Journal of Advances in Physical Chemistry
Vol.06 No.02(2017), Article ID:20512,8
pages
10.12677/JAPC.2017.62008
Theoretical Calculations of the Mechanism for the Catalytic Oxidation and the Shape-Selectivity in Ti-YNU-1 Zeolite
Yichen Wang, Mengzhao Li, Danhong Zhou*
College of Chemistry and Chemical Engineering, Liaoning Normal University, Dalian Liaoning

Received: Apr. 26th, 2017; accepted: May 13th, 2017; published: Mar. 16th, 2017

ABSTRACT
The catalytic performances and reaction mechanism of 1-hexene and cyclohexene epoxidations over active centers at the T3 and T6 sites in Ti-YNU-1/H2O2 system have been investigated by using density functional theory. All calculations were performed at the theoretical level of B3LYP/6- 31G(d,p). The results indicated that the active center at T3 is Ti3-η2 (OOH), and the active centers at T6 are Ti6-η2 (OOH)-H2O and Ti6-η2 (OOH)-CH3CN. The catalytic activity has the trend of Ti6-η2 (OOH)-H2O > Ti6-η2 (OOH)-CH3CN > Ti3-η2 (OOH). The activation barriers of 1-hexene epoxidation over different active centers are higher than that of cyclohexene epoxidation. With the increasing of Ti content in Ti-YNU-1, more Ti species are inserted into the T3 site in the 10-membered ring channels, which accretes the numbers of Ti3-η2 (OOH). Due to the distribution restrict for cyclohexene, the conversion of 1-hexene epoxidation is much higher than that of cyclohexene epoxidation.
Keywords:Density Functional Theory, Ti-YNU-1 Zeolite, Epoxidation Reaction Mechanism, Shap Selectivity, Distribution Control
Ti-YNU-1分子筛催化氧化机理及择形 选择性的理论计算
王译晨,李蒙召,周丹红*
辽宁师范大学化学化工学院,辽宁 大连

收稿日期:2017年4月26日;录用日期:2017年5月13日;发布日期:2017年5月16日

摘 要
采用密度泛函理论,研究了Ti-YNU-1/H2O2体系中T3和T6位活性中心上1-己烯和环己烯环氧化反应的催化性能和反应机理。所有计算都在B3LYP/6
关键词 :密度泛函理论,Ti-YNU-1分子筛,环氧化反应机理,择形选择性,扩散控制

Copyright © 2017 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
Ti-YNU-1分子筛作为一种新型的钛硅分子筛催化剂,具有与MWW分子筛相似的拓扑结构,经过结构修饰,其层间形成了扩展的12-MR (12-membered ring)孔道 [1] [2] [3] ,不仅更有利于大分子靠近活性位点,而且提高了作为氧化剂的H2O2水溶液的反应效率,为钛硅分子筛在大体积分子催化氧化方面的应用开辟了应用空间。Ti-YNU-1分子筛在催化C5~C12的环状烯烃氧化反应中显示出比Ti-Beta、TS-1、3D Ti-MWW和Ti-MOR分子筛更高的催化活性 [1] [3] 。Shen等人 [4] 通过实验研究了Ti-YNU-1对各种烯烃及其衍生物的环氧化反应的催化性能,结果表明,Ti-YNU-1可以比Ti-Beta更加高效和有选择性地催化H2O2水溶液中烯烃及其富电子取代衍生物。催化条件对Ti-YNU-1和Ti-Beta的催化性能有很大影响。Song等人 [5] 在实验中发现,随着Ti含量的增加,1-己烯转化率的增加程度要高于环己烯。他们认为这是由于1-己烯能在内层和中间层孔道中被氧化,而环己烯只能在中间层的超笼中被氧化,合成过程中更多的Ti物种被插入到10-MR(10-membered ring)正弦孔道中;还有一种可能是,层间Ti物种的氧化能力弱于内层空间的Ti物种。但是这些猜想还缺乏理论上的证明。
