Hans Journal of Medicinal Chemistry
Vol.
10
No.
02
(
2022
), Article ID:
50136
,
11
pages
10.12677/HJMCe.2022.102012
吡唑类化合物的合成及抗肿瘤活性分析
王玉琳,周庆发*
中国药科大学理学院,江苏 南京
收稿日期:2022年3月15日;录用日期:2022年3月31日;发布日期:2022年4月12日

摘要
吡唑是一类具有多种生理学活性的含氮五元杂环。在过去的十年里,越来越多的研究表明吡唑环类化合物可通过影响细胞分裂过程中关键酶,以及对特定肿瘤细胞系的抗增殖效应从而达到优秀的抗肿瘤效果。为进一步研究吡唑衍生物的抗肿瘤性质及其构效关系,以便充分开发这些化合物的抗肿瘤潜力,我们总结了合成吡唑及其衍生物的策略,并证明了这类化合物可以靶向发现新的抗肿瘤药物。
关键词
吡唑,吡唑衍生物,抗肿瘤活性

Synthesis and Antitumor Activity Analysis of Pyrazole Compounds
Yulin Wang, Qingfa Zhou*
College of Science, China Pharmaceutical University, Nanjing Jiangsu
Received: Mar. 15th, 2022; accepted: Mar. 31st, 2022; published: Apr. 12th, 2022

ABSTRACT
Pyrazoles are a class of nitrogen-containing five-membered heterocycles with various physiological activities. In the past decade, more and more studies have shown that pyrazole compounds can achieve excellent antitumor effects by affecting key enzymes in the process of cell division and antiproliferative effects on specific tumor cell lines. To further study the antitumor properties of pyrazole derivatives and their structure-activity relationships in order to fully exploit the antitumor potential of these compounds, we summarize strategies for the synthesis of pyrazoles and their derivatives, and demonstrate that such compounds can be targeted for discovery of new antitumor drugs.
Keywords:Pyrazoles, Pyrazole Derivatives, Antitumor Activity

Copyright © 2022 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
吡唑及其衍生物被认为是一种重要的药理活性支架,具有几乎所有类型的药理活性 [1] - [10]。这一基本骨架广泛存在于不同的治疗类药物中,如抗炎药塞来昔布、抗精神病药CDPPB、抗焦虑药扎来普隆、抗肥胖药利莫那班、COX-2选择性非甾体抗炎药(NSAIDs)替泊沙林(图1)等。这些均表明了吡唑骨架具有很大的药物潜力。在过去的十年中,许多科学家和研究人员报道了一系列具有良好抗癌活性的吡唑衍生物,表明吡唑基序是开发新型抗癌药物的有力工具。由于吡唑核在生物领域中的广阔应用,许多研究者对其骨架进行了化学和生物学研究。本文主要介绍吡唑类衍生物的合成方法及抗肿瘤活性分析。
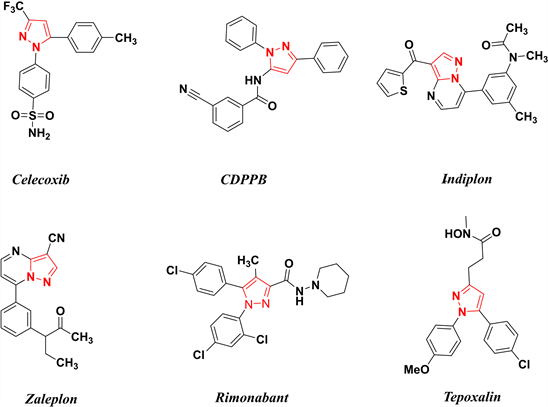
Figure 1. Some representative drugs containing pyrazole unit
图1. 一些包含吡唑骨架的代表性药物
2. 吡唑环类化合物的合成
吡唑是一类π电子过剩的芳香杂环,其环上的1、2、3、4四个位置可以被不同取代基部分或全部取代,形成一系列具有不同药理学活性的化合物。自从Knorr [11] 在1883年首次合成了吡唑化合物后,有关吡唑环的合成路径就在不断地发展。在这一部分,我们综述了目前常用的吡唑环类化合物的制备方法。
2.1. 肼及其衍生物与α,β-不饱和羰基化合物的环缩合反应
肼及其衍生物和α,β-不饱和羰基化合物的环合是制备多取代吡唑环的一种简便、快捷的方法。其中常用的α,β-不饱和羰基化合物有1,3-二羰基类、炔酮类、乙烯基甲酮类和携带易离去基团的乙炔基甲酮类等(图2)。
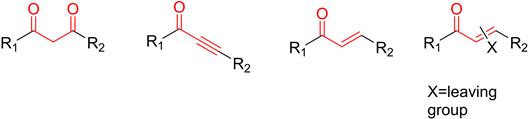
Figure 2. α,β-unsaturated carbonyl compounds used in the synthesis of pyrazoles
图2. 用于合成吡唑的α,β-不饱和羰基化合物
1,3-二羰基化合物与肼衍生物的环缩合是获得多取代吡唑的简单且快速的方法。1883年,Knorr等人 [11] 通过β-二酮和肼衍生物的环合首次合成了一对多取代吡唑环的区域异构体(图3(a))。随后,Girish等人 [12] 报道了一种用纳米ZnO催化剂来催化苯肼和乙酰乙酸乙酯的缩合,得到1,3,5-三取代吡唑衍生物的方法。这种方法用时少、容易操作,且具有较高的产率(95%) (图3(b))。为进一步探究由1,3-二羰基化合物制备具有高度区域选择性的多取代吡唑所需的合成条件,Gossselin和他的同事 [13] 研究了不同溶剂对此类反应的影响。他们发现1,3-二酮和芳基肼在非质子偶极溶剂中的环缩合效果优于其在极性质子溶剂(如乙醇)中的环缩合效果。在乙醇溶溶剂中,1,3-二酮8和芳基肼在常温条件下反应,生成区域异构体的等摩尔混合物。而在酰胺溶剂(DMF, NMP, DMAc)或尿素(DMPU, TMU)中,1,3-二酮8和芳基肼发生环合反应,可以59%~83%的产率生成相应的吡唑类化合物,并以98:2~ > 99.8:0.2的高选择性优先合成吡唑环化合物10。此外,他们还发现通过在酰胺溶剂或尿素中加入盐酸溶液,可以加速脱水步骤,从而提高产率(图3(c))。

Figure 3. Preparation of pyrazoles from 1,3-dicarbonyl compounds
图3. 由1,3-二羰基类化合物制备吡唑
炔酮类化合物和肼衍生物通过环缩合生成吡唑环的反应已有100年的历史。和1,3-二羰基化合物与肼衍生物的反应类似,该反应同样会形成两种区域异构体的混合物。Bishop等人 [14] 研究了乙炔酮12在乙醇中对甲基肼或芳基肼的环缩合,这提供了两种难以分离的区域异构吡唑14和15。他们发现肼的亲核性能显著影响吡唑物两种区域异构体生成的比例。使用芳基肼时,吡唑产物14/15的生成比例为87:13至99:1;使用甲基肼时,吡唑产物15/14的生成比例为93:3至97:3。这种区域选择性的差异可以通过以下事实来解释:携带甲基的氮更亲核,容易与乙炔酮的碳碳三键发生Michael加成,随后在分子内形成亚胺。在被芳基取代的肼的情况下,伯胺是最亲核的并与碳碳三键发生反应,随后仲胺进攻羰基生成最终产物14 (图4)。
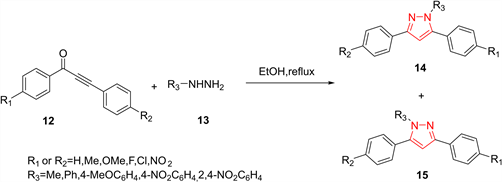
Figure 4. Preparation of pyrazoles from acetylenic ketones
图4. 由炔酮类化合物制备吡唑
α,β-乙烯基酮和芳基肼衍生物通过环合反应先生成吡唑啉,随后经过氧化形成吡唑环。Rao等人 [15] 报导了在Cu(OTf)3和1-丁基-3-甲基咪唑鎓六氟磷酸盐([bmim] (PF6))催化下,α,β-烯基酮16与肼类衍生物17生成吡唑化合物的反应。首先在Cu(OTF)2介导下将肼1,2-加成到查尔酮中,生成3-羟基吡唑啉A;随后A在Cu(OTF)2存在下进行消除以获得1,3,5-三芳基吡唑啉衍生物B;接着在Cu(OTF)2存在下进行B的氧化芳构化,产生相应的1,3,5-三芳基吡唑18。该反应方案通过查尔酮和芳基肼之间的一锅法进行加成–环缩合,以及氧化芳构化,使1,3,5-三芳基吡唑以较高产量生成(约82%)。此反应不需要额外的氧化试剂,且催化剂可以重复使用超过四个循环而不损失太多催化活性(图5)。
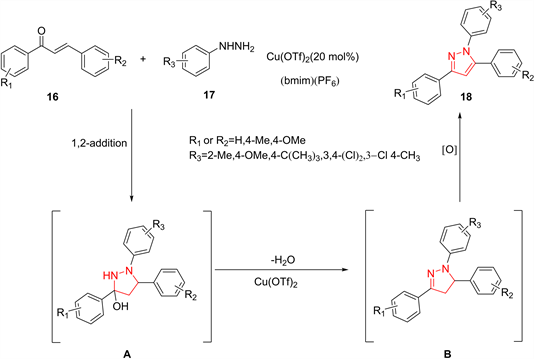
Figure 5. Preparation of pyrazoles from vinyl ketones
图5. 由乙烯基甲酮类化合物制备吡唑
具有离去基团的α,β-乙烯基酮与肼衍生物反应形成吡唑啉,在去除离去基后,可得到所需的吡唑类化合物。Katritzky等 [16] 报道了α-苯并三唑酮19和苯肼20的区域选择性缩合反应合成1-甲基(芳基)-3-苯基-5-烷基(芳基)吡唑22的反应。中间体吡唑啉21在碱性条件下去除苯并三唑后形成终产物吡唑。此反应的优点在于,苯并三唑基团的存才使得α位的质子酸性更强,从而允许在吡唑啉核的4位功能化,进一步生成四取代吡唑24 (图6)。
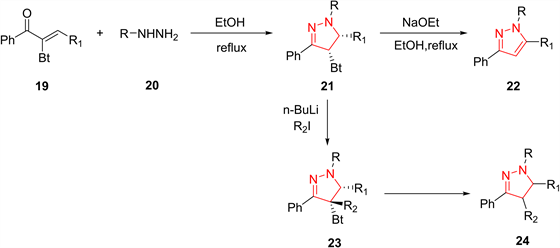
Figure 6. Preparation of pyrazoles from vinyl ketones having a leaving group
图6. 由携带易离去基团的乙炔基甲酮类化合物制备吡唑
2.2. 1,3-偶极子环合反应
合成吡唑环的另一种方法涉及烯烃或炔烃和1,3-偶极子的[3+2]环合。其中常用的1,3-偶极子包括重氮化合物、悉尼酮和腈亚胺。
