Advances in Clinical Medicine
Vol.
08
No.
07
(
2018
), Article ID:
26768
,
7
pages
10.12677/ACM.2018.87103
An Unusual Case of Metastasis of an Epithelioid Sarcoma to the Pleura and Bronchus: A Case Report and Literature Review
Bingqun Wu1, Shenhai Wei1, Jintao Tian1, Xiaoping Song1, Pengcheng Hu1, Yong Cui2*
1Department of Thoracic Surgery, The First Hospital of Tsinghua University, Beijing
2Department of Thoracic Surgery, Beijing Friendship Hospital Affiliated to Capital Medical University, Beijing

Received: Aug. 14th, 2018; accepted: Sep. 4th, 2018; published: Sep. 11th, 2018

ABSTRACT
We report a 31-year-old male patient who was diagnosed with right forearm epithelioid sarcoma 4 years ago. This hospitalization revealed pleural and bronchial metastases. This case report was informed by the patient and approved by the hospital ethics committee.
Keywords:Epithelioid Sarcoma, Pleural Metastasis, Bronchial Metastasis
上皮样肉瘤支气管胸膜转移1例并文献复习
吴炳群1,魏慎海1,田进涛1,宋小平1,胡鹏程1,崔永2*
1清华大学第一附属医院,胸外科,北京
2首都医科大学附属北京友谊医院,胸外科,北京

收稿日期:2018年8月14日;录用日期:2018年9月4日;发布日期:2018年9月11日

摘 要
我们报道一位31岁男性患者,4年前确诊为右前臂上皮样肉瘤,此次就诊发现其胸膜和支气管转移。该病例报道获患者本人知情同意,并经医院伦理委员会批准。
关键词 :上皮样肉瘤,胸膜转移,支气管转移

Copyright © 2018 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
上皮样肉瘤(Epithelioid sarcoma, Es)首先被Enzinge [1] 提出,为一罕见的恶性软组织肉瘤,多发于青少年和年轻男性患者,部位多为四肢远端,其发病率低,占所有软组织肉瘤的比例不到1%。有文献报道其发生肺囊性转移并单侧或双侧气胸 [2] [3] [4] [5] [6] ,但很少有报道其广泛胸膜转移 [7] ,至今,我们第一次发现其并发广泛胸膜转移并一侧支气管粘膜转移。在此,我们报道1例软组织肉瘤胸膜及支气管转移患者。
2. 正文
患者男,31岁,4年前确诊为右手及前臂软组织肉瘤,并于右肱骨中部行右上肢截肢术,术后进行放疗及化疗治疗。患者2个月前“感冒”后出现咳嗽咳痰,为黏白痰,偶有胸痛,无明显胸闷,无头痛头晕,无恶心呕吐,自诉服用“感冒药物”后咳嗽无明显好转,1天前突发胸闷,就诊于我院急诊,查胸片示:右侧液气胸。查体:胸廓无畸形,右侧呼吸运动减弱,右肺语颤减弱,未触及胸膜摩擦感,无皮下捻发感,右肺叩诊鼓音,左肺叩诊呈清音,右肺呼吸音弱,左肺呼吸音粗,双肺未闻及干湿罗音,语音传导右侧减弱,未闻及胸膜摩擦音。入院后进一步行胸部CT检查示:左肺上叶支气管截断,伴左上叶不张,占位?右肺液气胸,右肺多发结节(图1,图2)。考虑到患者左肺上叶不张,为进一步明确原因,行支气管镜检查,见左肺上叶支气管阻塞,支气管粘膜多发病变(图3),病理活检,考虑为上皮样肉瘤。胸腔闭式引流出淡红色血性积液,1周后患者咳嗽时仍有大量气体引流出,肺复张差,行胸腔镜探查并处理右侧液气胸。胸腔镜探查见:胸腔内淡血性胸腔积液,胸膜表面散在大小不等结节,最大者有黄豆粒大小(图4),脏层胸膜表面有大量纤维素沉着,肺膨胀不全。胸腔镜下取部分结节送冰冻,回报为恶性肿瘤。
病理学检查证实左支气管粘膜病变和胸膜结节均为上皮样肉瘤。H & E染色显示其为有核分裂和支气管上皮细胞的恶性肿瘤细胞(图5,图6,图7)。免疫组化分析证实肿瘤细胞细胞角蛋白(cytokeratin CK)、波形蛋白(Vimentin, Vim)、细胞细胞角蛋白8 (cytokeratin8, CK8),CD34均阳性(图8~图14)。
3. 讨论
上皮样肉瘤首先由Enzinger [1] 于1970年提出,是一种极度罕见的软组织肉瘤,占所有软组织肉瘤的比例不到1%。根据发生部位分为远端型(即经典型,主要位于四肢的末端)和近端型(发生于头颈部及躯干),以前者多见 [8] 。组织病理学方面,上皮样肉瘤是一种以形态学为主的上皮分化的间充质肿瘤 [8] 。免疫组化:广谱细胞角蛋白PCK抗体(pan-CK)、上皮膜抗原(EMA)、波形蛋白(Vimentin)、CD34 (about 50% of all) [9] [10] [11] [12] 。大多数上皮样肉瘤起源于远端浅表或深部组织 [12] ,多发于年轻成年男性 [1] 。

