Journal of Organic Chemistry Research
Vol.05 No.01(2017), Article ID:19995,6
pages
10.12677/JOCR.2017.51003
Synthesis of BODIPY Compounds and Their Modification Methods
Nanxiang Wang1*, Wen Zhen2
College of Material Science and Technology, Nanjing University of Aeronautics and Astronautics, Nanjing Jiangsu

Received: Mar. 7th, 2017; accepted: Mar. 25th, 2017; published: Mar. 28th, 2017

ABSTRACT
In recent years, BODIPY (Boron-dipyrromethene complex) compounds have been the focus of many studies in diverse areas. BODIPYs are fully recognized as excellent fluorescent materials in tunable laser, biological labeling, photosensitizers and photodynamic therapy of tumor (PDT), solid-state solar concentrators, electroluminescent devices, on-off fluorescent switches etc., and in particular the fluorophores in sensors and probes. This paper summarizes the synthesis of BODIPY compounds and its modification methods, hoping to help the relevant researchers.
Keywords:BODIPY, Synthesis and Modification, Method Summary
BODIPY类化合物的合成及其修饰方法概况
王南翔1*,甄文2
南京航空航天大学材料科学与技术学院,江苏 南京

收稿日期:2017年3月7日;录用日期:2017年3月25日;发布日期:2017年3月28日

摘 要
近年来,氟硼二吡咯(BODIPY)类荧光化合物在生物标记、肿瘤光动力治疗(PDT)光敏剂、固态太阳能聚光器电致发光器件、荧光开光等不同领域中都成为了研究的焦点,特别是在传感器和生物探针方面。本文总结了BODIPY类化合物的多种合成及其修饰方法,希望对相关研究人员有所帮助。
关键词 :BODIPY,合成及修饰,方法概况

Copyright © 2017 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
氟化硼络合二吡咯类(Boron-dipyrromethene,简称BODIPY)近些年来因其有着其优良的性质,在多方面都有很好的应用和发展 [1] - [13] 。在BODIPY的核心结构中,在络合的硼桥键和甲川桥键的共同作用下,将两侧的吡咯环固定在同一个平面上,使整个体系的骨架原子达到极好的共轭状态,为其吸收光能奠定了良好的基础。BODIPY的光谱峰宽比较窄,在光学领域和生物学领域进行分析检测时,光谱峰宽较窄,检测灵敏度较高,并且减少了待测样品的用量。测得的结果中,荧光光谱上会出现更明显的荧光吸收和发射的变化,这样更精确地测得了样品的含量 [14] [15] 。
BODIPY类荧光染料的光稳定性能非常优秀,当它被激发达到激发态时,不会因为很长时间的激发而在分析测定过程中发生激发态的分解或其他变化,这样测得的荧光光谱更加准确可信。BODIPY分子的荧光量子产率非常高,一般都能达到0.6。一般普通的染料在水溶液中其荧光量子产率会变低,有的甚至被猝灭。对于BODIPY分子来说,水溶液其荧光量子产率虽然有所下降,但它仍然能保持较高的荧光量子产率。由BODIPY的母体结构可知,他的母体结构上的取代基一般不会出现氨基或羧基这类对酸碱敏感的活性基团。因此外界的酸碱度对其影响会大幅度减小,因此BODIPY染料不会轻易变质。BODIPY类荧光染料对光的感应度很高,并且它还有高的摩尔消光系数,有些经过修饰后的BODIPY染料在水溶液中的溶解度也较高,再加上它在水中不易被猝灭这一优异的性能,使得BODIPY在光学、生物学、分析学领域有着极其广泛的应用。
2. BODIPY的基本合成方法
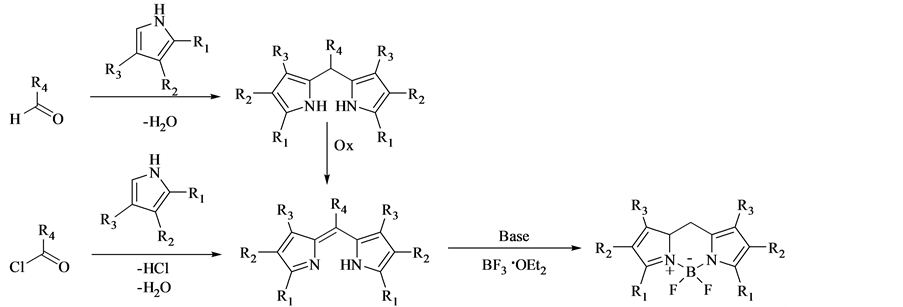
2.1. 醛与吡咯作为反应物合成F-BODIPY [16]
在TFA的催化作用下,首先经由吡咯和醛进行的缩合反应,然后再通过四氯苯醌或DDQ氧化,最后在碱存在的反应体系里与三氟化硼乙醚络合物进行配位反应生成目标产物F-BODIPY氟硼类荧光染料。上述方法是目前研究中用途最广泛的合成F-BODIPY氟硼类荧光染料的方法。副产物较多,产率较低都是该方法较为明显的缺点,合成步骤见反应式1。
2.2. 吡咯与含有吡咯环的醛或酮作为反应物合成F-BODIPY [17]
经由吡咯和含有吡咯环的醛或酮反应合成F-BODIPY染料,该反应合成的荧光染料呈现特殊的绿色。相对于其他合成方法,产率低是该方法的缺点(反应式2)。
2.3. 吡咯与酸酐或酰氯作为反应物合成F-BODIPY [18] [19]
吡咯与酸酐或酰氯反应合成F-BODIPY氟硼类荧光染料的步骤见反应式3,经由酸酐、酰氯形成二个吡咯环连接的桥梁,进而合成F-BODIPY氟硼类荧光染料。利用该方法的合成步骤中无需使用氧化剂,