对于钛硅分子筛催化性能的研究,实验上一般是从溶剂效应、分子筛的亲/疏水性等来分析,但只是停留在现象描述,对其本质还不清楚。而分子筛活性差异的本质还可能是活性中心微观结构的不同。实验研究表明,在Ti-YNU-1分子筛合成过程中,Ti(IV)主要落位在柱撑的T6位 [2] ,大部分另外添加的Ti物种被插入到了10-MR正弦孔道中 [5] 。这主要是由于T6位处于层间柱撑位点,Ti (IV)插入过多,会导致分子筛骨架的塌陷。因此,只能有少量的T6位被Ti(IV)取代。但是,目前现有的实验技术无法考察活性中心的微观结构,而理论计算能够从原子水平上确定分子筛不同孔道体系内骨架Ti (IV)的落位和微观结构,考察活性中心结构及稳定性,分析催化氧化机理等 [6] [7] [8] 。根据文献和我们前期的研究结果 [7] [8] ,在Ti-MWW分子筛上Ti最可能落位在超笼边缘的T1和10-MR正弦孔道中的T3位。作为活性中心的钛氧活性中间体存在不同的结构,而吸附溶剂的六配位活性中心比五配位的活性中心更加稳定 [9] [10] 。活性中心的结构和落位对于反应物的选择性和催化活性有可能呈现不同的结果。
本文采用密度泛函理论计算方法,考察Ti-YNU-1分子筛上不同活性中心对1-己烯和环己烯的催化氧化性能,从微观上揭示立体选择性的本质,为实验现象提供理论解释。
2. 模型的选取和计算方法
2.1. 模型的选取
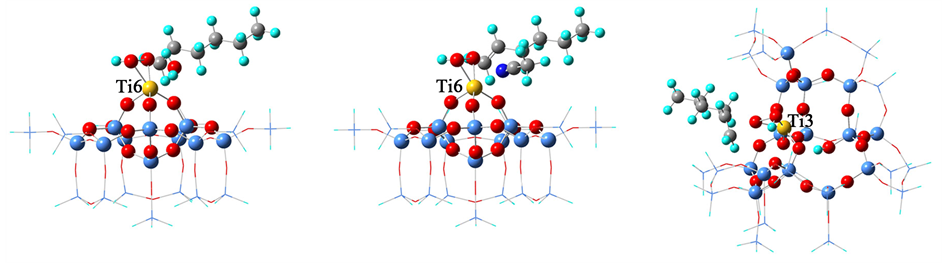
本文主要研究Ti-YNU-1分子筛中T3和T6位的Ti物种所形成的钛氧活性中间体的催化作用。为了模拟Ti-YNU-1分子筛中10-MR正弦孔道中的T3活性位点,从MWW晶体结构中截取36T簇模型,用Ti同晶取代T3位的Si,构建Ti3-η2 (OOH)活性中间体结构。尽管五配位的Ti3-η2 (OOH)可吸附一个水分子得到更稳定的六配位的活性中间体结构 [10] ,但是由于空间障碍所限,活性氧的位置不利于烯烃分子的进攻,因此Ti3-η2 (OOH)-H2O不可能是实际的活性中心,因此不予考虑。为了模拟Ti-YNU-1分子筛12-MR层间孔道的T6活性位点,从MWW晶体结构中截取24T簇模型,分别构建了六配位的Ti6-η2 (OOH)-H2O和Ti6-η2 (OOH)-CH3CN的活性中间体结构。所有簇模型均包含7层骨架原子,优化时最外三层骨架原子的坐标固定,只松弛优化内部四层骨架原子,具体方法可参考相关文献 [7] [11] 。几个活性中间体优化后的簇模型结构示于图1。
2.2. 计算方法
结构优化和过渡态的计算都在Gaussian 09 [12] 程序中完成,采用B3LYP [13] [14] 杂化泛函和6
3. 结果与讨论
3.1. 过渡态几何结构分析
Ti6-η2 (OOH)-H2O、Ti6-η2 (OOH)-CH3CN和Ti3-η2 (OOH)活性中心与1-己烯、环己烯发生环氧化反应的过渡态结构模型分别列于图2和图3。为了清楚起见,每个过渡态结构都给出了活性中心和烯烃分子反应的局部结构。从过渡态的模型可以看出,活性中心与烯烃分子反应后,六配位活性中心呈现扭曲的八面体构型,而五配位的Ti3-η2 (OOH)活性中心为三角双锥构型。各活性中心上1-己烯、环己烯环氧化反应过渡态的部分几何结构参数分别列于表1和表2。
 (a) (b) (c)
(a) (b) (c)
Figure 1. Cluster models of the Ti-hydroperoxo intermediates at the T6 and T3 sites in Ti-YNU-1 zeolite. (a) Ti6-η2 (OOH)-H2O; (b) Ti6-η2 (OOH)-CH3CN; (c) Ti3-η2 (OOH)
图1. Ti-YNU-1分子筛中T6和T3位钛氧活性中间体簇模型。(a) Ti6-η2 (OOH)-H2O; (b) Ti6-η2 (OOH)-CH3CN; (c) Ti3-η2 (OOH)
 (a) (b) (c)
(a) (b) (c)

 (a) (b) (c)
(a) (b) (c)
Figure 2. Optimized models for transition state of 1-hexene epoxidation on different active centers in Ti-YNU-1 zeolite. (a) Ti6-η2 (OOH)-H2O-TS; (b) Ti6-η2 (OOH)-CH3CN-TS; (c) Ti3-η2 (OOH)-TS
图2. Ti-YNU-1分子筛中各活性中心上1-己烯环氧化过渡态优化后的模型。(a) Ti6-η2 (OOH)-H2O-TS; (b) Ti6-η2 (OOH)-CH3CN-TS; (c) Ti3-η2 (OOH)-TS