2009年,何等人 [17] 以Zn(OTf)2为催化剂,通过苯基炔丙基25和乙基α-重氮乙酸酯26的1,3-偶极子环合反应生成了相应的吡唑类化合物。此反应条件温和、程序简单、产率良好、易于操作,这使得该方法在有机合成中具有广泛的用途(图7(a))。同年,Gioiello等人 [18] 报导了通过重氮基乙酸乙酯28和α-亚甲基羰基29的1,3-偶极子环合来制备多取代的吡唑-5-羧酸盐类的方法。此方法以1,8-二氮杂环[5,4,0]十一烯-7(DBU)为催化剂,以乙腈为溶剂,通过多米诺1,3-偶极环加成-水消除进行,具有良好的区域选择性和良好的产率(图7(b))。
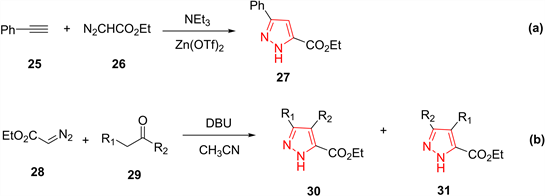
Figure 7. Preparation of pyrazoles from cycloaddition of diazocarbonyl compound
图7. 由重氮化合物的1,3-偶极子环合反应制备吡唑
2010年,Delaunay课题组 [19] 利用悉尼酮和炔类物质的环加成反应生成了1,3,4,5-多取代吡唑环。该反应在15 h内完成,得到了区域异构体5-碘吡唑34和35的3:1混合物,总收率为84%。用硅胶色谱法很容易分离出二者,并以63%的分离收率获得了主要异构体34 (图8)。

Figure 8. Preparation of pyrazoles from cycloaddition of sydnones
图8. 由悉尼酮的1,3-偶极子环合反应制备吡唑
腈亚胺是另一种常用于合成吡唑类化合物的1,3-偶极子。2006年,Oh等人 [20] 报道了由芳腙原位合成的腈亚胺与乙烯基衍生物进行1,3-偶极环加成反应,以72%的产率生成了1,3,5-多取代吡唑化合物38。该方法简单实用,所需原料简单易得,且合成的产物具有良好的区域选择性(图9)。
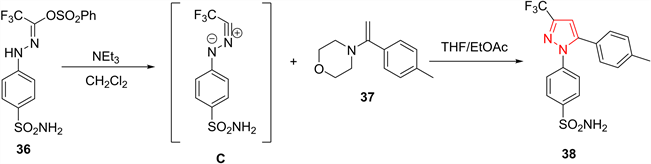
Figure 9. Preparation of pyrazoles from cycloaddition of nitrilimines
图9. 由腈亚胺的1,3-偶极子环合反应制备吡唑
2.3. 由各种杂环体系制备
一些其他杂环系统,如吡喃酮、呋喃酮、吡啶酮、咪唑酮、恶唑、四唑、三嗪、1,5-苯二氮卓类化合物,可通过反应生成相应的吡唑环类化合物(图10)。
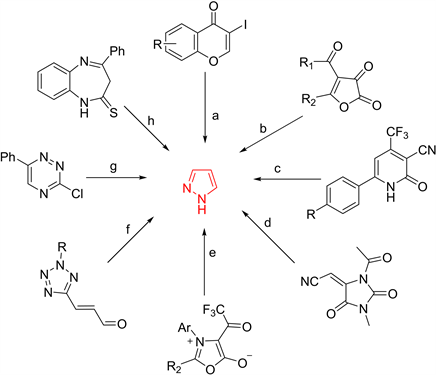
Figure 10. Some heterocyclic compounds used in the synthesis of pyrazoles
图10. 一些用于合成吡唑的杂环化合物
其中,吡喃酮是制备吡唑类药物中应用最广泛的杂环之一。在2004年,Yadav等人 [21] 以蒙脱石KSF为催化剂,使2,3-二氢-4H-吡喃-4-酮39与芳基肼在乙醇中发生缩合反应,以80%的产率得到了5-取代吡唑类化合物40 (图11(a))。2006年,Xie等人 [22] 开发了一种合成多芳基吡唑类化合物的通用方法。首先,芳基硼酸与色酮41进行铃木反应生成芳基取代的色酮,然后在水合肼的作用下以48%~95%的产率产生相应的3,4-二芳基吡唑42 (图11(b))。
2005年,Ilham等人 [23] 以呋喃-2,3-二酮43为底物,在苯回流下,与芳基肼发生反应,以45–65%的产率合成了吡唑-3-肼类45 (图11(c))。此外,Narsaiah等人 [24] 通过3-氰基-4-三氟甲基-6-苯基-2(1H)-吡啶酮46与水合肼在回流下反应,以中等产率生成5-三氟甲基-3-苯基吡唑47(图11(d))。类似地,3,5-二酰基-1,4-二氢吡啶48与肼在乙醇中于140℃反应,以良好的收率得到了双吡唑基甲烷49 (图11(e))。
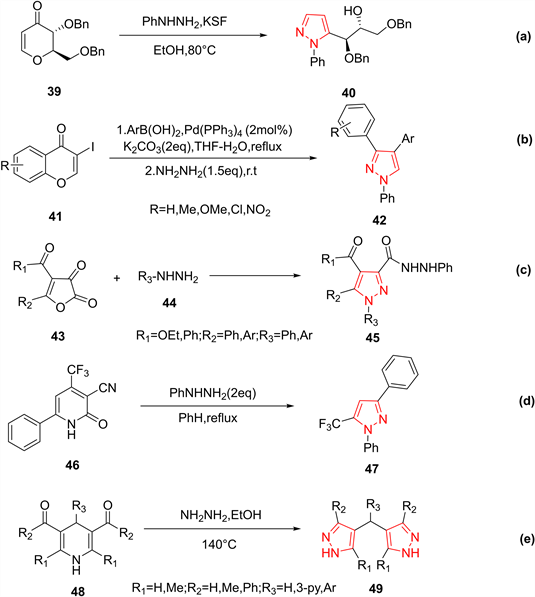
Figure 11. Examples of the preparation of pyrazoles from heterocyclic compounds
图11. 一些由杂环化合物制备吡唑的例子
3. 吡唑环类化合物的抗肿瘤活性分析
吡唑环化合物作为一种重要的含氮杂环化合物,因具有广谱的药学活性和独特的高效低毒的生物活性,使其成为抗肿瘤领域的研究热点。
BRAF是一种人类基因,属于RAS/RAF/MEK/ERK/MAP激酶通路,它介导细胞对生长信号的反应。它在一些人类癌症中会发生突变,其中缬氨酸取代600位谷氨酸(V600E;正式确定为V599E)已被确定为人类癌症中最常见的突变。因此,通过抑制BRAFV600E来靶向蛋白激酶途径在癌症治疗中是很重要的,特别是黑色素瘤。基于此,Liu等人 [25] 制备了4,5-二氢-2H-吡唑-2-羟基苯基衍生物50作为BRAFV600E抑制剂,并进一步证实其对WM266.5 (人黑色素瘤细胞系)和MCF-7 (人乳腺癌细胞系)具有显著的抗增殖作用。其中,化合物50a和50b的抑制活性最强(50a:对MCF-7的半抑制浓度为1.31 μM,对WM266.5的半抑制浓度为0.45 μM;50b:对MCF-7的半抑制浓度为0.97 μM,对WM266.5的半抑制浓度为0.72 μM) (图12)。
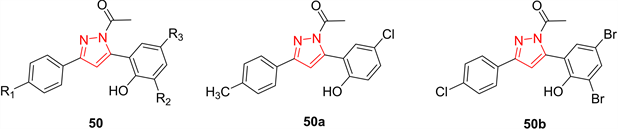
Figure 12. Compound 50 and its representative compounds
图12. 化合物50及其代表化合物
细胞周期蛋白依赖性激酶(CDKs)是一个丝氨酸–苏氨酸蛋白激酶家族,它不仅控制着细胞周期事件的起始、进展和完成,还参与调节神经细胞的转录、mRNA的加工和分化。细胞周期控制缺失是导致细胞异常增殖是癌症的关键特征之一,因而抑制CDKs有望成为控制肿瘤生长的最有效策略。Sun等人 [26] 制备了一系列1,3-二苯基-N-(苯基胺基甲酰胺)-1H-吡唑-4-羧酸酰胺衍生物51,发现其对A549 (癌性人肺泡基底上皮细胞)具有抗增殖活性,且对CDK2,4,6具有抑制活性。在合成的化合物中,51a的抑制活性最强,其对A549的半抑制浓度为0.75 μM,对CDK2、CDK4和CDK6的半抑制浓度分别为25、35和25 nM。SAR研究表明,以OMe和Me基团为供电子取代基的化合物的活性优于具有吸电子取代基的化合物(图13)。
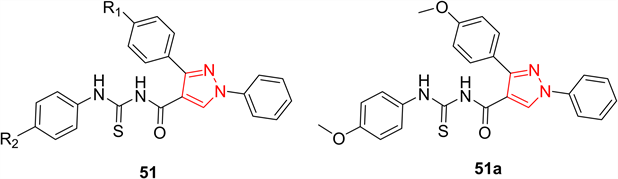
Figure 13. Compound 51 and its representative compounds
图13. 化合物51及其代表化合物
丙酮酸激酶(PK)是糖酵解的关键介质,是催化糖酵解最后一步的限速酶。肿瘤细胞在快速增长过程中通过过量表达低活性形式的丙酮酸激酶M2 (PKM2),将葡萄糖氧化代谢转换为有氧糖酵解,为其生长提供足够的物质积累和能量。研究表明PKM2的激活剂能将有氧糖酵解转化为氧化代谢并防止癌症生长。Xu等人 [27] 制备了一系列3-取代-1H-吡唑-5-羧基酰胺化合物52作为hPKM2激活剂并对其进行抗肿瘤活性初筛,筛选出抗肿瘤活性最强的化合物52a,其对A549和NCI-H1299细胞系的半抑制浓度值分别为0.26和0.22 µM (图14)。
极光激酶是一个高度保守的丝氨酸/苏氨酸蛋白激酶家族,在调节有丝分裂和细胞分裂完成的许多关键过程中发挥着关键作用。其中极光激酶A参与了中心体的成熟和分离、双极纺锤体的组装和有丝分裂的进入,其过量表达可导致遗传不稳定和肿瘤发生。因此,靶向抑制极光激酶A已成为癌症治疗中一种有吸引力的治疗策略。在2012年,Li等人 [28] 设计、合成了一系列N,1,3-三苯基-1H-吡唑-4-羧基酰胺衍生物53,并评价了其潜在的抗增殖活性和极光激酶A抑制活性。在所合成的化合物中,化合物53a对HCT116和MCF-7细胞系具有最高的生物活性,半抑制浓度值分别为0.39 ± 0.06 μM和0.46 ± 0.04 μM,与阳性对照相当。化合物53a也具有显著的极光激酶A抑制活性(半抑制浓度 = 0.16 ± 0.03 μM)。根据初步结果,推测具有较强的极光激酶A抑制活性的化合物53a可能是一种潜在的抗癌药物(图15)。
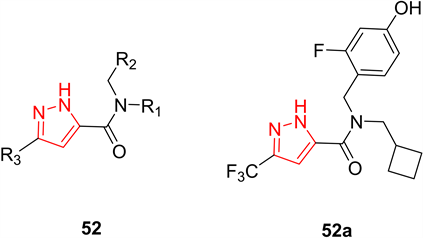
Figure 14. Compound 52 and its representative compounds
图14. 化合物52及其代表化合物
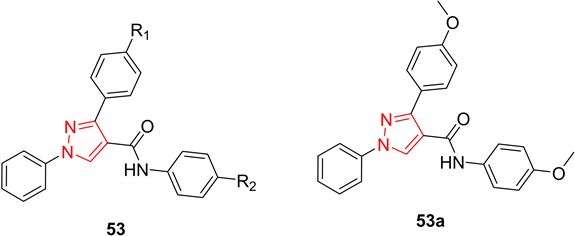
Figure 15. Compound 53 and its representative compounds
图15. 化合物53及其代表化合物
吡唑类化合物还可通过其优异的抗细胞增殖作用来发挥抗癌作用。细胞增殖是导致细胞数量增加的过程,是个体整体生长的重要过程之一。细胞的异常生长和过度增殖可导致肿瘤的发生。Huang等人 [29] 报道了几种具有抗增殖活性的吡唑罗[3,4-d]嘧啶衍生物。这些化合物对一系列人类癌细胞系显示出抗增殖活性,包括肺癌(NCl-H226)、鼻咽癌(NPC-TW01)和T细胞白血病(Jurkat)细胞。其中,化合物54a对NCI-H226和NPC-TW01有最好的抗癌活性,其半抑制浓度分别为18 μM和23 μM (图16)。
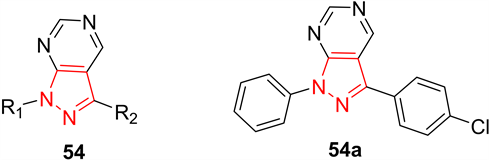
Figure 16. Compound 54 and its representative compounds
图16. 化合物54及其代表化合物
4. 总结和展望
杂环化合物因其显著的化学多样性,已经成为当今新药发展的主流。而在所有杂环化合物中,含氮杂环是最重要的组成部分。吡唑类化合物作为含氮杂环的一种,因具有广阔的药物活性受到了药学研究者的广泛关注。随着相关研究的不断深入,越来越多的证据证实吡唑环类衍生物为药物发现开拓了一个十分具有发展前景的领域。在这里,我们总结了一些常用的合成吡唑环类化合物的方法。这些条件温和、操作简单、产率良好,为获得高度功能化的吡唑衍生物库奠定基础。与此同时,由于多取代吡唑环类化合物合成策略的发展和吡唑类衍生物自身所具有的高效低毒生物活性,近年来,以吡唑环类化合物为母核,设计并合成抗肿瘤先导药物渐渐成为抗肿瘤领域的研究热点。许多研究者从靶点出发,对吡唑衍生物进行活性基团拼接,通过改善不同立体结构的取代基,对结构进行化学修饰,随后进行生物活性分析,获得了很多高活性、高选择性的吡唑类化合物。这些所报道的化合物的SARs揭示了进一步了解取代基的模式,包括供电子、吸电子和一些杂环部分,在调节基本骨架的生物潜力方面起着关键作用。