Scheme 1. The pyrrole and aldehyde condensation oxidation to BODIPY
反应式1. 吡咯和醛缩合氧化配位成BODIPY


Scheme 2. Synthesis of BODIPY by pyrrolidone or pyrrolidine and pyrrole
反应式2. 吡咯酮或吡咯醛和吡咯合成BODIPY

Scheme 3. Synthesis of BODIPY by acid chloride or acid anhydride and pyrrole
反应式3. 酰氯或酸酐和吡咯合成BODIPY
适合用于8位取代的F-BODIPY氟硼类荧光染料的合成。
3. BODIPY衍生物的合成
3.1. BODIPY2,6位的修饰
2007年,在BODIPY的母体上,Loudet [20] 等人通过亲电取代反应在其2位和6位引入Br、SO3Na、NO2、I等,得到了BODIPY2,6位的修饰产物。在此基础上,他们还提出了用钯催化的合成方法,该方法可直接在BODIPY母体的2,6位增加取代基,增长了体系的共轭,吸收波长红移,见图1。
3.2. BODIPY3,5位上的修饰
通过金属催化,通过亲核取代反应取代3,5位的氯原子,从而引入其他官能团 [21] 。利用该方法可以合成3,5位取代的BODIPY衍生物(见图2)。
3.3. BODIPY8位上的修饰
图3为BODIPY8位修饰的产物。8位取代的BODIPY衍生物是由Loudet [22] 等人提出,经由吡咯和戊二酸酐缩合,在有机碱存在下和三氟化硼乙醚配位得到的8位含有羧基的衍生物。另外,他们设计出经由吡咯和醛的缩合反应合成8位取代衍生物。
3.4. 硼原子的取代
Ulrich [23]等人报道的文献中,他们设计合成了在硼原子修饰的BODIPY衍生物,在BODIPY的中心硼原子上引入炔基、芳基和烯基等官能,见图4。