 (a) (b) (c)
(a) (b) (c)

 (a) (b) (c)
(a) (b) (c)
Figure 3. Optimized models for transition state of cyclohexene epoxidation on different active centers in Ti-YNU-1 zeolite. (a) Ti6-η2 (OOH)-H2O-TS; (b) Ti6-η2 (OOH)-CH3CN-TS; (c) Ti3-η2 (OOH)-TS
图3. Ti-YNU-1分子筛中各活性中心上环己烯环氧化过渡态优化后的模型。(a) Ti6-η2 (OOH)-H2O-TS; (b) Ti6-η2 (OOH)-CH3CN-TS; (c) Ti3-η2 (OOH)-TS
Table 1. Geometric parameters of transition state for 1-hexene epoxidation on different active centers in Ti-YNU-1/H2O2 system (Å)
表1. Ti-YNU-1/H2O2体系不同活性中心上1-己烯环氧化反应过渡态的几何结构参数(Å)
a括号内的数据表示形成过渡态后键长的变化。
Table 2. Geometric parameters of transition state for cyclohexene epoxidation on different active centers in Ti-YNU-1/H2O2 system (Å)
表2. Ti-YNU-1/H2O2体系不同活性中心上环己烯环氧化反应过渡态的几何结构参数(Å)
a括号内的数据表示形成过渡态后键长的变化。
从表1中可以看出,在1-己烯环氧化反应的过渡态结构中,Ti3-η2 (OOH)活性中心的Ti-Oα键长增加了0.069 Å,Ti-Oβ距离减小了0.217 Å,Oα-Oβ键长增加了0.379 Å,活性Oα原子与C=C双键上两个C原子的距离分别为2.015和2.348 Å。Ti6-η2 (OOH)-H2O和Ti6-η2 (OOH)-CH3CN活性中心结构具有相似的变化趋势,但变化范围更小,说明五配位的活性中心确实不如六配位的活性中心稳定,与文献 [10] [18] [19] [20] 研究相一致。在过渡态结构中活性Oα原子与1-己烯C=C双键末端C原子的距离更近,考虑这主要是由于直链烯烃尾部与分子筛骨架存在一定的排斥作用。而在环己烯环氧化反应的过渡态结构中(表2),虽然各活性中心的键长变化均小于1-己烯环氧化反应,但是活性中心的键长变化趋势与1-己烯环氧化反应相同,并且同样是Ti3-η2 (OOH)活性中心的键长变化最大。不同的是,其过渡态结构中活性Oα原子与环己烯C=C双键原子的距离几乎相同。结果表明,在Ti-YNU-1分子筛各活性中心上不论是发生1-己烯还是环己烯的环氧化反应都遵循相同的反应机理,即烯烃C=C双键与从Oα方向进攻活性中心,Oα原子逐渐转移到C=C双键上,形成过渡态后Ti-Oα和Oα-Oβ键长增加,Ti-Oβ距离减小,且Ti-Oα和Ti-Oβ的键长大小相近,活性中心有恢复到TiOH物种的趋势,这与Ti-MWW和TS-1分子筛上液相烯烃环氧化的机理相一致 [10] [15] [20] 。
3.2. 过渡态能量分析
某种烯烃分子环氧化反应的转化率高可能有两种原因,一种是各活性中心的活性相同,能够催化该烯烃分子的活性中心数量多导致其转化率高;另一种是活性中心数量一定,活性中心活性高导致其转化率高。本文以1-己烯、环己烯为底物分子,考察了Ti-YNU-1/H2O2体系中不同活性中心上烯烃环氧化反应的活化能,计算结果列于图4。
首先,通过比较相同活性中心上1-己烯和环己烯环氧化反应的活化能发现:各活性位点上1-己烯环氧化反应的活化能垒要高于环己烯,这是由于相同碳原子数的环状烯烃和链状烯烃相比,环状烯烃的C=C双键是更加富电子的,有利于C=C双键到σO-O*过氧反键的电子转移,从而完成环氧化过程。其中,Ti6-η2 (OOH)-H2O和Ti6-η2 (OOH)-CH3CN活性中心上1-己烯环氧化反应的活化能分别为35.00和43.92 kJ/mol,而Xu等人 [21] 通过阿伦尼乌兹公式得出Ti-MWW/H2O2/CH3CN体系中1-己烯环氧化反应的表观活化能

Figure 4. The activation energies of 1-hexene and cyclohexene epoxidations over different active centers in Ti-YNU-1
图4. Ti-YNU-1中不同活性中心上1-己烯和环己烯环氧化反应的活化能
