Advances in Clinical Medicine
Vol.
10
No.
06
(
2020
), Article ID:
36215
,
6
pages
10.12677/ACM.2020.106166
Research Perspectives of NLRP3 in the Pathogenesis of Cardiovascular Diseases
Meng Zheng1, Di Lu1, Limei Li1, Qing Li1, Ligong Bian1, Hongcai Yang2, Xi Huang1, Jiaoyun Wang1, Jiazhi Guo1*
1Biomedical Engineering Research Center, Kunming Medical University, Kunming Yunnan
2The First Affiliated Hospital of Kunming Medical University, Kunming Yunnan

Received: May 25th, 2020; accepted: Jun. 15th, 2020; published: Jun. 22nd, 2020

ABSTRACT
NLRP3 inflammasome is a key multiprotein signaling platform that tightly controls inflammatory responses. NLRP3 has been identified to play a central role in the pathological progression of certain cardiovascular diseases, such as hypertension, vascular damage span ning atherosclerosis and other myocardial infarction. The study of the NLRP3 inflammasome in these cardiovascular disease states may lead to new treatment strategies. This review outlines current insights into NLRP3 inflammasome research associated with cardiovascular diseases and discusses the questions that remain in this field.
Keywords:NLRP3, Inflammation, IL-1β, Caspase-1, Cardiovascular Diseases

NLRP3在心血管疾病中的研究进展
郑梦1,陆地1,李丽梅1,李庆1,边立功1,杨洪财2,黄曦1,王娇云1,郭家智1*
1昆明医科大学生物医学工程研究中心,云南 昆明
2昆明医科大学第一附属医院,云南 昆明

收稿日期:2020年5月25日;录用日期:2020年6月15日;发布日期:2020年6月22日

摘 要
NLRP3炎症小体是一种与炎症反应息息相关的大分子多蛋白复合物。NLRP3炎症小体的组装和激活与高血压、动脉粥样硬化、心肌梗死等多种心血管疾病病理过程相关。研究NLRP3炎症小体在心血管相关疾病中的作用,可以为心血管疾病提供新的治疗方案。本文主要对NLRP3炎症小体在心血管疾病上的研究进展做一简要概述。
关键词 :NLRP3,炎症小体,IL-1β,Caspase-1,心血管疾病

Copyright © 2020 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
炎症小体是一种多蛋白复合物,通过激活Caspase-1和释放促炎性因子IL-1,对病原相关分子模式(PAMPs)和危险/损伤分子相关模式(DAMPs),氧化应激(ROS),胆固醇结晶和环境刺激做出响应。到目前为止,已经发现了多种类型的炎症小体。但NLRP3炎症小体的研究最为广泛。NLRP3能够识别非微生物引起的危险信号,也可在各种疾病条件下导致无菌的炎症反应发生。最近的研究表明,NLRP3炎症小体的激活与心血管疾病的病理过程有紧密联系 [1] [2] [3]。
2. NLRP3的结构与激活
NLRP3炎症小体是一种先天性免疫受体,通常由三部分组成:1) C端亮氨酸重复序列(LRRs),N端的热蛋白样结构域(PYD)和中间的NACHT结构域;2) 包含PYD和C端的Caspase募集结构域(CARD)的凋亡相关斑点样蛋白(ASC);3) 由CARD和Caspase域组成的半胱氨酸蛋白酶前体 [4],具体结构见图1。NLRP3
