Journal of Organic Chemistry Research
Vol.05 No.02(2017), Article ID:20536,8
pages
10.12677/JOCR.2017.52011
The Michael Addition Reaction of Indoles and α, β-Unsaturated Ketones Catalyzed by Brønsted Acidic Ionic Liquid
Xuecheng Ma, Xiumei Liu, Chenjiang Liu*
The Key Laboratory of Oil and Gas Fine Chemicals, Ministry of Education & Xinjiang Uygur Autonomous Region, Physics and Chemistry Detecting Center, Xinjiang University, Urumqi Xinjiang
*通讯作者。

Received: Apr. 27th, 2017; accepted: May 14th, 2017; published: May 17th, 2017

ABSTRACT
The Michael addition reaction of indoles with α, β-unsaturated ketones was studied using brønsted acidic ionic liquid [ThiN(CH2)4SO3][p-CH3PhSO3] as catalyst. A series of β-indolone derivatives were obtained in up to 99% yield. The catalyst ionic liquid used in the approach can be easily recycled and reused for at least three cycles without decreasing its catalytic activity. The approach is characterized by mild condition and simple operation. It provides a new method for the synthesis of β-indole derivatives.
Keywords:Ionic Liquid, Catalysis, Indole, α, β-Unsaturated Ketone, Michael Addition
Brønsted酸性离子液体催化吲哚与α,β-不饱和酮的Michael加成反应
马雪成,刘秀梅,刘晨江*
石油天然气精细化工教育部&自治区重点实验室,新疆大学理化测试中心,新疆 乌鲁木齐

收稿日期:2017年4月27日;录用日期:2017年5月14日;发布日期:2017年5月17日

摘 要
本文以Brønsted酸性离子液体[ThiN(CH2)4SO3][p-CH3PhSO3]催化吲哚与α,β-不饱和酮发生Michael加成反应,得到了一系列β-吲哚酮化合物,最高产率可达99%。离子液体催化剂循环使用3次以上,催化活性没有明显下降。该方法具有条件温和、操作简单的特点,为β-吲哚酮化合物的合成提供了一种新的方法。
关键词 :离子液体,催化,吲哚,α, β-不饱和酮,Michael加成

Copyright © 2017 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
众所周知,吲哚作为一类具有独特结构和生物活性的化合物,在药物、功能材料和香料等领域应用广泛 [1] [2] [3] [4] [5] 。β-吲哚酮化合物在吲哚化学的研究中具有重要地位,迈克尔加成反应是合成该类化合物的有效途径之一 [6] [7] [8] [9] [10] 。
近年来,离子液体因具有低毒、低挥发性、良好的溶解性能和可循环使用等优点被广泛关注 [11] [12] [13] [14] 。离子液体作为反应溶剂、催化剂或促进剂应用于有机合成反应,可以高效、高选择性且条件温和的实现化合物之间的转化,符合绿色化学的理念 [15] [16] 。基于本课题组在离子液体领域的研究基础 [17] [18] ,本文采用Brønsted酸性功能化噻唑硫酮离子液体[ThiN(CH2)4SO3][p-CH3PhSO3]催化吲哚与α,β-不饱和酮发生迈克尔加成反应,以82%~99%的产率得到了一系列β-吲哚酮化合物。本方法优点在于底物普适性好、产物产率高,催化剂可以循环使用3次以上催化活性无明显下降。
2. 实验部分
2.1. 仪器与试剂
瑞士Buchi B-540型熔点仪;德国Bruker Equinox 55红外光谱仪(KBr压片);美国Varian inova-400型核磁共振仪(TMS为内标,CDCl3、D2O或DMSO-d6为溶剂);美国HP1100液相色谱质谱仪。所有试剂均为市售分析纯,用前未经处理直接使用。
2.2. Brønsted酸性离子液体[ThiN(CH2)4SO3][p-CH3PhSO3]合成
Brønsted酸性离子液体[ThiN(CH2)4SO3][p-CH3PhSO3]的合成参照文献 [19]