文章引用
王玉琳,周庆发. 吡唑类化合物的合成及抗肿瘤活性分析
Synthesis and Antitumor Activity Analysis of Pyrazole Compounds[J]. 药物化学, 2022, 10(02): 122-132. https://doi.org/10.12677/HJMCe.2022.102012
参考文献
- 1. Abdellatif, K.R.A. and Fadaly, W.A. (2017) Design, Synthesis, Cyclooxygenase Inhibition and Biological Evaluation of New 1,3,5-Triaryl-4,5-Dihydro-1H-Pyrazole Derivatives Possessing Amino/Methanesulfonyl Pharmacophore. Bioor-ganic Chemistry, 70, 57-66. https://doi.org/10.1016/j.bioorg.2016.11.008
- 2. Abdellatif, K.R.A., Abdelall, E.K.A., Lamie, P.F., Labib, M.B., El-Nahaas, E.S. and Abdelhakeem, M.M. (2020) New Pyrazole Derivatives Pos-sessing Amino/Methanesulphonyl Pharmacophore with Good Gastric Safety Profile: Design, Synthesis, Cyclooxygenase Inhibition, Anti-Inflammatory Activity and Histopathological Studies. Bioorganic Chemistry, 95, Article ID: 103540. https://doi.org/10.1016/j.bioorg.2019.103540
- 3. Abdel-Maksoud, M.S., El-Gamal, M.I., Gamal El-Din, M.M., Choi, Y., Choi, J., Shin, J.S., Kang, S.Y., Yoo, K.H., Lee, K.T., Baek, D. and Oh, C.H. (2018) Synthesis of New Tri-arylpyrazole Derivatives Possessing Terminal Sulfonamide Moiety and Their Inhibitory Effects on PGE2 and Nitric Ox-ide Productions in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Molecules, 23, Article No. 2556. https://doi.org/10.3390/molecules23102556
- 4. El-Shoukrofy, M.S., Abd El Razik, H.A., AboulWafa, O.M., Bayad, A.E. and El-Ashmawy, I.M. (2019) Pyrazoles Containing Thiophene, Thienopyrimidine and Thienotriazolopy-rimidine as COX-2 Selective Inhibitors: Design, Synthesis, in Vivo Anti-Inflammatory Activity, Docking and in Silico Chemo-Informatic Studies. Bioorganic Chemistry, 85, 541-557. https://doi.org/10.1016/j.bioorg.2019.02.036
- 5. Farooq, M., Sharma, A., Almarhoon, Z., Al-Dhfyan, A., El-Faham, A., Taha, N.A., Wadaan, M.A.M., Torre, B.G. and Albericio, F. (2019) Design and Synthesis of Mono-and Di-Pyrazolyl-s-Triazine Derivatives, Their Anticancer Profile in Human Cancer Cell Lines, and in Vivo Toxicity in Zebrafish Embryos. Bioorganic Chemistry, 87, 457-464. https://doi.org/10.1016/j.bioorg.2019.03.063
- 6. Florentino, I.F., Silva, D.P.B., Cardoso, C.S., Menegatti, R., de Carvalho, F.S., Liao, L.M., Pinto, P.M., Peigneur, S., Costa, E.A. and Tytgat, J. (2019) Antinociceptive Effects of New Pyrazoles Compounds Mediated by the ASIC-1α Channel, TRPV-1 and μMOR Receptors. Biomedicine & Pharma-cotherapy, 115, Article ID: 108915. https://doi.org/10.1016/j.biopha.2019.108915
- 7. Oliveira, D.H., Sousa, F.S.S., Birmann, P.T., Alves, D., Jacob, R.G., Savegnago, L. (2020) Antinociceptive and Anti-Inflammatory Effects of 4-(Arylchalcogenyl)-1H-Pyrazoles Con-taining Selenium or Sulfur. Pharmacological Reports, 72, 36-46. https://doi.org/10.1007/s43440-019-00001-4
- 8. Pratik, K., Arun, K., Neha, S., Bhumika, Y., Anshuman, S. and Kumar, G.S. (2018) Synthesis, Characterization of Ethyl 5-(Substituted)-1H-Pyrazole-3-Carboxylate Derivative as Potent Anti-inflammatory Agents. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 17, 32-38. https://doi.org/10.2174/1871523017666180411155240
- 9. Taher, A.T., Mostafa Sarg, M.T., El-Sayed Ali, N.R. and Hilmy Elnagdi, N. (2019) Design, Synthesis, Modeling Studies and Biological Screening of Novel Pyrazole Deriva-tives as Potential Analgesic and Anti-Inflammatory Agents. Bioorganic Chemistry, 89, Article ID: 103023. https://doi.org/10.1016/j.bioorg.2019.103023
- 10. Yao, H., Guo, Q., Wang, M., Wang, R. and Xu, Z. (2021) Discovery of Pyrazole N-Aryl Sulfonate: A Novel and Highly Potent Cyclooxygenase-2 (COX-2) Selective Inhibitors. Bioorganic & Medicinal Chemistry, 46, Article ID: 116344. https://doi.org/10.1016/j.bmc.2021.116344
- 11. Knorr, L. (1883) Einwirkung von acetessigester auf phenylhydra-zin. European Journal of Inorganic Chemistry, 16, 2597-2599. https://doi.org/10.1002/cber.188301602194
- 12. Girish, Y.R., Kumar, K.S.S., Manasa, H.S. and Shashikanth, S. (2014) ZnO: An Ecofriendly, Green Nano-Catalyst for the Synthesis of Pyrazole Derivatives under Aqueous Media. Journal of the Chinese Chemical Society, 61, 1175-1179. https://doi.org/10.1002/jccs.201400170