为21.03 kJ/mol,明显低于我们计算的活化能垒。其次,通过比较不同活性中心上相同烯烃分子的活化能发现,Ti3-η2 (OOH)活性中心的催化活性要低于Ti6-η2 (OOH)-H2O和Ti6-η2 (OOH)-CH3CN活性中心,这主要是由于T3位属于内层空间的活性位点,存在较大的空间障碍,不利于形成稳定的过渡态结构。Ti6-η2 (OOH)-H2O和Ti6-η2 (OOH)-CH3CN活性中心上环己烯环氧化反应的活化能分别为24.23和32.50 kJ/mol,而Ti3-η2 (OOH)活性中心与环己烯反应的活化能为56.61 kJ/mol。计算结果可以与Kwon等人 [16] 通过实验和阿伦尼乌兹公式计算得到的TS-1分子筛上气相环己烯环氧化反应的表观活化能相比较(40 ± 2 kJ/mol)。此外,Vandichel等人 [22] 以叔丁基过氧化氢做氧化剂,在VO(acac)2催化剂上进行的环己烯环氧化反应的表观活化能为48.60 kJ/mol。可以看出Ti-YNU-1分子筛上T6位活性中心的催化活性更高,并且计算结果在合理范围内。
此外,我们必须提到,虽然在Ti3-η2 (OOH)活性中心上环己烯环氧化反应的活化能低于1-己烯的活化能,但是由于T3位存在于10-MR正弦孔道内,体积较大的环己烯比1-己烯更难扩散到T3位的活性中心。Clerici等人 [23] 曾提出,扩散效应是1-己烯反应速率远远大于环己烯的决定因素。因此,我们可以推测,1-己烯环氧化可以在层间的T6位和内层的T3位活性中心上进行,而环己烯的环氧化只能在T6位的活性中心进行。随着Ti含量增加,更多的Ti插入到了10-MR正弦孔道中的T3位,使1-己烯转化率增加程度高于环己烯,因此可以为实验现象提出合理解释 [5] 。
4. 结论
本文应用密度泛函理论计算,对Ti-YNU-1分子筛中1-己烯和环己烯环氧化反应进行了研究。确定了Ti3-η2 (OOH)、Ti6-η2 (OOH)-H2O和Ti6-η2 (OOH)-CH3CN活性中间体模型结构,计算了各活性中心上两种烯烃环氧化反应的过渡态和活化能。结果表明,在各种活性中心上,1-己烯反应的活化能大于环己烯反应活化能,活性中间体的催化活性为Ti6-η2 (OOH)-H2O > Ti6-η2 (OOH)-CH3CN > Ti3-η2 (OOH)。Ti-YNU-1分子筛中随着Ti含量的增加,更多的Ti插入到T3位,导致Ti3-η2 (OOH)活性中心位点增多,但是由于孔道空间障碍,限制了较大体积的环己烯的扩散,只有1-己烯能够在该活性中心发生环氧化反应。因此,随着Ti含量的增加,1-己烯转化率增加程度高于环己烯。
致 谢
感谢国家自然科学基金(21343010)项目的资助。
文章引用
王译晨,李蒙召,周丹红. Ti-YNU-1分子筛催化氧化机理及择形选择性的理论计算
Theoretical Calculations of the Mechanism for the Catalytic Oxidation and the Shape-Selectivity in Ti-YNU-1 Zeolite[J]. 物理化学进展, 2017, 06(02): 60-67. http://dx.doi.org/10.12677/JAPC.2017.62008
参考文献 (References)
- 1. Fan, W.B., Wu, P., Namba, S., et al. (2004) A Titanosilicate That Is Structurally Analogous to an MWW-Type Lamellar Precursor. Angewandte Chemie International Edition, 43, 236-240. https://doi.org/10.1002/anie.200352723
- 2. Ruan, J.F., Wu, P., Slater, B., et al. (2005) Structure Elucidation of the Highly Active Titanosilicate Catalyst Ti-YNU- 1. Angewandte Chemie International Edition, 44, 6719-6723. https://doi.org/10.1002/anie.200501939
- 3. Fan, W.B., Wu, P., Namba, S., et al. (2006) Synthesis and Catalytic Properties of a New Titanosilicate Molecular Sieve with the Structure Analogous to MWW-Type Lamellar Precursor. Journal of Catalysis, 243, 183-191. https://doi.org/10.1016/j.jcat.2006.07.003
- 4. Shen, X.H., Fan, W.B., He, Y., et al. (2011) Epoxidation of Alkenes and their Derivatives over Ti-YNU-1. Applied Catalysis A-general, 401, 37-45. https://doi.org/10.1016/j.apcata.2011.04.044