2.3. α,β-不饱和酮的合成
α,β-不饱和酮的合成参照文献 [20] 。
2.4. β-吲哚酮化合物3a-3z的合成及结构分析
吲哚化合物(1 mmol)、α,β-不饱和酮化合物(1 mmol)、Brønsted酸性离子液体催化剂[ThiN(CH2)4SO3][p-CH3PhSO3] (10 mol%)和乙腈(5 mL)在80℃下磁力搅拌反应5 h。反应结束后,待反应混合物冷至室温,加入碎冰搅拌至碎冰溶化并析出固体,过滤,用蒸馏水洗涤固体得到粗产物。粗产物经硅胶柱层析分离纯化得到目标产物纯品,反应式如式1所示。所得化合物结构经1H NMR,13C NMR,IR和MS确征。
未知化合物的结构表征如下:
化合物3h:粉色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm): 7.99 (s, 1H), 7.90-7.92 (m, 2H), 7.92 (d, J = 7.6 Hz, 2H), 7.46-7.52 (m, 1H), 7.39 (d, J = 7.6 Hz, 1H), 7.27-7.32 (m, 1H), 7.14 (t, J = 8.0 Hz, 1H), 6.88-7.03 (m, 6H), 5.03 (t, J = 8.0 Hz, 1H), 3.84 (s, 3H), 3.60-3.76 (m, 2H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 196.91, 163.47, 162.53, 160.10, 140.01, 139.98, 136.63, 130.37, 130.12, 129.29, 129.21, 126.48, 122.23, 121.29, 119.47, 119.44, 119.33, 115.24, 115.03, 113.73, 111.15, 76.70, 55.47, 44.80, 37.64; IR (KBr), υmax/cm-1: 3390, 3048, 2908, 2838, 1659, 1598, 1507, 1419, 1355, 1253, 1218, 1180, 1020, 980, 837, 742, 591, 534; ESI-MS: m/z (%) = 396 (100) [M+Na] +.
化合物3j:土黄色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm): 7.88 (d, J = 12.0 Hz, 3H), 6.86-7.48 (m, 10H), 4.98 (t, J = 8.0 Hz, 1H), 4.04-4.09 (m, 2H), 3.59-3.77 (m, 2H), 2.17 (s, 6H ), 1.40 (t, J = 4.0 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 197.16, 162.76, 141.82, 136.59, 136.38, 134.27, 130.38, 130.10, 129.59, 129.17, 126.74, 124.97, 121.99, 121.34, 119.73, 119.61, 119.28, 114.08, 111.01, 76.69, 63.71, 44.97, 37.89, 19.89, 19.32, 14.66; IR (KBr), υmax/cm-1: 3335, 3041, 2982, 2936, 2889, 1649, 1592, 1420, 1357, 1259, 1171, 1041, 816, 741, 610; ESI-MS: m/z (%) = 420 (100) [M+Na] +.
化合物3k:紫红色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm): 8.05 (s, 1H), 7.87-7.91 (m, 2H), 6.86-7.50 (m, 10H), 5.01 (s, 1H), 4.04-4.10 (m, 2H), 3.56-3.75 (m, 2H), 1.41 (t, J = 8.0 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 196.46, 163.02, 158.75, 156.30, 141.95, 141.92, 136.63, 132.64, 130.37, 129.77, 128.45, 128.37, 126.33, 122.36, 121.31, 119.59, 119.28, 118.67, 116.29, 116.07, 114.22, 111.24, 108.93, 108.72, 76.71, 63.79, 44.57, 37.34, 14.65; IR (KBr), υmax/cm-1: 3331, 3058, 2983, 2935, 2895, 1645, 1598, 1493, 1334, 1246, 1172, 1042, 980, 813, 738, 612; ESI-MS: m/z (%) = 490 (100) [M+Na] +.