- 13. O’Shea, P., Gosselin, F., Webster, R., Reamer, R., Tillyer, R. and Grabowski, E. (2006) Highly Regioselective Synthesis of 1-Aryl-3,4,5-Substituted Pyrazoles. Synlett, 2006, 3267-3270. https://doi.org/10.1055/s-2006-956487
- 14. Bishop, B., Brands, K., Gibb, A. and Kennedy, D. (2003) Regioselec-tive Synthesis of 1,3,5-Substituted Pyrazoles from Acetylenic Ketones and Hydrazines. Synthesis, 2004, 43-52. https://doi.org/10.1055/s-2003-44376
- 15. Rao, V.K., Tiwari, R., Chhikara, B.S., Shirazi, A.N., Parang, K. and Kumar, A. (2013) Copper Triflate-Mediated Synthesis of 1,3,5-Triarylpyrazoles in [Bmim][PF6] Ionic Liquid and Evalu-ation of Their Anticancer Activities. RSC Advances, 3, 15396-15403. https://doi.org/10.1039/c3ra41830h
- 16. Kat-ritzky, A.R., Wang, M., Zhang, S., Voronkov, M.V. and Steel, P.J. (2001) Regioselective Synthesis of Polysubstituted Pyrazoles and Isoxazoles. The Journal of Organic Chemistry, 66, 6787-6791. https://doi.org/10.1021/jo0101407
- 17. He, S., Chen, L., Niu, Y.N., Wu, L.Y. and Liang, Y.M. (2009) 1,3-Dipolar Cycloaddition of Diazoacetate Compounds to Terminal Alkynes Promoted by Zn(OTf)2: An Efficient Way to the Preparation of Pyrazoles. Tetrahedron Letters, 50, 2443-2445. https://doi.org/10.1016/j.tetlet.2009.03.030
- 18. Gioiello, A., Khamidullina, A., Fulco, M.C., Venturoni, F., Zlot-sky, S. and Pellicciari, R. (2009) New One-Pot Synthesis of Pyrazole-5-Carboxylates by 1,3-Dipole Cycloadditions of Ethyl Diazoacetate with α-Methylene Carbonyl Compounds. Tetrahedron Letters, 50, 5978-5980. https://doi.org/10.1016/j.tetlet.2009.07.152
- 19. Delaunay, T., Genix, P., Es-Sayed, M., Vors, J.P., Monteiro, N. and Balme, G. (2010) A Modular Sydnone Cycloaddition/Suzuki-Miyaura Cross-Coupling Strategy to Unsymmetrical 3,5-Bis (Hetero) Aromatic Pyrazoles. Organic Letters, 12, 3328-3331. https://doi.org/10.1021/ol101087j
- 20. Oh, L.M. (2006) Synthesis of Celecoxib via 1,3-Dipolar Cycloaddition. Tetrahedron Letters, 47, 7943-7946. https://doi.org/10.1016/j.tetlet.2006.08.138
- 21. Yadav, J.S., Reddy, B.V.S., Srinivas, M., Prabhakar, A. and Jagadeesh, B. (2004) Montmorillonite KSF Clay-Promoted Synthesis of Enantiomerically Pure 5-Substituted Pyrazoles from 2,3-Dihydro-4H-Pyran-4-Ones. Tetrahedron Letters, 45, 6033-6036. https://doi.org/10.1016/j.tetlet.2004.06.031
- 22. Xie, F., Cheng, G. and Hu, Y. (2006) Three-Component, One-Pot Reaction for the Combinatorial Synthesis of 1,3,4-Substituted Pyrazoles. Journal of Combinatorial Chemistry, 8, 286-288. https://doi.org/10.1021/cc050159d
- 23. Ilhan, I.Ö., Saripinar, E. and Akçamur, Y. (2005) Synthesis of Some Pyrazole-3-Carboxylic Acid Hydrazide and Pyrazolopyridazine Compounds. Journal of Heterocyclic Chemistry, 42, 117-120. https://doi.org/10.1002/jhet.5570420117
- 24. Krishnaiah, A. and Narsaiah, B. (2002) A Novel Ap-proach to the Synthesis of 5 Trifluoromethyl-3-Substituted Pyrazoles. Journal of Fluorine Chemistry, 115, 9-11. https://doi.org/10.1016/S0022-1139(01)00501-2
- 25. Liu, J.J., Zhang, H., Sun, J., Wang, Z.C., Yang, Y.S., Li, D.D., Zhang, F., Gong, H.B. and Zhu, H.L. (2012) Synthesis, Biological Evaluation of Novel 4,5-Dihydro-2H-Pyrazole 2-Hydroxyphenyl Derivatives as BRAF Inhibitors. Bioorganic & Medicinal Chemistry, 20, 6089-6096. https://doi.org/10.1016/j.bmc.2012.08.020
- 26. Sun, J., Lv, X.H., Qiu, H.Y., Wang, Y.T., Du, Q.R., Li, D.D., Yang, Y.H. and Zhu, H.L. (2013) Synthesis, Biological Evaluation and Molecular Docking Studies of Pyrazole Deriva-tives Coupling with a Thiourea Moiety as Novel CDKs Inhibitors. European Journal of Medicinal Chemistry, 68, 1-9. https://doi.org/10.1016/j.ejmech.2013.07.003
- 27. Xu, Y., Liu, X.H., Saunders, M., Pearce, S., Foulks, J.M., Par-nell, K.M., Clifford, A., Nix, R.N., Bullough, J., Hendrickson, T.F., Wright, K., McCullar, M.V., Kanner, S.B. and Ho, K.K. (2014) Discovery of 3-(Trifluoromethyl)- 1H-Pyrazole-5-Carboxamide Activators of the M2 Isoform of Pyruvate Kinase (PKM2). Bioorganic & Medicinal Chemistry, 24, 515-519. https://doi.org/10.1016/j.bmcl.2013.12.028
- 28. Li, X., Lu, X., Xing, M., Yang, X.H., Zhao, T.T., Gong, H.B. and Zhu, H.L. (2012) Synthesis, Biological Evaluation, and Molecular Docking Studies of N,1,3-Triphenyl-1H-Pyrazole-4-Carboxamide Derivatives as Anticancer Agents. Bioorganic & Medicinal Chemistry, 22, 3589-3593. https://doi.org/10.1016/j.bmcl.2012.04.066