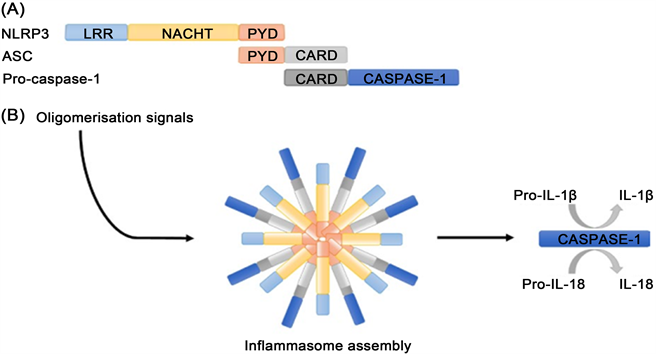
Figure 1. The structure of NLRP3 inflammasome [9]. (A) NLRP3 Inflammasome components; (B) Activation of oligomer NLRP3 inflammasome promotes Caspase-1 activation and IL-1 maturation
图1. NLRP3炎性小体的结构 [9]。(A) NLRP3炎性小体组成成分;(B) 低聚体NLRP3炎症小体的激活促进Caspase-1的活化和IL-1β的成熟的过程
与ASC相互作用启动炎症小体的形成;ASC通过招募和激活Caspase前体来生成具有活性的Caspase-1。Caspase-1是IL-1β的转化酶,将IL-1β前体剪切成具有活性的IL-1β [5]。NLRP3炎症小体的激活需要两个过程:激活过程和触发过程 [6]。NLRP3的激活过程是指TLRs或细胞因子(肿瘤坏死因子TNF-α)识别PAMPs和DAMPs或环境刺激引起核因子κB (NF-κB)的激活,进一步引起NLRP3、IL-1β前体、IL-18前体以及转录蛋白修饰(如NLRP3去泛素化和ASC磷酸化)的表达和激活 [7]。NLRP3的触发过程主要以NLRP3、ASC和Caspase-1前体的寡聚化为特征,诱导Caspase-1的活化和IL-1β的成熟,最终导致炎症小体的形成 [8]。
3. NLRP3炎症小体与疾病
3.1. NLRP3炎症小体与高血压
高血压是一种由多基因遗传和环境因素引起的复杂疾病。炎症小体的活性在高血压的病理发生和发展中起着至关重要的作用。NF-κB是NLRP3的一种有效激活剂,它可以诱发高血压的炎症反应 [10];抑制NF-κB的活性后,NLRP3和Caspase-1的活性下降,促炎因子和氧化应激的表达降低,从而抑制高血压的发生 [11]。Krishnan等人 [12] 观察到高血压患者的血清中IL-1β的含量升高,说明高血压的病理过程与炎症发生存在一定的联系。IL-1β是一种由NLRP3炎症小体激活后调控的多效促炎因子。IL-1β抑制肾素–血管紧张素系统的激活,降低室旁核中ROS的产生,从而减弱高血压诱导的心血管损害 [13]。血管紧张素II (Angiotensin II, Ang II)在调节高血压相关的炎症过程中起着关键作用 [14]。Ang II是肾素–血管紧张素系统激活后产生的一种血管收缩肽,通过激活1型和2型受体发挥生理效应 [15]。小鼠体内注射Ang II 7天后,NLRP3激活型炎症小体增多、IL-1β以及其它促炎细胞因子的表达增加 [16]。在不影响血压的情况下,阻断NLRP3炎症小体的激活可以显著性减弱Ang II诱导的心肌纤维化,这说明NLRP3炎症小体可以引起心脏重构 [16]。因此,NLRP3炎症小体/IL-1β可能成为高血压性心脏病的一种新型的治疗靶点。未来进一步的研究应该着重于对NLRP3蛋白家族的组装和激活,以更具体地阐明其在高血压进展过程中的功能作用。
3.2. NLRP3炎症小体与动脉粥样硬化
动脉粥样硬化的特征是动脉壁脂质沉积和炎性细胞膨胀。这种慢性病理状态是许多心血管疾病发生的根本原因 [17]。动脉粥样硬化斑块被认为是一种与先天免疫系统有关的炎症性病变,慢性炎症在其发生进展中起着重要作用 [18]。研究报道,调节IL-1β前体释放的途径与动脉粥样硬化病变的进展密切相关 [19]。Zheng等人 [20] 报道NLRP3沉默后,动脉粥样硬化斑块将更稳定,说明NLRP3炎症小体参与动脉粥样硬化的发生。Duewell等人 [21] 对低密度脂蛋白受体(LDLR-)或骨髓中NLRP3−/−,ASC−/−,IL-1α/β−/−缺乏的野生型小鼠进行8周的高脂肪饮食后,发现NLRP3−/−,ASC−/−和IL-1α/β−/−的骨髓组织中IL-18表达下降,动脉粥样硬化病变进程减慢。高脂饮食喂养LDLR缺乏和骨髓组织中Caspase-1/11缺乏的小鼠,动脉粥样硬化斑块明显减少 [22],证实了胆固醇结晶可以激活NLRP3炎症小体,说明NLRP3炎性小体在动脉粥样硬化中发挥重要作用。Abderrazak等人 [23] 发现在高脂肪喂养的载脂蛋白E2Ki小鼠上,NLRP3炎症小体抑制剂能够抑制胆固醇结晶诱导的NLRP3炎症小体的激活,动脉粥样硬化病变明显减少。以上均说明NLRP3炎症小体调节的信号通路可以调控动脉粥样硬化中胆固醇结晶诱导的炎症反应。然而,随着研究的不断深入,Menu等人报道了相反的结果 [24]。他们对Apoe−/−小鼠进行高脂饮食喂养11周后,发现NLRP3−/−、ASC−/−或Caspase-1−/−小鼠,细胞浸润、斑块稳定性以及动脉粥样硬化均无显著变化,造成这种差异的机制尚未确定。Baldrighi等人 [24] 认为这可能与Apoe−/−小鼠高脂饮食持续时间有关。也有研究报道,这些差异可能是由于使用了不同的小鼠模型。因此,在Apoe−/−小鼠模型中,IL-1在动脉粥样硬化发展中的作用尚未完全确定。