化合物3l:淡黄色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm): 7.92-7.99 (m, 2H), 6.80-7.39 (m, 9H), 5.46 (t, J = 8.0 Hz, 1H), 4.06-4.11 (m, 2H), 3.72-3.84 (m, 1H), 3.53-3.76 (m, 2H), 1.55 (s, 1H), 1.41 (t, J = 8.0 Hz, 3H);
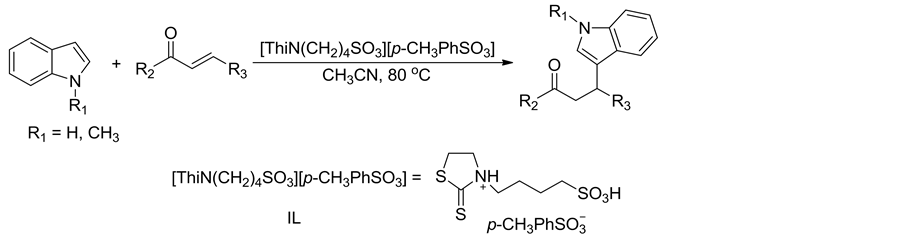
Scheme 1. Synthesis of β-indolone compounds
式1. β-吲哚酮化合物的合成
化合物3m:褐色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.94 (s, 2H), 7.71 (d, J = 8.0 Hz, 1H), 7.00-7.06 (m, 9H), 4.98 (t, J = 8.0 Hz, 1H), 3.61-3.75 (m, 2H), 2.18 (s, 6H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 196.57, 196.02, 141.09, 137.43, 136.68, 136.57, 134.62, 133.15, 130.61, 130.15, 129.69, 129.06, 127.09, 126.51, 124.88, 122.20, 121.28, 119.46, 111.10, 58.49, 45.27, 37.89, 19.90, 19.33; IR (KBr), υmax/cm-1: 3393, 3040, 2922, 1677, 1580, 1453, 1387, 1276, 1190, 1026, 880, 802, 741, 523; ESI-MS: m/z (%) = 444 (45) [M+Na] +.
化合物3o:淡粉色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.91-7.94 (m, 2H), 7.16-7.57 (m, 7H), 7.24 (t, J = 3.6 Hz, 1H), 6.99-7.03 (m, 1H), 6.90-6.95 (m, 2H), 6.83 (s, 1H), 5.02 (t, J = 8.0 Hz, 1H), 3.75 (d, J = 9.6 Hz, 2H), 3.74 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 196.57, 141.09, 137.43, 136.68, 136.57, 134.62, 133.15, 130.61, 130.15, 129.69, 129.06, 127.09, 126.51, 124.88, 122.20, 121.28, 119.46, 119.27, 111.10, 76.69, 45.27, 37.89, 19.90, 19.33; IR (KBr), υmax/cm-1: 3058, 2878, 1673, 1569, 1505, 1448, 1308, 1223, 1155, 1012, 976, 830, 742, 690; ESI-MS: m/z (%) = 380 (100) [M+Na] +.
化合物3q:草绿色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.92 (d, J = 7.6 Hz, 2H), 7.46-7.52 (m, 4H), 7.19-7.32 (m, 5H), 7.11-7.15 (m, 1H), 7.01 (t, J = 1.6 Hz, 1H), 6.84 (s, 1H), 5.12-5.16 (m, 1H), 3.74 (s, 3H), 3.32-3.3.52 (m, 2H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 188.35, 164.42, 163.01, 161.89, 138.54, 138.53, 136.34, 136.23, 130.92, 130.50, 129.86, 129.83, 129.07, 128.98, 124.37, 124.34, 117.69, 117.44, 114.81, 114.59, 114.31, 76.69, 63.80, 14.67.; IR (KBr), υmax/cm-1: 3056, 2909, 2823, 1673, 1592, 1471, 1371, 1237, 1196, 1071, 998, 881, 737, 686; ESI-MS: m/z (%) = 442 (100) [M+Na] +.
化合物3t:土黄色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.75-7.79 (m, 2H), 7.54-7.57 (m, 2H), 7.43 (d, J = 8.0 Hz, 1H), 7.14-7.35 (m, 7H), 6.99-7.03 (m, 1H), 6.81 (s, 1H), 5.02 (t, J = 8.0 Hz, 1H), 3.73-3.79 (m, 2H), 3.72 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 197.54, 144.10, 137.30, 135.80, 131.83, 129.61, 128.45, 128.12, 127.72, 126.87, 126.33, 126.18, 121.73, 119.50, 118.89, 117.58, 109.30, 109.22, 45.22, 38.17, 32.71; IR (KBr), υmax/cm-1: 3054, 2885, 1679, 1580, 1481, 1397, 1243, 1194, 1069, 972, 828, 740, 697; ESI-MS: m/z (%) = 442 (75) [M+Na] +.