Figure 1. 2,6-substituted BODIPY derivatives
图1. 2,6位取代的BODIPY衍生物结构

Figure 2. 3,5-substituted BODIPY derivatives
图2. 3,5位取代的BODIPY衍生物

Figure 3. 8-substituted BODIPY derivatives
图3. 8位取代的BODIPY衍生物
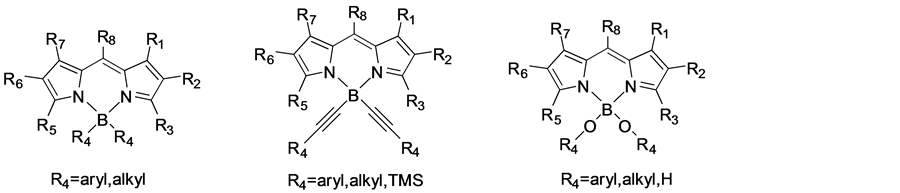
Figure 4. Boron atom substituted BODIPY derivatives
图4. 硼原子取代的BODIPY衍生物
4. 总结
在过去的几十年中,BODIPY类化合物随着一代代科研工作者的不懈努力对其合成及其修饰方法不断改善,使得拥有更优良的性能,使其更加全面深入地发展,在今后BODIPY类化合物的研究工作中,相信一定会有更多的合成及修饰方法的出现,使得BODIPY类化合物有着更大的拓展延伸。
致谢
江苏省高校优势学科建设工程项目。
文章引用
王南翔,甄 文. BODIPY类化合物的合成及其修饰方法概况
Synthesis of BODIPY Compounds and Their Modification Methods[J]. 有机化学研究, 2017, 05(01): 15-20. http://dx.doi.org/10.12677/JOCR.2017.51003
参考文献 (References)
- 1. Boens, N., Leen, V. and Dehaen, W. (2012) Fluorescent Indicators Based on Bodipy. Chemical Society Reviews, 41, 1130-1172. https://doi.org/10.1039/C1CS15132K
- 2. Liu, Q., Yin, B., Yang, T., et al. (2013) A General Strategy for Biocompatible, High-Effective up Conversion Nanocapsules Based on Triplet-Triplet Annihilation. Journal of the American Chemical Society, 135, 5029-5037. https://doi.org/10.1021/ja3104268
- 3. Huang, L. and Zhao, J. (2013) C60-Bodipy Dyad Triplet Photo Sensitizers as Organic Photo Catalysts for Photo Catalytic Tandem Oxidation/[3+2] Cycloaddition Reactions to Prepare Pyr-rolo[2,1-a]isoquinoline. Chemical Communications, 49, 3751-3753. https://doi.org/10.1039/c3cc41494a
- 4. Benstead, M., Mehl, G.H. and Boyle, R.W. (2011) 4,4’-Difluoro-4-bora-3a,4a-diaza-s-indacenes (BODIPYs) as Components of Novel Light Active Materials. Tetrahedron, 67, 3573-3601. https://doi.org/10.1016/j.tet.2011.03.028
- 5. Olivier, J.-H., Barbera, J., Bahaidarah, E., et al. (2012) Self-Assembly of Charged Bodipy Dyes To Form Cassettes That Display Intracomplex Electronic Energy Transfer and Accrete into Liquid Crystals. Journal of the American Chemical Society, 134, 6100-6103. https://doi.org/10.1021/ja3007935
- 6. Nepomnyashchii, A.B. and Bard, A.J. (2012) Electrochemistry and Elec-trogenerated Chemiluminescence of Bodipy Dyes. Accounts of Chemical Research, 45, 1844-1853. https://doi.org/10.1021/ar200278b
- 7. Kamkaew, A., Lim, S.H., Lee, H.B., et al. (2013) Bodipy Dyes in Photo-dynamic Therapy. Chemical Society Reviews, 42, 77-88. https://doi.org/10.1039/C2CS35216H
- 8. He, H., Si, L., Zhong, Y., et al. (2012) Iodized Bodipy as a Long Wavelength Light Sensitizer for the Near-Infrared Emission of Yt-terbium(iii) Ion. Chemical Communications, 48, 1886-1888. https://doi.org/10.1039/c2cc17037j
- 9. Huang, L., Yu, X., Wu, W., et al. (2012) Styryl Bodipy-C60 Dyads as Efficient Heavy-Atom-Free Organic Triplet Photosensitizers. Organic Letters, 14, 2594-2597. https://doi.org/10.1021/ol3008843