- 5. Song, S.S., Wang, P.F., He, Y., et al. (2012) Preparation, Characterization and Catalytic Properties of Ti-Rich Ti- YNU-1. Microporous and Mesoporous Materials, 159, 74-80. https://doi.org/10.1016/j.micromeso.2012.04.009
- 6. Yang, G., Zhou, L.J., Liu, X.C., et al. (2011) Density Functional Calculations on the Distribution, Acidity, and Catalysis of TiIV and TiIII Ions in MCM-22 Zeolite. Chemistry—A European Journal, 17, 1614-1621. https://doi.org/10.1002/chem.201002241
- 7. Zhou, D.H., Zhang, H.J., Zhang, J.J., et al. (2014) Density Functional Theory Investigations into the Structure and Spectroscopic Properties of the Ti4+ Species in Ti-MWW Zeolite. Microporous and Mesoporous Materials, 195, 216- 226. https://doi.org/10.1016/j.micromeso.2014.04.037
- 8. 李娜, 蒋艳娇, 乔溢铭, 等. Ti-MWW分子筛正弦孔道内骨架钛物种的结构和红外振动光谱的理论计算[J]. 无机化学学报, 2015, 31(5): 901-907.
- 9. 李娜. Ti-MWW/H2O2催化剂中钛氧活性中心的结构及电子光谱的理论计算[D]: [硕士学位论文]. 大连: 辽宁师范大学, 2015.
- 10. 邹晶, 范志琳, 姜丽莎, 等. Ti-MWW分子筛钛氧活性中心与溶剂分子吸附作用的理论研究[J]. 物理化学学报, 2016, 32(4): 935-942.
- 11. Qiao, Y.M., Fan, Z.L., Jiang, Y.J., et al. (2015) Structures and Vibrational Spectra of Ti-MWW Zeolite upon Adsorption of H2O and NH3: A Density Functional Theory Study. Chinese Journal of Catalysis, 36, 1733-1741. https://doi.org/10.1016/S1872-2067(15)60900-7
- 12. Frisch, M.J., Trucks, G.W., Schlegel, H.B., et al. (2010) Gaussian 09 revision D.01. Gaussian Inc. Wallingford, CT.
- 13. Lee, C., Yang, W. and Parr, R.G. (1988) Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Physical Review B, 37, 785-789. https://doi.org/10.1103/PhysRevB.37.785
- 14. Becke, A.D. (1988) Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Physical Review A, 38, 3098-3100. https://doi.org/10.1103/PhysRevA.38.3098
- 15. Fang, X.Q., Wang, Y.N., Deng, X.J., et al. (2011) Reaction Dynamics Behavior of Epoxidation of Allyl Chloride with Hydrogen Peroxide Catalyzed by Ti-MWW. Chinese Journal of Catalysis, 32, 333-339. https://doi.org/10.3724/sp.j.1088.2011.00820
- 16. Kwon, S., Schweitzer, N.M., Park, S., et al. (2015) A Kinetic Study of Vapor-Phase Cyclohexene Epoxidation by H2O2 over Mesoporous TS-1. Journal of Catalysis, 326, 107-115. https://doi.org/10.1016/j.jcat.2015.04.005
- 17. 高焕新, 卢文奎, 陈庆龄. 钛硅分子筛TS-1催化氯丙烯环氧化反应动力学研究[J]. 催化学报, 2002, 23(1): 3-8.