化合物3u:粉色粉末;1H NMR (CDCl3, 400 MHz, TMS): δ (ppm) : 7.83-7.85 (m, 2H), 7.16-7.43 (m, 7H), 7.01 (t, J = 7.6 Hz, 1H), 6.77-6.80 (m, 3H), 4.97 (t, J = 8.0 Hz, 1H), 3.74 (s, 3H), 3.71 (s, 3H), 3.62-3.70 (m, 2H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 188.07, 146.42, 140.47, 137.96, 137.35, 137.09, 133.19, 132.12, 130.68, 130.41, 130.32, 129.77, 127.48, 126.38, 119.66, 76.68, 19.95, 19.77; IR (KBr), υmax/cm-1: 3050, 2934, 2834, 1677, 1585, 1508, 1466, 1250, 1173, 1092, 1035, 1009, 976, 829, 802, 740; 7.88-7.92 (d, 2H, CH), 7.18-7.21 (d, 2H, CH), 7.04-7.06 (d, 2H, CH); ESI-MS: m/z (%) = 426 (98) [M+Na] +.
化合物3v:粉色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.75 (d, J = 8.8 Hz, 2H), 7.53 (d, J = 8.8 Hz, 2H), 7.46 (d, J = 8.0 Hz, 1H), 6.99-7.25 (m, 7H), 6.79 (s, 1H), 3.62-3.66 (m, 2H), 3.76 (s, 3H), 2.18 (s, 6H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 197.64, 141.50, 137.27, 136.47, 135.86, 134.42, 131.77, 129.62, 129.08, 128.00, 126.92, 126.12, 124.82, 121.63, 119.50, 118.81, 117.89, 109.15, 77.30, 77.19, 76.99, 76.67, 45.36, 37.71, 32.67, 19.89, 19.32; IR (KBr), υmax/cm-1: 3044, 2915, 1677, 1580, 1478, 1377, 1248, 1197, 1069, 1004, 975, 816, 744; ESI-MS: m/z (%) = 468 (100) [M+Na] +.
化合物3w:粉色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.92-7.96 (m, 2H), 6.80-7.41 (m, 10H), 5.46 (t, J = 8.0 Hz, 1H), 4.09 (dd, 2H, J = 12.0, 7.2 Hz), 3.73-3.75 (m, 1H), 3.72 (s, 3H), 3.54-3.60 (m, 1H), 1.45 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 196.30, 162.91, 162.01, 159.54, 137.87, 137.83, 137.29, 133.97, 133.87, 130.40, 129.89, 129.81, 129.69, 126.92, 126.55, 121.79, 119.42, 118.94, 116.94, 116.70, 116.16, 114.14, 113.93, 109.19, 76.66, 63.73, 43.95, 34.36, 32.75, 14.65; IR (KBr), υmax/cm-1: 3051, 2981, 2902, 1668, 1599, 1483, 1315, 1244, 1177, 1040, 904, 846, 745, 600; ESI-MS: m/z (%) = 458 (100) [M+Na] +.
化合物3x:砖红色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.89-7.92 (m, 2H), 7.46 (d, J = 8.0 Hz, 1H), 6.86-7.25 (m, 8H), 6.80 (s, 1H), 4.97 (t, J = 8.0 Hz, 1H), 4.07 (d, J = 7.2 Hz, 2H), 3.61-3.75 (m, 5H), 2.18 (s, 6H), 1.42 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz):δ (ppm) : 197.08, 162.73, 141.97, 137.28, 136.37, 134.21, 130.37, 130.08, 129.58, 129.16, 127.06, 126.16, 124.90, 121.51, 119.64, 118.70, 118.24, 114.04, 109.08, 76.68, 63.69, 45.08, 37.73, 32.67, 19.91, 19.34, 14.67; IR (KBr), υmax/cm-1: 3044, 2974, 2929, 1667, 1599, 1472, 1309, 1247, 1174, 1045, 838, 742, 610; ESI-MS: m/z (%) = 434 (100) [M+Na] +.