- 29. Huang, Y.Y., Wang, L.Y., Chang, C.H., Kuo, Y.H., Kaneko, K., Takayama, H., Kimura, M., Juang, S.H. and Wong, F.F. (2012) One-Pot Synthesis and Antiproliferative Evaluation of Pyrazolo[3,4-d]Pyrimidine Derivatives. Tetrahedron, 68, 9658-9664. https://doi.org/10.1016/j.tet.2012.09.054
- 30. Abdellatif, K.R.A. and Fadaly, W.A. (2017) Design, Synthesis, Cyclooxygenase Inhibition and Biological Evaluation of New 1,3,5-Triaryl-4,5-Dihydro-1H-Pyrazole Derivatives Possessing Amino/Methanesulfonyl Pharmacophore. Bioor-ganic Chemistry, 70, 57-66. https://doi.org/10.1016/j.bioorg.2016.11.008
- 31. Abdellatif, K.R.A., Abdelall, E.K.A., Lamie, P.F., Labib, M.B., El-Nahaas, E.S. and Abdelhakeem, M.M. (2020) New Pyrazole Derivatives Pos-sessing Amino/Methanesulphonyl Pharmacophore with Good Gastric Safety Profile: Design, Synthesis, Cyclooxygenase Inhibition, Anti-Inflammatory Activity and Histopathological Studies. Bioorganic Chemistry, 95, Article ID: 103540. https://doi.org/10.1016/j.bioorg.2019.103540
- 32. Abdel-Maksoud, M.S., El-Gamal, M.I., Gamal El-Din, M.M., Choi, Y., Choi, J., Shin, J.S., Kang, S.Y., Yoo, K.H., Lee, K.T., Baek, D. and Oh, C.H. (2018) Synthesis of New Tri-arylpyrazole Derivatives Possessing Terminal Sulfonamide Moiety and Their Inhibitory Effects on PGE2 and Nitric Ox-ide Productions in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Molecules, 23, Article No. 2556. https://doi.org/10.3390/molecules23102556
- 33. El-Shoukrofy, M.S., Abd El Razik, H.A., AboulWafa, O.M., Bayad, A.E. and El-Ashmawy, I.M. (2019) Pyrazoles Containing Thiophene, Thienopyrimidine and Thienotriazolopy-rimidine as COX-2 Selective Inhibitors: Design, Synthesis, in Vivo Anti-Inflammatory Activity, Docking and in Silico Chemo-Informatic Studies. Bioorganic Chemistry, 85, 541-557. https://doi.org/10.1016/j.bioorg.2019.02.036
- 34. Farooq, M., Sharma, A., Almarhoon, Z., Al-Dhfyan, A., El-Faham, A., Taha, N.A., Wadaan, M.A.M., Torre, B.G. and Albericio, F. (2019) Design and Synthesis of Mono-and Di-Pyrazolyl-s-Triazine Derivatives, Their Anticancer Profile in Human Cancer Cell Lines, and in Vivo Toxicity in Zebrafish Embryos. Bioorganic Chemistry, 87, 457-464. https://doi.org/10.1016/j.bioorg.2019.03.063
- 35. Florentino, I.F., Silva, D.P.B., Cardoso, C.S., Menegatti, R., de Carvalho, F.S., Liao, L.M., Pinto, P.M., Peigneur, S., Costa, E.A. and Tytgat, J. (2019) Antinociceptive Effects of New Pyrazoles Compounds Mediated by the ASIC-1α Channel, TRPV-1 and μMOR Receptors. Biomedicine & Pharma-cotherapy, 115, Article ID: 108915. https://doi.org/10.1016/j.biopha.2019.108915
- 36. Oliveira, D.H., Sousa, F.S.S., Birmann, P.T., Alves, D., Jacob, R.G., Savegnago, L. (2020) Antinociceptive and Anti-Inflammatory Effects of 4-(Arylchalcogenyl)-1H-Pyrazoles Con-taining Selenium or Sulfur. Pharmacological Reports, 72, 36-46. https://doi.org/10.1007/s43440-019-00001-4
- 37. Pratik, K., Arun, K., Neha, S., Bhumika, Y., Anshuman, S. and Kumar, G.S. (2018) Synthesis, Characterization of Ethyl 5-(Substituted)-1H-Pyrazole-3-Carboxylate Derivative as Potent Anti-inflammatory Agents. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 17, 32-38. https://doi.org/10.2174/1871523017666180411155240
- 38. Taher, A.T., Mostafa Sarg, M.T., El-Sayed Ali, N.R. and Hilmy Elnagdi, N. (2019) Design, Synthesis, Modeling Studies and Biological Screening of Novel Pyrazole Deriva-tives as Potential Analgesic and Anti-Inflammatory Agents. Bioorganic Chemistry, 89, Article ID: 103023. https://doi.org/10.1016/j.bioorg.2019.103023
- 39. Yao, H., Guo, Q., Wang, M., Wang, R. and Xu, Z. (2021) Discovery of Pyrazole N-Aryl Sulfonate: A Novel and Highly Potent Cyclooxygenase-2 (COX-2) Selective Inhibitors. Bioorganic & Medicinal Chemistry, 46, Article ID: 116344. https://doi.org/10.1016/j.bmc.2021.116344
- 40. Knorr, L. (1883) Einwirkung von acetessigester auf phenylhydra-zin. European Journal of Inorganic Chemistry, 16, 2597-2599. https://doi.org/10.1002/cber.188301602194
- 41. Girish, Y.R., Kumar, K.S.S., Manasa, H.S. and Shashikanth, S. (2014) ZnO: An Ecofriendly, Green Nano-Catalyst for the Synthesis of Pyrazole Derivatives under Aqueous Media. Journal of the Chinese Chemical Society, 61, 1175-1179. https://doi.org/10.1002/jccs.201400170
- 42. O’Shea, P., Gosselin, F., Webster, R., Reamer, R., Tillyer, R. and Grabowski, E. (2006) Highly Regioselective Synthesis of 1-Aryl-3,4,5-Substituted Pyrazoles. Synlett, 2006, 3267-3270. https://doi.org/10.1055/s-2006-956487
