appli-cation. Electrochimica Acta, 349, Article ID: 136381. https://doi.org/10.1016/j.electacta.2020.136381
- 43. Bian, T., Zhang, H., Jiang, Y.Y., Jin, C.H., Wu, J.B., Yang, H., et al. (2015) Epitaxial Growth of Twinned Au-Pt Core-Shell Star-Shaped Decahedra as Highly Durable Electrocata-lysts. Nano Letters, 15, 7808-7815. https://doi.org/10.1021/acs.nanolett.5b02960
- 44. Shi, M.Q., Song, G.H., Yang, P.P., Chu, Y.-Q. and Ma, C.-A. (2015) A New Biomass Template to Prepare Multi-Channel Structure of WO3 and Its Application for Methanol Electro-Oxidation. Materials Letters, 153, 124-127. https://doi.org/10.1016/j.matlet.2015.04.014
- 45. Arammesh, N., Hoseini, S.J., Shansavari, H.R., Masoud Nabav-izadeh, S., Bahrami, M., Reza Halvagar, M., et al. (2020) PtSn Nanoalloy Thin Films as Anode Catalysts in Methanol Fuel Cells. Inorganic Chemistry, 59, 10688-10698. https://doi.org/10.1021/acs.inorgchem.0c01147
- 46. Khotseng, L., Bangisa, A., Modibedi, R.M. and Linkov, V. (2016) Electrochemical Evaluation of Pt-Based Binary Catalysts on Various Supports for the Direct Methanol Fuel Cell. Electrocatalysis, 7, 1-12. https://doi.org/10.1007/s12678-015-0282-x
- 47. Wang, W., Lu, X., Zhu, M., Cao, Z., Li, C., Gao, Y., et al. (2015) Rod-Shaped CeO2 Intercalated Graphene for Supporting Pt Composite as Anode Catalysts for DMFCs. Electrochimica Acta, 176, 1338-1342. https://doi.org/10.1016/j.electacta.2015.07.057
- 48. Lei, W., Li, M., He, L., Meng, X., Mu, Z., Yu, Y., et al. (2020) A General Strategy for Bimetallic Pt-Based Nano-Branched Structures as Highly Active and Stable Oxygen Reduction and Methanol Oxidation Bifunctional Catalysts. Nano Research, 13, 638-645. https://doi.org/10.1007/s12274-020-2666-3
- 49. Guo, R.H., Wang, J., An, S.L., Zhang, J., Zhou, G. and Guo, L. (2020) Effect of Cerium Oxide Prepared under Different Hydrothermal Time on Electrocatalytic Performance of Pt-Based Anode Catalysts. Journal of Rare Earths, 38, 384-394. https://doi.org/10.1016/j.jre.2019.05.010
- 50. Guo, T., Xiang, H., Li, W., Li, H., Chen, H., Liu, S. et al. (2020) Synthesis of Ultrathin and Composition-Tunable PdPt Porous Nanowires with Enhanced Electrocatalytic Performance. ACS Sustainable Chemistry & Engineering, 8, 2901-2909. https://doi.org/10.1021/acssuschemeng.9b07189
- 51. Sahoo, M.K., Shanmugam, R., Umeshbabu, E. and Ranga Rao, G. (2020) Activated ZrC Promotes the Methanol Electro-oxidation Activity and Enhances Poison Tolerance of Pt Nanoparticles in Acidic Medium. ChemistrySelect, 5, 7205-7216. https://doi.org/10.1002/slct.202001581
- 52. Wang, H., Zhang, K., Qiu, J., Wu, J., Shao, J., Wang, H., et al. (2020) Ternary PtFeCo Alloys on Grapheme with High Electrocatalytic Activities for Methanol Oxidation. Nanoscale, 12, 9824-9832. https://doi.org/10.1039/D0NR00757A
- 53. Zhao, F., Ye, J.Y., Yuan, Q., Yang, X., and Zhou, Z. (2020) Realizing a CO-Free Pathway and Enhanced Durability in Highly Dispersed Cu-Doped PtBi Nanoalloys towards Methanol Full Electrooxidation. Journal of Materials Chemistry A, 8, 11564-11572. https://doi.org/10.1039/D0TA03330H
- 54. Zheng, Y., Zhai, Y., Tu, M., Huang, X., Shu, M., Guo, X., et al. (2020) Bimetals and Semiconductor Support Synergistic Interaction Effects for Superior Electrochemical Catalysis. Na-noscale, 12, 4719-4728. https://doi.org/10.1039/C9NR09608F
- 55. Yuan, C., Gao, H., Xu, Q., Song, X., Zhai, C. and Zhu, M. (2020) Pt Decorated 2D/3D Heterostructure of Bi2WO6 Nanosheet/Cu2S Snowflake for Improving Electrocatalytic Methanol Oxi-dation with Visible-Light Assistance. Applied Surface Science, 521, Article ID: 146431. https://doi.org/10.1016/j.apsusc.2020.146431
- 56. Ren, B., Lu, J., Wang, Y., Gu, X., Xu, B.B., Fu, Y., et al. (2020) Half-Sphere Shell Supported Pt Catalyst for Electrochemical Methanol Oxidation. Journal of the Electrochemical Society, 167, Article ID: 084510. https://doi.org/10.1149/1945-7111/ab8dde
- 57. Basumatary, P., Konwar, D. and Yoon, Y.S. (2020) Conductivi-ty-Tailored PtNi/MoS2 3D Nanoflower Catalyst via Sc doping as a Hybrid Anode for a Variety of Hydrocarbon Fuels in Proton Exchange Membrane Fuel Cells. Applied Catalysis B: Environmental, 267, Article ID: 118724. https://doi.org/10.1016/j.apcatb.2020.118724
- 58. Guo, J., Li, F., Sun, Y., Zhang, X. and Tang, L. (2015) Oxygen-Incorporated MoS2 Ultrathin Nanosheets Grown on Graphene for Efficient Electrochemical Hydrogen Evolution. Journal of Power Sources, 291, 195-200. https://doi.org/10.1016/j.jpowsour.2015.05.034
- 59. Javan, H., Asghari, E., Ashassi, S.H. and Moradi-Haghighi, M. (2020) Nickel Nanoparticles Decorated on Carbon Quantum Dots as a Novel Non-Platinum Catalyst for Methanol Oxidation; A Green, Low-Cost, Electrochemically-Synthesized Electrocatalyst. Chemical Engineering science, 217, Arti-cle ID: 115534. https://doi.org/10.1016/j.ces.2020.115534
NOTES
*通讯作者。