化合物3y:粉色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.51 (d, J = 7.6 Hz, 1H), 7.12-7.37 ( m, 7H), 7.00-7.04 (m, 3H), 6.83 (s, 1H), 4.92 (t, J = 8.0 Hz, 1H), 3.74 (s, 3H), 3.54-3.70 (m, 1H), 2.34 ( s, 3H), 2.29 (s, 3H); 13C NMR (CDCl3, 100 MHz):δ (ppm) : 201.35, 144.80, 141.99, 138.65, 137.30, 134.91, 132.90, 132.26, 130.25, 130.05, 129.77, 128.64, 127.35, 126.66, 126.26, 126.15, 121.93, 119.27, 119.08, 116.67, 109.31, 76.68, 47.58, 37.76, 32.74, 21.36, 21.10; IR (KBr), υmax/cm-1: 3051, 2958, 2917, 1668, 1561, 1466, 1347, 1290, 1199, 1124, 1029, 978, 818, 741, 6890; ESI-MS: m/z (%) = 437 (100) [M+H+], 458 (93) [M+Na] +.
化合物3z:黄色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.94 (d, J = 1.6 Hz, 1H), 7.70 (dd, J = 12, 2.0 Hz, 1H), 7.45-7.49 (m, 3H), 7.16-7.26 (m, 5H), 7.00-7.09 (m, 5H), 6.79 (s, 1H), 4.94 (t, J = 8.0 Hz, 1H), 3.66-3.73 (m, 3H), 2.18 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 196.54, 141.27, 137.40, 137.28, 136.68, 136.56, 134.56, 133.13, 130.59, 130.16, 129.69, 129.06, 127.09, 126.86, 126.13, 124.83, 121.71, 119.47, 118.89, 117.73, 109.20, 45.42, 37.78, 32.70, 19.91, 19.34; IR (KBr), υmax/cm-1: 3085, 3013, 2934, 2891, 1678, 1579, 1463, 1387, 1243, 1197, 1027, 815, 744, 677; ESI-MS: m/z (%) = 458 (100) [M+Na] +.
3. 结果讨论
3.1. 优化反应条件
以吲哚和3-(4-甲基苯基)-1-(4-甲氧基苯基)-1-丙酮在乙腈中的反应为模型,对反应条件进行了优化,实验结果见表1。首先考察了催化剂Brønsted酸性离子液体[ThiN(CH2)4SO3][p-CH3PhSO3]的用量对反应的影响(Table 1, entries 1-4)。当不加催化剂时,无法得到目标产物(Table 1, entry 1)。当催化剂用量为15 mol%时,催化效率最高,产率可达98% (Table 1, entry 4),考虑到反应的经济性,确定催化剂的用量为10 mol% (Table 1, entry 3)。其次考察了反应时间、溶剂种类等对该反应的影响(Table 1, entries 5-10)。通过筛选,确定反应的最佳条件为:离子液体催化剂[ThiN(CH2)4SO3][p-CH3PhSO3]用量10 mol%,乙腈为反应溶剂,反应时间5 h,反应温度80℃。
3.2. 反应底物普适性研究
在最佳反应条件下,对反应底物的普适性进行研究,结果见表2。研究发现α, β-不饱和酮的苯环上连有供电子的Me、Et、OMe或OEt等基团或是吸电子的卤原子时,反应都能够顺利的进行,所得产物产率为82%~99% (Table 2, 3b-3l, 3o-3x)。当α, β-不饱和酮的苯环上均为双取代时,也能以优秀的产率得到相应的目标产物(Table 2, 3m、3y、3z)。此外,无论是吲哚还是N-甲基吲哚都能与各种不同取代的α, β-不饱和酮平稳的反应。以上结果表明,该反应底物的普适性好。
4. 催化剂的循环使用性研究
在最佳反应条件下,以吲哚和3-(4-甲基苯基)-1-(4-甲氧基苯基)-1-丙酮为模型反应,考察了离子液体
Table 1. Optimization of reaction conditionsa