- 43. Bishop, B., Brands, K., Gibb, A. and Kennedy, D. (2003) Regioselec-tive Synthesis of 1,3,5-Substituted Pyrazoles from Acetylenic Ketones and Hydrazines. Synthesis, 2004, 43-52. https://doi.org/10.1055/s-2003-44376
- 44. Rao, V.K., Tiwari, R., Chhikara, B.S., Shirazi, A.N., Parang, K. and Kumar, A. (2013) Copper Triflate-Mediated Synthesis of 1,3,5-Triarylpyrazoles in [Bmim][PF6] Ionic Liquid and Evalu-ation of Their Anticancer Activities. RSC Advances, 3, 15396-15403. https://doi.org/10.1039/c3ra41830h
- 45. Kat-ritzky, A.R., Wang, M., Zhang, S., Voronkov, M.V. and Steel, P.J. (2001) Regioselective Synthesis of Polysubstituted Pyrazoles and Isoxazoles. The Journal of Organic Chemistry, 66, 6787-6791. https://doi.org/10.1021/jo0101407
- 46. He, S., Chen, L., Niu, Y.N., Wu, L.Y. and Liang, Y.M. (2009) 1,3-Dipolar Cycloaddition of Diazoacetate Compounds to Terminal Alkynes Promoted by Zn(OTf)2: An Efficient Way to the Preparation of Pyrazoles. Tetrahedron Letters, 50, 2443-2445. https://doi.org/10.1016/j.tetlet.2009.03.030
- 47. Gioiello, A., Khamidullina, A., Fulco, M.C., Venturoni, F., Zlot-sky, S. and Pellicciari, R. (2009) New One-Pot Synthesis of Pyrazole-5-Carboxylates by 1,3-Dipole Cycloadditions of Ethyl Diazoacetate with α-Methylene Carbonyl Compounds. Tetrahedron Letters, 50, 5978-5980. https://doi.org/10.1016/j.tetlet.2009.07.152
- 48. Delaunay, T., Genix, P., Es-Sayed, M., Vors, J.P., Monteiro, N. and Balme, G. (2010) A Modular Sydnone Cycloaddition/Suzuki-Miyaura Cross-Coupling Strategy to Unsymmetrical 3,5-Bis (Hetero) Aromatic Pyrazoles. Organic Letters, 12, 3328-3331. https://doi.org/10.1021/ol101087j
- 49. Oh, L.M. (2006) Synthesis of Celecoxib via 1,3-Dipolar Cycloaddition. Tetrahedron Letters, 47, 7943-7946. https://doi.org/10.1016/j.tetlet.2006.08.138
- 50. Yadav, J.S., Reddy, B.V.S., Srinivas, M., Prabhakar, A. and Jagadeesh, B. (2004) Montmorillonite KSF Clay-Promoted Synthesis of Enantiomerically Pure 5-Substituted Pyrazoles from 2,3-Dihydro-4H-Pyran-4-Ones. Tetrahedron Letters, 45, 6033-6036. https://doi.org/10.1016/j.tetlet.2004.06.031
- 51. Xie, F., Cheng, G. and Hu, Y. (2006) Three-Component, One-Pot Reaction for the Combinatorial Synthesis of 1,3,4-Substituted Pyrazoles. Journal of Combinatorial Chemistry, 8, 286-288. https://doi.org/10.1021/cc050159d
- 52. Ilhan, I.Ö., Saripinar, E. and Akçamur, Y. (2005) Synthesis of Some Pyrazole-3-Carboxylic Acid Hydrazide and Pyrazolopyridazine Compounds. Journal of Heterocyclic Chemistry, 42, 117-120. https://doi.org/10.1002/jhet.5570420117
- 53. Krishnaiah, A. and Narsaiah, B. (2002) A Novel Ap-proach to the Synthesis of 5 Trifluoromethyl-3-Substituted Pyrazoles. Journal of Fluorine Chemistry, 115, 9-11. https://doi.org/10.1016/S0022-1139(01)00501-2
- 54. Liu, J.J., Zhang, H., Sun, J., Wang, Z.C., Yang, Y.S., Li, D.D., Zhang, F., Gong, H.B. and Zhu, H.L. (2012) Synthesis, Biological Evaluation of Novel 4,5-Dihydro-2H-Pyrazole 2-Hydroxyphenyl Derivatives as BRAF Inhibitors. Bioorganic & Medicinal Chemistry, 20, 6089-6096. https://doi.org/10.1016/j.bmc.2012.08.020
- 55. Sun, J., Lv, X.H., Qiu, H.Y., Wang, Y.T., Du, Q.R., Li, D.D., Yang, Y.H. and Zhu, H.L. (2013) Synthesis, Biological Evaluation and Molecular Docking Studies of Pyrazole Deriva-tives Coupling with a Thiourea Moiety as Novel CDKs Inhibitors. European Journal of Medicinal Chemistry, 68, 1-9. https://doi.org/10.1016/j.ejmech.2013.07.003
- 56. Xu, Y., Liu, X.H., Saunders, M., Pearce, S., Foulks, J.M., Par-nell, K.M., Clifford, A., Nix, R.N., Bullough, J., Hendrickson, T.F., Wright, K., McCullar, M.V., Kanner, S.B. and Ho, K.K. (2014) Discovery of 3-(Trifluoromethyl)- 1H-Pyrazole-5-Carboxamide Activators of the M2 Isoform of Pyruvate Kinase (PKM2). Bioorganic & Medicinal Chemistry, 24, 515-519. https://doi.org/10.1016/j.bmcl.2013.12.028
- 57. Li, X., Lu, X., Xing, M., Yang, X.H., Zhao, T.T., Gong, H.B. and Zhu, H.L. (2012) Synthesis, Biological Evaluation, and Molecular Docking Studies of N,1,3-Triphenyl-1H-Pyrazole-4-Carboxamide Derivatives as Anticancer Agents. Bioorganic & Medicinal Chemistry, 22, 3589-3593. https://doi.org/10.1016/j.bmcl.2012.04.066
- 58. Huang, Y.Y., Wang, L.Y., Chang, C.H., Kuo, Y.H., Kaneko, K., Takayama, H., Kimura, M., Juang, S.H. and Wong, F.F. (2012) One-Pot Synthesis and Antiproliferative Evaluation of Pyrazolo[3,4-d]Pyrimidine Derivatives. Tetrahedron, 68, 9658-9664. https://doi.org/10.1016/j.tet.2012.09.054