- 10. Hepp, A., Ulrich, G., Schmechel, R., et al. (2004) Highly Efficient Energy Transfer to a Novel Organic Dye in OLED Devices. Synthetic Metals, 146, 11-15.
- 11. Shi, W.-J., Liu, J.-Y. and Ng, D.K.P. (2012) A Highly Selective Colorimetric and Fluorescent Probe for Cu2+ and Hg2+ Ions Based on a Distyryl BODIPY with Two Bis(1,2,3-triazole)amino Receptors. Chemistry—An Asian Journal, 7, 196-200. https://doi.org/10.1002/asia.201100598
- 12. Bonaccorsi, P., Aversa, M.C., Barattucci, A., et al. (2012) Artificial Light-Harvesting Antenna Systems Grafted on a Carbohyate Drdrate Platform. Chemical Commu-nications, 48, 10550-10552. https://doi.org/10.1039/c2cc35555h
- 13. Chan, L., Tritsch, J.R. and Zhu, X.Y. (2012) Harvesting Singlet Fission for Solar Energy Conversion: One- versus Two-Electron Transfer from the Quantum Me-chanical Superposition. Journal of the American Chemical Society, 134, 18295-18302.
- 14. Ziessel, R., Ulrich, G. and Harriman, A. (2007) The Chemistry of Bodipy: A New El Dorado for Fluorescence Tools. New Journal of Chemistry, 31, 496-501. https://doi.org/10.1039/b617972j
- 15. Ulrich, G., Ziessel, R. and Harriman, A. (2008) The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angewandte Chemie International Edition, 47, 1184-1201. https://doi.org/10.1002/anie.200702070
- 16. Lee, S., Park, K., Kim, K.W., et al. (2008) Activatable Imaging Probes with Amplified Fluorescent Signals. Chemical Communications, 36, 4250-4260. https://doi.org/10.1039/b806854m
- 17. Shane, O.M., Michael, J.H., Lorcan, T.A., et al. (2005) Supramolecular Photonic Therapeutic Agents. Journal of the American Chemical Society, 127, 16360-16361. https://doi.org/10.1021/ja0553497
- 18. Li, Z., Mintzer, E. and Bittman, R. (2006) First Synthesis of Free Choles-terol-BODIPY Conjugates. Journal of Organic Chemistry, 71, 1718-1721. https://doi.org/10.1021/jo052029x
- 19. Boyer, J.H., Haag, A.M., Sathyamoorthi, G., et al. (1993) Pyrrome-thene-BF2 Complexes as Laser Dyes. Heteroatom Chemistry, 4, 39-49. https://doi.org/10.1002/hc.520040107
- 20. Loudet, A. and Burgess, K. (2007) BODIPY Dyes and Their Deriva-tives: Syntheses and Spectroscopic Properties. Chemical Reviews, 107, 4891-4932. https://doi.org/10.1021/cr078381n
- 21. 刘汉壮. 基于硼—二吡咯亚甲基荧光探针的合成和性质研究[D]: [博士学位论文]. 南京: 南京大学, 2011.
- 22. Loudet, A., Bandichhor, R., Wu, L.X. and Burgess, K. (2008) Function-alized BF2 Chelated Azadipyrromethene Dyes. Tetrahedron, 64, 3642-3654. https://doi.org/10.1016/j.tet.2008.01.117
- 23. Ulrich, G. and Ziessel, R. (2008) The Chemistry of Fluorescent BODIPY Dyes: Versatility Unsurpassed. Angewandte Chemie International Edition, 47, 1184-1201. https://doi.org/10.1002/anie.200702070
NOTES
*通讯作者。