表1. 反应条件优化a
a反应条件:1a (1 mmol),2a (1 mmol),催化剂IL:[ThiN(CH2)4SO3][p-CH3PhSO3],溶剂 (5 mL),80℃;b分离产率。
Table 2. Research of substrate scopea
表2. 底物的普适性研究a
a反应条件:吲哚或取代吲哚(1 mmol),α,β-不饱和酮(1 mmol),[ThiN(CH2)4SO3][p-CH3PhSO3] (10 mol%),80℃,5 h,乙腈(5mL)。b分离产率。
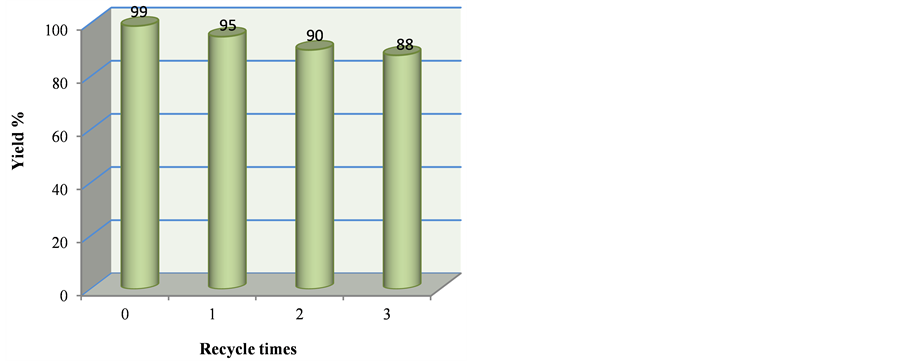
Figure 1. Recycling research of ionic liquid [ThiN(CH2)4SO3][p-CH3PhSO3]
图1. 离子液体[ThiN(CH2)4SO3][p-CH3PhSO3]的循环性研究
催化剂([ThiN(CH2)4SO3][p-CH3PhSO3])的循环使用效果,结果见图1。具体操作是:将15 mL乙酸乙酯分三次加入到除去粗产物后的滤液中萃取残留的有机物,然后将水相中的水旋除,真空干燥至恒重,即得回收的离子液体催化剂,然后直接用于下一次催化循环实验。研究发现该离子液体催化剂循环使用3次,相应产物的产率分别是95%、90%、88%,表明离子液体具有良好的循环使用效果。
5. 总结
本文开展了吲哚和α,β-不饱和酮在Brønsted酸性离子液体[ThiN(CH2)4SO3][p-CH3PhSO3]催化下的Michael加成反应,以82%~99%的产率合成了β-吲哚酮化合物,具有产物收率高、催化剂可循环使用的特点。
基金项目
国家自然科学基金(No. 21572195, 21262035, 21162025)。
文章引用
马雪成,刘秀梅,刘晨江. Br∅nsted酸性离子液体催化吲哚与α,β-不饱和酮的Michael加成反应
The Michael Addition Reaction of Indoles and α, β-Unsaturated Ketones Catalyzed by Br∅nsted Acidic Ionic Liquid[J]. 有机化学研究, 2017, 05(02): 86-93. http://dx.doi.org/10.12677/JOCR.2017.52011
参考文献 (References)
- 1. Humphrey, G.R. and Kuethe, J.T. (2006) Practical Methodologies for the Synthesis of Indoles. Chemical Reviews, 106, 2875-2911. https://doi.org/10.1021/cr0505270
- 2. Sundberg, R.J. (1996) Indoles. Academic Press, Lon-don.
- 3. Finefield, J.M., Frisvad, J.C., Sherman, D.H. and Williams, R.M. (2012) Fungal Origins of the Bicyclo [2.2.2] Diazaoctane Ring System of Prenylated Indole Alkaloids. Natural Product Reports, 75, 812-833. https://doi.org/10.1021/np200954v
- 4. Taber, D.F. and Tirunahari, P.K. (2011) Indole Synthesis: A Review and Proposed Classification. Tetrahedron, 67, 7195-7210. https://doi.org/10.1016/j.tet.2011.06.040
- 5. Li, Y.X., Wang, H.X., Ali, S., Xia, X.F. and Liang, Y.M. (2012) Iodine-Mediated Regioselective C2-Amination of Indoles and a Concise Total Synthesis of (±)-Folicanthine. Chemical Communications, 48, 2343-2345. https://doi.org/10.1039/c2cc16637b
- 6. Shiri, M. (2012) Indoles in Multicomponent Processes (MCPs). Chemical Reviews, 112, 3508-3549.