- 59. Abdellatif, K.R.A. and Fadaly, W.A. (2017) Design, Synthesis, Cyclooxygenase Inhibition and Biological Evaluation of New 1,3,5-Triaryl-4,5-Dihydro-1H-Pyrazole Derivatives Possessing Amino/Methanesulfonyl Pharmacophore. Bioor-ganic Chemistry, 70, 57-66. https://doi.org/10.1016/j.bioorg.2016.11.008
- 60. Abdellatif, K.R.A., Abdelall, E.K.A., Lamie, P.F., Labib, M.B., El-Nahaas, E.S. and Abdelhakeem, M.M. (2020) New Pyrazole Derivatives Pos-sessing Amino/Methanesulphonyl Pharmacophore with Good Gastric Safety Profile: Design, Synthesis, Cyclooxygenase Inhibition, Anti-Inflammatory Activity and Histopathological Studies. Bioorganic Chemistry, 95, Article ID: 103540. https://doi.org/10.1016/j.bioorg.2019.103540
- 61. Abdel-Maksoud, M.S., El-Gamal, M.I., Gamal El-Din, M.M., Choi, Y., Choi, J., Shin, J.S., Kang, S.Y., Yoo, K.H., Lee, K.T., Baek, D. and Oh, C.H. (2018) Synthesis of New Tri-arylpyrazole Derivatives Possessing Terminal Sulfonamide Moiety and Their Inhibitory Effects on PGE2 and Nitric Ox-ide Productions in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Molecules, 23, Article No. 2556. https://doi.org/10.3390/molecules23102556
- 62. El-Shoukrofy, M.S., Abd El Razik, H.A., AboulWafa, O.M., Bayad, A.E. and El-Ashmawy, I.M. (2019) Pyrazoles Containing Thiophene, Thienopyrimidine and Thienotriazolopy-rimidine as COX-2 Selective Inhibitors: Design, Synthesis, in Vivo Anti-Inflammatory Activity, Docking and in Silico Chemo-Informatic Studies. Bioorganic Chemistry, 85, 541-557. https://doi.org/10.1016/j.bioorg.2019.02.036
- 63. Farooq, M., Sharma, A., Almarhoon, Z., Al-Dhfyan, A., El-Faham, A., Taha, N.A., Wadaan, M.A.M., Torre, B.G. and Albericio, F. (2019) Design and Synthesis of Mono-and Di-Pyrazolyl-s-Triazine Derivatives, Their Anticancer Profile in Human Cancer Cell Lines, and in Vivo Toxicity in Zebrafish Embryos. Bioorganic Chemistry, 87, 457-464. https://doi.org/10.1016/j.bioorg.2019.03.063
- 64. Florentino, I.F., Silva, D.P.B., Cardoso, C.S., Menegatti, R., de Carvalho, F.S., Liao, L.M., Pinto, P.M., Peigneur, S., Costa, E.A. and Tytgat, J. (2019) Antinociceptive Effects of New Pyrazoles Compounds Mediated by the ASIC-1α Channel, TRPV-1 and μMOR Receptors. Biomedicine & Pharma-cotherapy, 115, Article ID: 108915. https://doi.org/10.1016/j.biopha.2019.108915
- 65. Oliveira, D.H., Sousa, F.S.S., Birmann, P.T., Alves, D., Jacob, R.G., Savegnago, L. (2020) Antinociceptive and Anti-Inflammatory Effects of 4-(Arylchalcogenyl)-1H-Pyrazoles Con-taining Selenium or Sulfur. Pharmacological Reports, 72, 36-46. https://doi.org/10.1007/s43440-019-00001-4
- 66. Pratik, K., Arun, K., Neha, S., Bhumika, Y., Anshuman, S. and Kumar, G.S. (2018) Synthesis, Characterization of Ethyl 5-(Substituted)-1H-Pyrazole-3-Carboxylate Derivative as Potent Anti-inflammatory Agents. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 17, 32-38. https://doi.org/10.2174/1871523017666180411155240
- 67. Taher, A.T., Mostafa Sarg, M.T., El-Sayed Ali, N.R. and Hilmy Elnagdi, N. (2019) Design, Synthesis, Modeling Studies and Biological Screening of Novel Pyrazole Deriva-tives as Potential Analgesic and Anti-Inflammatory Agents. Bioorganic Chemistry, 89, Article ID: 103023. https://doi.org/10.1016/j.bioorg.2019.103023
- 68. Yao, H., Guo, Q., Wang, M., Wang, R. and Xu, Z. (2021) Discovery of Pyrazole N-Aryl Sulfonate: A Novel and Highly Potent Cyclooxygenase-2 (COX-2) Selective Inhibitors. Bioorganic & Medicinal Chemistry, 46, Article ID: 116344. https://doi.org/10.1016/j.bmc.2021.116344
- 69. Knorr, L. (1883) Einwirkung von acetessigester auf phenylhydra-zin. European Journal of Inorganic Chemistry, 16, 2597-2599. https://doi.org/10.1002/cber.188301602194
- 70. Girish, Y.R., Kumar, K.S.S., Manasa, H.S. and Shashikanth, S. (2014) ZnO: An Ecofriendly, Green Nano-Catalyst for the Synthesis of Pyrazole Derivatives under Aqueous Media. Journal of the Chinese Chemical Society, 61, 1175-1179. https://doi.org/10.1002/jccs.201400170
- 71. O’Shea, P., Gosselin, F., Webster, R., Reamer, R., Tillyer, R. and Grabowski, E. (2006) Highly Regioselective Synthesis of 1-Aryl-3,4,5-Substituted Pyrazoles. Synlett, 2006, 3267-3270. https://doi.org/10.1055/s-2006-956487