Figure 1. Right hydropneumothorax, left upper lobe bronchial cutoff and the upper lobe of the left lung atelectasis
图1. 右侧液气胸,左肺上叶支气管阻塞,伴左上叶不张

Figure 2. Right hydropneumothorax, multiple lung nodules
图2. 右侧液气胸,右肺多发结节
 (a)
(a)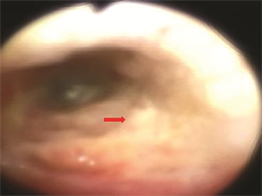 (b)
(b)
Figure 3. Bronchoscopy: left upper lobe bronchus congestion, bronchial mucosal lesions
图3. 支气管镜:左肺上叶支气管阻塞,支气管粘膜病变
 (a)
(a) (b)
(b)
Figure 4. The pleural surface scattered sizes nodules, the surface of visceral pleura has a lot of cellulose, and atelectasis
图4. 壁层胸膜表面散在大小不等结节,脏层胸膜表面有大量纤维素沉着,肺膨胀不全
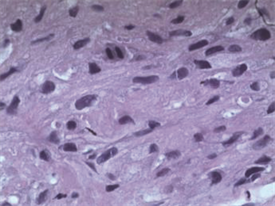
Figure 5. (Bronchial mucosal lesions) malignant cells with nuclear division (H & E stain, ×400)
图5. (支气管粘膜病变)具有核分裂的恶性肿瘤细胞(H & E 染色,×400)

Figure 6. (Bronchial mucosal lesions) malignant cells and bronchial epithelial cells (H & E stain, ×100)
图6. (支气管粘膜病变)恶性肿瘤细胞和支气管上皮细胞(H & E染色,×100)

Figure 7. (Pleural nodules) malignant cells (H & E stain, ×100)
图7. (胸膜结节)恶性肿瘤细胞(H & E染色,×100)

Figure 8. (Bronchial mucosal lesions) positive for Vim (immunohistochemistry ×200)
图8. (支气管粘膜病变)免疫组织化学分析示Vim阳性(×200)
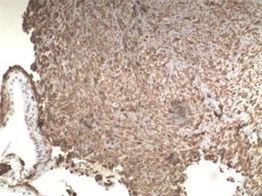
Figure 9. Positive for Vim with bronchial epithelial cells (immunohistochemistry × 100)
图9. (支气管粘膜病变)免疫组织化学分析示:Vim阳性并带有支气管上皮细胞(×100)

Figure 10. Positive for CD34 (immunohistochemistry ×200)
图10. (支气管粘膜病变) 免疫组织化学分析示CD34阳性(×200)
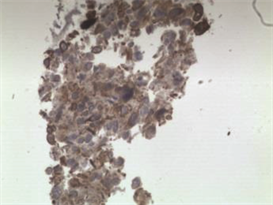
Figure 11. Positive for CK (immunohistochemistry ×200)
图11. (支气管粘膜病变)免疫组织化学分析示CK阳性(×200)
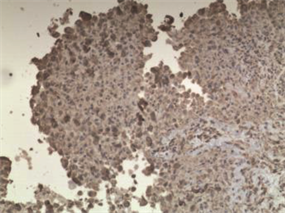
Figure 12. (Pleural nodules) positive for Vim (immunohistochemistry ×100)
图12. (胸膜结节)免疫组织化学分析示Vim阳性(×100)