- 7. Aksenov, A.V., Aksenov, N.A., Dzhandigova, Z.V., Aksenov, D.A., Voskressensky, L.G., Nenajdenkoc, V.G. and Rubin, M. (2016) Direct Reductive Coupling of Indoles to Nitrostyrenes en Route to (Indol-3-YL) Acetamides. RSC Advances, 6, 93881-93886. https://doi.org/10.1039/C6RA21399E
- 8. Romano, C., Jia, M.Q., Monari, M., Manoni, E. and Bandini, M. (2014) Metal-Free Enantioselective Electrophilic Activation of Al-lenamides: Stereoselective Dearomatization of Indoles. Angewandte Chemie International Edition, 53, 1-5.
- 9. Wu, K.K., Wu, P., Wang, L.D., Chen, J.P., Sun, C.L. and Yu, Z.K. (2014) Tunable Brønsted Acidity-Dependent Alkylation and Alkenylation of Indoles. Advanced Synthesis & Catalysis, 356, 3871-3880. https://doi.org/10.1002/adsc.201400477
- 10. Ballini, R., Clemente, R.R., Palmieri, A. and Petrini, M. (2006) Conjugate Addition of Indoles to Nitroalkenes Promoted by Basic Alumina in Solventless Conditions. Advanced Syn-thesis & Catalysis, 348, 191-196. https://doi.org/10.1002/adsc.200505339
- 11. Li, Z., Friedrich, A. and Taubert, A. (2008) Gold Microcrystal Syn-thesis via Reduction of HAuCl4 by Cellulose in the Ionic Liquid 1-Butyl-3-Methyl Imidazolium Chloride. Journal of Materials Chemistry, 18, 1008.
- 12. Zakrzewska, M.E., Bogel-Łukasik, E. and Bogel-Łukasik, R. (2011) Ionic Liq-uid-Mediated Formation of 5-Hydrox- ymethylfurfurals-A Promising Biomass-Derived Building Block. Chemical Re-views, 111, 397-417. https://doi.org/10.1021/cr100171a
- 13. Jason, P.H. and Tom, W. (2011) Room-Temperature Ionic Liquids: Sol-vents for Synthesis and Catalysis. Chemical Reviews, 111, 3508-3565. https://doi.org/10.1021/cr1003248
- 14. Zhang, Q. and Jeanne, M.S. (2014) Energetic Ionic Liquids as Explosives and Propellant Fuels: A New Journey of Ionic Liquid Chemistry. Chemical Reviews, 114, 10527-10572.
- 15. Ding, Z.Y., Wang, T.L., He, Y.M., Chen, F., Zhou, H.F., Fan, Q.H., Guo, Q.X. and Chan, A.S.C. (2013) Highly Enantiose-lective Synthesis of Chiral Tetrahydroquinolines and Tetrahydroisoquinolines by Ruthenium-Catalyzed Asymmetric Hydrogenation in Ionic Liquid. Advanced Synthesis & Catalysis, 355, 3727-3735. https://doi.org/10.1002/adsc.201300698
- 16. Taheri, A., Lai, B.B., Cheng, C. and Gu, Y.L. (2015) Brønsted Acid Ionic Liquid-Catalyzed Reductive Friedel-Crafts Alkylation of Indoles and Cyclic Ketones without Using an External Reductant. Green Chemistry, 17, 812-816.