- 18. Wang, L.L., Xiong, G., Su, J., et al. (2012) In Situ UV Raman Spectroscopic Study on the Reaction Intermediates for Propylene Epoxidation on TS-1. The Journal of Physical Chemistry C, 116, 9122-9131. https://doi.org/10.1021/jp3017425
- 19. Xiong, G., Cao, Y.Y., Guo, Z.D., et al. (2016) The Roles of Different Titanium Species in TS-1 Zeolite in Propylene Epoxidation Studied by In Situ UV Raman Spectroscopy. Physical Chemistry Chemical Physics, 18, 190-196. https://doi.org/10.1039/C5CP05268H
- 20. 周丹红, 姜丽莎, 范志琳, 等. TS-1/H2O2催化活性中心结构及活性预测[J]. 辽宁师范大学学报(自然科学版), 2016, 39(1): 70-76.
- 21. Xu, L., Huang, D.-D., Li, C.G., et al. (2015) Construction of Unique Six-Coordinated Titanium Species with an Organic Amine Ligand in Titanosilicate and Their Unprecedented High Efficiency for Alkene Epoxidation. Chemical Communications, 51, 9010-9013. https://doi.org/10.1039/C5CC02321A
- 22. Vandichel, M., Leus, K., Van Der Voort, P., et al. (2012) Mechanistic Insight into the Cyclohexene Epoxidation with VO(acac)2 and Tert-Butyl Hydroperoxide. Journal of Catalysis, 294, 1-18. https://doi.org/10.1016/j.jcat.2012.06.002
- 23. Clerici, M.G. and Ingallina, P. (1993) Epoxidation of Lower Olefins with Hydrogen Peroxide and Titanium Silicalite. Journal of Catalysis, 140, 71-83. https://doi.org/10.1006/jcat.1993.1069
- 24. Fan, W.B., Wu, P., Namba, S., et al. (2004) A Titanosilicate That Is Structurally Analogous to an MWW-Type Lamellar Precursor. Angewandte Chemie International Edition, 43, 236-240. https://doi.org/10.1002/anie.200352723
- 25. Ruan, J.F., Wu, P., Slater, B., et al. (2005) Structure Elucidation of the Highly Active Titanosilicate Catalyst Ti-YNU- 1. Angewandte Chemie International Edition, 44, 6719-6723. https://doi.org/10.1002/anie.200501939
- 26. Fan, W.B., Wu, P., Namba, S., et al. (2006) Synthesis and Catalytic Properties of a New Titanosilicate Molecular Sieve with the Structure Analogous to MWW-Type Lamellar Precursor. Journal of Catalysis, 243, 183-191. https://doi.org/10.1016/j.jcat.2006.07.003
- 27. Shen, X.H., Fan, W.B., He, Y., et al. (2011) Epoxidation of Alkenes and their Derivatives over Ti-YNU-1. Applied Catalysis A-general, 401, 37-45. https://doi.org/10.1016/j.apcata.2011.04.044
- 28. Song, S.S., Wang, P.F., He, Y., et al. (2012) Preparation, Characterization and Catalytic Properties of Ti-Rich Ti- YNU-1. Microporous and Mesoporous Materials, 159, 74-80. https://doi.org/10.1016/j.micromeso.2012.04.009
- 29. Yang, G., Zhou, L.J., Liu, X.C., et al. (2011) Density Functional Calculations on the Distribution, Acidity, and Catalysis of TiIV and TiIII Ions in MCM-22 Zeolite. Chemistry—A European Journal, 17, 1614-1621. https://doi.org/10.1002/chem.201002241
- 30. Zhou, D.H., Zhang, H.J., Zhang, J.J., et al. (2014) Density Functional Theory Investigations into the Structure and Spectroscopic Properties of the Ti4+ Species in Ti-MWW Zeolite. Microporous and Mesoporous Materials, 195, 216- 226. https://doi.org/10.1016/j.micromeso.2014.04.037
- 31. 李娜, 蒋艳娇, 乔溢铭, 等. Ti-MWW分子筛正弦孔道内骨架钛物种的结构和红外振动光谱的理论计算[J]. 无机化学学报, 2015, 31(5): 901-907.