Figure 13. (Pleural nodules) positive for CD34 (immunohistochemistry ×100)
图13. (胸膜结节)免疫组织化学分析示:CD34阳性(×100)

Figure 14. (Pleural nodules) positive for CK (immunohistochemistry ×100)
图14. (胸膜结节)免疫组织化学分析示CK阳性(×100)
通常为惰性,但临床上时有局部复发和淋巴结转移,常见转移部位依次为肺,局部淋巴结,头皮,骨,脑,肝,胸膜 [13] 。显微镜下,中央坏死周围有嗜酸性细胞质和周边梭形的轻度或非典型多角形细胞,上皮样肉瘤通常是多结节的 [14] [15] [16] 。关于治疗,首选手术治疗,广泛切除或根治性手术是必不可少的,建议进行预防性淋巴结清扫,并辅助放疗、系统化疗及免疫治疗 [12] [17] [18] [19] 。
上皮样肉瘤的形态异质性常常导致转移标本的诊断困难。从临床管理方面来说,只需要区分转移性肉瘤与癌和黑素瘤,因为目前治疗转移性肉瘤的方法主要不是手术、化疗和放疗,部分原因是鉴于肉瘤的罕见和极端多样性,难以在临床试验中获得足够数量的患者。有多种罕见类型的肉瘤可见于转移标本,包括肺泡软组织肉瘤,透明细胞肉瘤,上皮样血管内皮瘤,上皮样血管肉瘤和颗粒细胞瘤 [20] 。罕见的是,大量的胸膜和支气管转移可能是肉瘤的第一个征兆。这些实体即使可能,也很难仅基于细胞形态学来辨别。然而,在出现恶性渗出之前,这些肉瘤患者通常有已知的原发肿瘤病史。在大多数情况下,病理学家通过在形态学上与原发性肿瘤进行比较,并进行免疫过氧化物酶研究,将两者结合起来,可以得出准确的诊断结果。总之,来自胸膜和支气管的转移性上皮样肉瘤的细胞病理学与切除的原发肿瘤的组织病理学有很好的相关性。上皮样肉瘤是一种罕见的肿瘤,了解其在支气管粘膜病变和胸膜中的细胞形态和免疫组织化学特征,患者的临床病史以及辅助研究可能会得出准确的诊断。
总之,支气管和广泛胸膜转移性上皮样肉瘤极为罕见,其病理和免疫组化表现与手术切除的原发肿瘤的组织病理学有很好的相关性。
文章引用
吴炳群,魏慎海,田进涛,宋小平,胡鹏程,崔 永. 上皮样肉瘤支气管胸膜转移1例并文献复习
An Unusual Case of Metastasis of an Epithelioid Sarcoma to the Pleura and Bronchus: A Case Report and Literature Review[J]. 临床医学进展, 2018, 08(07): 615-621. https://doi.org/10.12677/ACM.2018.87103
参考文献
- 1. Enzinger, F.M. (1970) Epithelioid Sarcoma: A Sarcoma Simulating a Granulomaor a Carcinoma. Cancer, 26, 1029-1041. https://doi.org/10.1002/1097-0142(197011)26:5<1029::AID-CNCR2820260510>3.0.CO;2-R
- 2. Hasegawa, S., Inui, K., Kamakari, K., et al. (1999) Pulmonary Cysts as the Sole Metastatic Manifestation of Soft Tissue Sarcoma: Case Report and Consideration of the Pathogenesis. Chest, 116, 263-265. https://doi.org/10.1378/chest.116.1.263
- 3. Chan, D.P., Griffith, J.F., Lee, T.W., et al. (2003) Cystic Pulmonary Metastases from Epithelioid Cell Sarcoma. The Annals of Thoracic Surgery, 75, 1652-1654. https://doi.org/10.1016/S0003-4975(02)05018-X
- 4. Kikuchi, E., Kinoshita, I., Yamazaki, K., et al. (2006) Epi-thelioid Sarcoma Presenting as Pulmonary Cysts with Cancer Antigen 125 Expression. Respirology, 11, 826-829. https://doi.org/10.1111/j.1440-1843.2006.00925.x
- 5. Barnoud, R., Collardeau-Frachon, S., de la Roche, E., et al. (2010) Lung Metastases of Epithelioid Sarcoma Revealed by Bilateral Spontaneous Pneumothorax: A Pathological Diagnosis. Annales de Pathologie, 30, 139-142. https://doi.org/10.1016/j.annpat.2010.01.004
- 6. Liu, Y., Ma, X., Zang, D., et al. (2011) Epithelioid Sarcoma with Osteoporosis and Pneumothorax. European Journal of Dermatology, 21, 296-297.
- 7. Jeon, S.-Y., Yhim, H.-Y. and Lee, N.-R. (2016) Epithelioid Sarcoma with Spontaneous Pneumothorax and Massive Pleural Effusion. The Korean Journal of Internal Medicine, 31, 191-193.