- 17. Cao, D.W., Zhang, Y.H., Liu, C.J., Wang, B., Sun, Y.D., Abdukadera, A., Hu, H.Y. and Liu, Q. (2016) Ionic liquid Promoted Diazenylation of N-Heterocyclic Compounds with Aryltriazenes under Mild Conditions. Organic Letters, 18, 2000-2003. https://doi.org/10.1021/acs.orglett.6b00605
- 18. Li, H., Liu, C.J., Zhang, Y.H., Sun, Y.D., Wang, B. and Liu, W.B. (2015) GREEN METHOd for the Synthesis of Chromeno [2,3-c]pyrazol-4(1H) Ones through Ionic Liquid Promoted Directed Annulation of 5-(Aryloxy)-1h-Pyra- zole-4-Carbaldehydes in Aqueous Media. Organic Letters, 17, 932-935. https://doi.org/10.1021/acs.orglett.5b00033
- 19. 刘晨江, 张永红. 一种阳离子为1,3-四氢噻唑-2-硫酮类的新型Brønsted酸性离子液体的制备方法[P]. 中国专利, CN102229579, 2011.
- 20. 李在国, 王清民, 黄君珉. 有机中间体制备[M]. 第2版. 北京: 化学工业出版社, 1996: 51.
- 21. Jaber, N.A., Bougasim, A.S.A. and Karah, M.M.S. (2012) Study of Michael Addition on Chalcones and or Chalcone Analogues. Journal of Saudi Chemical Society, 16, 45-53.
- 22. Ekbote, S.S., Panda, A.G., Bhor, M.D. and Bhanage, B.M. (2009) Polyvinylsulfonic Acid as a Novel Brønsted Acid Catalyst for Michael Addition of Indoles to α,β-Unsaturated Ketones. Catalysis Communications, 10, 1569-1573. https://doi.org/10.1016/j.catcom.2009.04.011
- 23. Gao, Y.H., Yang, L., Zhou, W., Xu, L.W. and Xia, C.G. (2009) Highly Efficient Bimetallic Iron-Palladium Catalyzed Michael-Type Friedel-Crafts Reactions of Indoles with Chalcones. Apply Organic Chemistry, 23, 114-118.
- 24. Azizi, N., Arynasab, F. and Saidi, M.R. (2006) Efficient Friedel-Crafts Alkylation of Indoles and Pyrrole with Enones and Nitroalkene in Water. Organic & Biomolecular Chemistry, 4, 4275-4277.
- 25. Huang, Z. H., Zou, J. P. and Jiang, W. Q. (2006) Gallium(III) Triiodide Catalyzed Conjugate Addition of Indoles with α,β-Unsaturated Ketones. Tetrahedron Letters, 47, 7965-7968. https://doi.org/10.1016/j.tetlet.2006.08.108
- 26. Yu, C.J. and Liu, C.J. (2009) Conjugate Addition of Indoles to α,β-Unsaturated Ketones Using a Brønsted Acid Ionic Liquid as an Efficient Catalyst. Molecules, 14, 3222-3228.
- 27. Damodiran, M., Kumar, R.S., Sivakumar, P.M., Doble, M. and Perumal, P. (2009) A Simple Protocol for the Michael Addition of Indoles with Electron Deficient Olefins Catalysed by TBAHS in Aqueous Media and their Broad Spectrum Antibacterial Activity. Journal of Chemical Sciences, 121, 65-73. https://doi.org/10.1007/s12039-009-0007-x
- 28. Gu, D.G., Ji, S.J., Wang, H.X. and Xu, Q.Y. (2008) Acidic Ionic Liquid—Catalyzed Highly Efficient Reaction of Indoles to α,β-Unsaturated Ketones. Synthetic Communications, 38, 1212-1223. https://doi.org/10.1080/00397910701866304
- 29. Bartoli, G., Bartolacci, M., Bosco, M., Foglia, G., Giuliani, A. and Marcantoni, E. (2003) The Michael Addition of Indoles to α,β-Unsaturated Ketones Catalyzed by CeCl3•7H2O-NaI Combination Supported on Silica Gel. Journal of Organic Chemistry, 68, 4594-4597.
- 30. Murugan, R., Karthikeyan, M., Perumal, P.T. and Reddy, B.S.R. (2005) A Mild Efficient and Improved Protocol for the Synthesis of Novel Indolyl Crown Ethers, Di (Indolyl) Pyrazolyl Methanes and 3-Alkylated Indoles Using H4[Si(W3o10)3]. Tetrahedron, 61, 12275-12281. https://doi.org/10.1016/j.tet.2005.09.108