- 72. Bishop, B., Brands, K., Gibb, A. and Kennedy, D. (2003) Regioselec-tive Synthesis of 1,3,5-Substituted Pyrazoles from Acetylenic Ketones and Hydrazines. Synthesis, 2004, 43-52. https://doi.org/10.1055/s-2003-44376
- 73. Rao, V.K., Tiwari, R., Chhikara, B.S., Shirazi, A.N., Parang, K. and Kumar, A. (2013) Copper Triflate-Mediated Synthesis of 1,3,5-Triarylpyrazoles in [Bmim][PF6] Ionic Liquid and Evalu-ation of Their Anticancer Activities. RSC Advances, 3, 15396-15403. https://doi.org/10.1039/c3ra41830h
- 74. Kat-ritzky, A.R., Wang, M., Zhang, S., Voronkov, M.V. and Steel, P.J. (2001) Regioselective Synthesis of Polysubstituted Pyrazoles and Isoxazoles. The Journal of Organic Chemistry, 66, 6787-6791. https://doi.org/10.1021/jo0101407
- 75. He, S., Chen, L., Niu, Y.N., Wu, L.Y. and Liang, Y.M. (2009) 1,3-Dipolar Cycloaddition of Diazoacetate Compounds to Terminal Alkynes Promoted by Zn(OTf)2: An Efficient Way to the Preparation of Pyrazoles. Tetrahedron Letters, 50, 2443-2445. https://doi.org/10.1016/j.tetlet.2009.03.030
- 76. Gioiello, A., Khamidullina, A., Fulco, M.C., Venturoni, F., Zlot-sky, S. and Pellicciari, R. (2009) New One-Pot Synthesis of Pyrazole-5-Carboxylates by 1,3-Dipole Cycloadditions of Ethyl Diazoacetate with α-Methylene Carbonyl Compounds. Tetrahedron Letters, 50, 5978-5980. https://doi.org/10.1016/j.tetlet.2009.07.152
- 77. Delaunay, T., Genix, P., Es-Sayed, M., Vors, J.P., Monteiro, N. and Balme, G. (2010) A Modular Sydnone Cycloaddition/Suzuki-Miyaura Cross-Coupling Strategy to Unsymmetrical 3,5-Bis (Hetero) Aromatic Pyrazoles. Organic Letters, 12, 3328-3331. https://doi.org/10.1021/ol101087j
- 78. Oh, L.M. (2006) Synthesis of Celecoxib via 1,3-Dipolar Cycloaddition. Tetrahedron Letters, 47, 7943-7946. https://doi.org/10.1016/j.tetlet.2006.08.138
- 79. Yadav, J.S., Reddy, B.V.S., Srinivas, M., Prabhakar, A. and Jagadeesh, B. (2004) Montmorillonite KSF Clay-Promoted Synthesis of Enantiomerically Pure 5-Substituted Pyrazoles from 2,3-Dihydro-4H-Pyran-4-Ones. Tetrahedron Letters, 45, 6033-6036. https://doi.org/10.1016/j.tetlet.2004.06.031
- 80. Xie, F., Cheng, G. and Hu, Y. (2006) Three-Component, One-Pot Reaction for the Combinatorial Synthesis of 1,3,4-Substituted Pyrazoles. Journal of Combinatorial Chemistry, 8, 286-288. https://doi.org/10.1021/cc050159d
- 81. Ilhan, I.Ö., Saripinar, E. and Akçamur, Y. (2005) Synthesis of Some Pyrazole-3-Carboxylic Acid Hydrazide and Pyrazolopyridazine Compounds. Journal of Heterocyclic Chemistry, 42, 117-120. https://doi.org/10.1002/jhet.5570420117
- 82. Krishnaiah, A. and Narsaiah, B. (2002) A Novel Ap-proach to the Synthesis of 5 Trifluoromethyl-3-Substituted Pyrazoles. Journal of Fluorine Chemistry, 115, 9-11. https://doi.org/10.1016/S0022-1139(01)00501-2
- 83. Liu, J.J., Zhang, H., Sun, J., Wang, Z.C., Yang, Y.S., Li, D.D., Zhang, F., Gong, H.B. and Zhu, H.L. (2012) Synthesis, Biological Evaluation of Novel 4,5-Dihydro-2H-Pyrazole 2-Hydroxyphenyl Derivatives as BRAF Inhibitors. Bioorganic & Medicinal Chemistry, 20, 6089-6096. https://doi.org/10.1016/j.bmc.2012.08.020
- 84. Sun, J., Lv, X.H., Qiu, H.Y., Wang, Y.T., Du, Q.R., Li, D.D., Yang, Y.H. and Zhu, H.L. (2013) Synthesis, Biological Evaluation and Molecular Docking Studies of Pyrazole Deriva-tives Coupling with a Thiourea Moiety as Novel CDKs Inhibitors. European Journal of Medicinal Chemistry, 68, 1-9. https://doi.org/10.1016/j.ejmech.2013.07.003
- 85. Xu, Y., Liu, X.H., Saunders, M., Pearce, S., Foulks, J.M., Par-nell, K.M., Clifford, A., Nix, R.N., Bullough, J., Hendrickson, T.F., Wright, K., McCullar, M.V., Kanner, S.B. and Ho, K.K. (2014) Discovery of 3-(Trifluoromethyl)- 1H-Pyrazole-5-Carboxamide Activators of the M2 Isoform of Pyruvate Kinase (PKM2). Bioorganic & Medicinal Chemistry, 24, 515-519. https://doi.org/10.1016/j.bmcl.2013.12.028
- 86. Li, X., Lu, X., Xing, M., Yang, X.H., Zhao, T.T., Gong, H.B. and Zhu, H.L. (2012) Synthesis, Biological Evaluation, and Molecular Docking Studies of N,1,3-Triphenyl-1H-Pyrazole-4-Carboxamide Derivatives as Anticancer Agents. Bioorganic & Medicinal Chemistry, 22, 3589-3593. https://doi.org/10.1016/j.bmcl.2012.04.066
- 87. Huang, Y.Y., Wang, L.Y., Chang, C.H., Kuo, Y.H., Kaneko, K., Takayama, H., Kimura, M., Juang, S.H. and Wong, F.F. (2012) One-Pot Synthesis and Antiproliferative Evaluation of Pyrazolo[3,4-d]Pyrimidine Derivatives. Tetrahedron, 68, 9658-9664. https://doi.org/10.1016/j.tet.2012.09.054
NOTES
*通讯作者。