- 32. 李娜. Ti-MWW/H2O2催化剂中钛氧活性中心的结构及电子光谱的理论计算[D]: [硕士学位论文]. 大连: 辽宁师范大学, 2015.
- 33. 邹晶, 范志琳, 姜丽莎, 等. Ti-MWW分子筛钛氧活性中心与溶剂分子吸附作用的理论研究[J]. 物理化学学报, 2016, 32(4): 935-942.
- 34. Qiao, Y.M., Fan, Z.L., Jiang, Y.J., et al. (2015) Structures and Vibrational Spectra of Ti-MWW Zeolite upon Adsorption of H2O and NH3: A Density Functional Theory Study. Chinese Journal of Catalysis, 36, 1733-1741. https://doi.org/10.1016/S1872-2067(15)60900-7
- 35. Frisch, M.J., Trucks, G.W., Schlegel, H.B., et al. (2010) Gaussian 09 revision D.01. Gaussian Inc. Wallingford, CT.
- 36. Lee, C., Yang, W. and Parr, R.G. (1988) Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Physical Review B, 37, 785-789. https://doi.org/10.1103/PhysRevB.37.785
- 37. Becke, A.D. (1988) Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Physical Review A, 38, 3098-3100. https://doi.org/10.1103/PhysRevA.38.3098
- 38. Fang, X.Q., Wang, Y.N., Deng, X.J., et al. (2011) Reaction Dynamics Behavior of Epoxidation of Allyl Chloride with Hydrogen Peroxide Catalyzed by Ti-MWW. Chinese Journal of Catalysis, 32, 333-339. https://doi.org/10.3724/sp.j.1088.2011.00820
- 39. Kwon, S., Schweitzer, N.M., Park, S., et al. (2015) A Kinetic Study of Vapor-Phase Cyclohexene Epoxidation by H2O2 over Mesoporous TS-1. Journal of Catalysis, 326, 107-115. https://doi.org/10.1016/j.jcat.2015.04.005
- 40. 高焕新, 卢文奎, 陈庆龄. 钛硅分子筛TS-1催化氯丙烯环氧化反应动力学研究[J]. 催化学报, 2002, 23(1): 3-8.
- 41. Wang, L.L., Xiong, G., Su, J., et al. (2012) In Situ UV Raman Spectroscopic Study on the Reaction Intermediates for Propylene Epoxidation on TS-1. The Journal of Physical Chemistry C, 116, 9122-9131. https://doi.org/10.1021/jp3017425
- 42. Xiong, G., Cao, Y.Y., Guo, Z.D., et al. (2016) The Roles of Different Titanium Species in TS-1 Zeolite in Propylene Epoxidation Studied by In Situ UV Raman Spectroscopy. Physical Chemistry Chemical Physics, 18, 190-196. https://doi.org/10.1039/C5CP05268H
- 43. 周丹红, 姜丽莎, 范志琳, 等. TS-1/H2O2催化活性中心结构及活性预测[J]. 辽宁师范大学学报(自然科学版), 2016, 39(1): 70-76.
- 44. Xu, L., Huang, D.-D., Li, C.G., et al. (2015) Construction of Unique Six-Coordinated Titanium Species with an Organic Amine Ligand in Titanosilicate and Their Unprecedented High Efficiency for Alkene Epoxidation. Chemical Communications, 51, 9010-9013. https://doi.org/10.1039/C5CC02321A
- 45. Vandichel, M., Leus, K., Van Der Voort, P., et al. (2012) Mechanistic Insight into the Cyclohexene Epoxidation with VO(acac)2 and Tert-Butyl Hydroperoxide. Journal of Catalysis, 294, 1-18. https://doi.org/10.1016/j.jcat.2012.06.002
- 46. Clerici, M.G. and Ingallina, P. (1993) Epoxidation of Lower Olefins with Hydrogen Peroxide and Titanium Silicalite. Journal of Catalysis, 140, 71-83. https://doi.org/10.1006/jcat.1993.1069
NOTES
*通讯作者。