ROS在动脉粥样硬化斑块进展的关系中发挥着重要作用,ROS的过度生成会诱导凝集素样氧化低密度脂蛋白受体-1 (LOX-1)的表达。LOX-1的活化反过来诱导ROS的释放,形成正反馈回路,在动脉粥样硬化的发生发展中发挥重要作用 [25] [26]。研究报道,用脂多糖刺激人THP-1巨噬细胞,可显著诱导LOX-1和ROS的表达,并启动线粒体DNA (mtDNA)损伤,进而造成受损的mtDNA积累,然后引起NLRP3炎症小体激活,造成细胞自噬发生。ROS抑制剂和自噬诱导因子均能降低NLRP3蛋白的蛋白表达;相反,自噬抑制剂增强了NLRP3蛋白的表达。这些发现为NLRP3在加速动脉粥样硬化中的重要作用提供了新的见解,也为动脉粥样硬化的治疗指明了新的潜在分子靶点。
3.3. NLRP3炎症小体与心肌梗死
心血管疾病,尤其是急性心肌梗死(MI)和中风,是全世界死亡和残疾的主要原因 [27]。心肌梗死会引发强烈而复杂的炎症反应以对心脏进行修复和重建 [8]。目前已证明,对炎症信号的干预可以减少心肌梗死面积 [28] [29] [30]。抑制IL-1β的表达可明显减少小鼠急性心肌梗死后的心脏肥大和心肌功能障碍的发生 [31]。IL-10是一种抗炎细胞因子,通过激活M2巨噬细胞极化,可抑制心肌纤维化,改善心肌重构,促进心肌梗死后小鼠心肌伤口愈合 [28]。目前NLRP3被认为是心肌缺血中模式识别受体中的一个高度可信的候选受体,可识别多种危险信号并诱导炎症反应发生 [32]。VanHout等人 [33] 报道,在猪心肌梗死模型中,NLRP3炎症小体抑制剂MCC950可显著降低梗死灶的大小,并保留了心肌功能,这说明抑制NLRP3炎症小体的活化可能对急性心肌梗死患者具有潜在的治疗作用。虽然心肌缺血再灌注可以改善心肌损伤的程度,但同时也有加重心肌损伤的可能。Marchetti等 [34] 报道,在大鼠心肌缺血再灌注模型中,抑制NLRP3炎症小体的活性可以减少心肌的梗死。Liu等人 [35] 发现,对C57BL/6J小鼠进行心肌缺血再灌注发现,NLRP3的表达增加、Casepase-1的活性提高、IL-1β和IL-18的表达也都显著上升。随后,在C57BL/6J小鼠心肌内注射NLRP3抑制剂BAY-11-7028或NLRP3 siRNA,可以显著减轻心肌缺血再灌注引起的心肌损伤。Kawaguchi等人 [36] 通过使用短暂性阻断心脏左前降支的方法来构建心肌缺血再灌注损伤模型。在这个模型中,与野生型小鼠相比,ASC−/−或Caspase-1−/−小鼠的炎性细胞因子和趋化因子的表达都显著降低。这些小鼠在心肌梗死后,梗死灶大小、心肌梗死后纤维化、左室功能障碍均有显著降低。这些发现提示了NLRP3炎症小体可能在心肌缺血再灌注损伤的病理生理学中发挥重要作用。随着对不同心肌损伤模型中NLRP3炎症小体研究的深入,Sandanger等人 [7] 发现,在冠状动脉结扎后的缺血性心肌中,NLRP3炎症小体在心肌成纤维细胞中可以显著升高(p < 0.05)。在NLRP3−/−小鼠中,缺血再灌注引起的心肌功能障碍和损伤均得到相应的改善,而在ASC−/−小鼠中没有得到体现,说明NLRP3和ASC在缺血再灌注心肌损伤中可能发挥不同的作用。Inoue等人 [37] 也发现,在NLRP3−/−小鼠中,肝缺血再灌注引起的肝损伤得到相应的改善,而在ASC−/−小鼠中没有得到体现。这些结果都说明,炎症小体的组成成分如NLRP3和ASC都可以发挥独立的作用。因此,进一步确定NLRP3和ASC的生理功能,可更好地了解疾病的进展,为治疗心肌梗死提供新的策略。
4. 未来展望
NLRP3炎症小体在高血压、动脉粥样硬化和心肌梗死等心血管疾病的发生发展中发挥着重要作用。NLRP3是炎性小体形成和免疫应答启动的关键参与者。在感染、受伤等期间,NLRP3和IL-1β的活化可以调节DAMPs和PAMPs。了解NLRP3蛋白蛋白体组装和活化的分子机制,可为心血管疾病的安全治疗提供新的靶点。综上所述,NLRP3炎性小体在心血管疾病中靶向治疗具有良好的前景,进一步阐明NLRP在心血管疾病中的确切作用可以使治疗方法得到优化。
基金项目
国家自然科学基金(81860326);云南省教育厅科学研究基金项目(No. 2018Y046)资助。
文章引用
郑 梦,陆 地,李丽梅,李 庆,边立功,杨洪财,黄 曦,王娇云,郭家智. NLRP3在心血管疾病中的研究进展
Research Perspectives of NLRP3 in the Pathogenesis of Cardiovascular Diseases[J]. 临床医学进展, 2020, 10(06): 1102-1107. https://doi.org/10.12677/ACM.2020.106166