- 8. Thway, K., Jones, R., Noujaim, J. and Fisher, C. (2016) Epithelioid Sarcoma: Diagnostic Features and Genetics. Advances in Anatomic Pathology, 23, 41-49. https://doi.org/10.1097/PAP.0000000000000102
- 9. Cho, W. and Balarezo, F. (2018) Expression of CD34 and β-Catenin in Malignant Rhabdoid Tumor of the Liver Mimicking Proximal-Type Epithelioid Sarcoma. Journal of Pa-thology and Translational Medicine, 52, 195-197. https://doi.org/10.4132/jptm.2017.05.15
- 10. Li, L., Xia, Q., Rao, Q., et al. (2014) Molecular Genetics and Im-munophenotype of INI1/SMARCB in Epithelioid Sarcoma. Zhonghua Bing Li Xue Za Zhi, 43, 389-393.
- 11. Lynch, M., Graber, E., Johnson, T. and Clarke, L. (2015) Epithelioid Sarcoma Resembling Benign Fibrous Histiocytoma. Cutis, 95, 83-86.
- 12. Xing, Y., Pan, Z., Li, Y., et al. (2011) Diagnosis and Treatment of Epithelioid Sarcoma. Zhonghua Zhong Liu Za Zhi, 33, 872-874.
- 13. Wolf, P.S., Flum, D.R., Tanas, M.R., et al. (2008) Epithelioid Sarcoma: The University of Washington Experience. The American Journal of Surgery, 196, 407-412. https://doi.org/10.1016/j.amjsurg.2007.07.029
- 14. Sakharpe, A., Lahat, G., Gulamhusein, T., et al. (2011) Epi-thelioid Sarcoma and Unclassified Sarcoma with Epithelioid Features: Clinicopathological Variables, MolecularMarkers, and a New Experimental Model. Oncologist, 16, 512-522. https://doi.org/10.1634/theoncologist.2010-0174
- 15. Nunes, L.F., Fiod, N.J., Vasconcelos, R.A., et al. (2010) Epithelioid Sarcoma: Clinical Behavior, Prognostic Factors and Survival. Revista do Colégio Brasileiro de Cirurgiões, 37, 251-255.
- 16. Armah, H.B. and Parwani, A.V. (2009) Epithelioidsarcoma. Archives of Pathology & Laboratory Medicine, 133, 814-819.
- 17. Grimer, R., Judson, I., Peake, D. and Seddon, B. (2010) Guidelines for the Management of Soft Tissue Sarcomas. Sarcoma, 506182. https://doi.org/10.1155/2010/506182
- 18. Frezza, A., Jones, R., Lo Vullo, S., et al. (2018) Anthracycline, Gemcitabine, and Pazopanib in Epithelioid Sarcoma: A Multi-Institutional Case Series. JAMA Oncology. https://doi.org/10.1001/jamaoncol.2018.0219
- 19. Levy, A., Le Péchoux, C., Terrier, P., et al. (2014) Epithelioid Sarcoma: Need for a Multimodal Approach to Maximize the Chances of Curative Conservative Treatment. Annals of Surgical Oncology, 21, 269-276. https://doi.org/10.1245/s10434-013-3247-4
- 20. Cibas, E.S. and Ducatman, B.S. (2009) Cytology, Diagnostic Principles and Clinical Correlates. 3rd Edition, Sauders, Elsevier.
NOTES
*通讯作者。