参考文献
- 1. Bullón, P., Cano-García, F.J., Alcocer-Gómez, E., et al. (2017) Could NLRP3-Inflammasome Be a Cardiovascular Risk Biomarker in Acute Myocardial Infarction Patients? Antioxidants & Redox Signaling, 27, 269-275. https://doi.org/10.1089/ars.2016.6970
- 2. Hoseini, Z., Sepahvand, F., Rashidi, B., et al. (2018) NLRP3 Inflammasome: Its Regulation and Involvement in Atherosclerosis. Journal of Cellular Physiology, 233, 2116-2132. https://doi.org/10.1002/jcp.25930
- 3. Toldo, S., Mezzaroma, E., Mauro, A.G., et al. (2015) The Inflammasome in Myocardial Injury and Cardiac Remodeling. Antioxidants & Redox Signaling, 22, 1146. https://doi.org/10.1089/ars.2014.5989
- 4. Rongbin, Z., Yazdi, A.S., Philippe, M., et al. (2011) A Role for Mitochondria in NLRP3 Inflammasome Activation. Nature, 469, 221. https://doi.org/10.1038/nature09663
- 5. Franchi, L. and Núñez, G. (2012) Immunology. Orchestrating Inflammasomes. Science, 337, 1299-1300. https://doi.org/10.1126/science.1229010
- 6. Elliott, E.I. and Sutterwala, F.S. (2015) Initiation and Perpetuation of NLRP3 Inflammasome Activation and Assembly. Immunological Reviews, 265, 35-52. https://doi.org/10.1111/imr.12286
- 7. Stutz, A., Kolbe, C.C., Stahl, R., et al. (2017) NLRP3 Inflammasome Assembly Is Regulated by Phosphorylation of the Pyrin Domain. Journal of Experimental Medicine, 214, 1725. https://doi.org/10.1084/jem.20160933
- 8. Frangogiannis, N.G. (2014) The Inflammatory Response in Myocardial Injury, Repair, and Remodelling. Nature Reviews Cardiology, 11, 255-265. https://doi.org/10.1038/nrcardio.2014.28
- 9. Hughes, M.M. and O’neill, L.J. (2018) Metabolic Regulation of NLRP3. Immunological Reviews, 281, 88-98. https://doi.org/10.1111/imr.12608
- 10. Yu, X.J., Zhang, D.M., Jia, L.L., et al. (2015) Inhibition of NF-kappaB Activity in the Hypothalamic Paraventricular Nucleus Attenuates Hypertension and Cardiac Hypertrophy by Modulating Cytokines and Attenuating Oxidative Stress. Toxicology and Applied Pharmacology, 284, 315-322.
- 11. Qi, J., Yu, X.J., Shi, X.L., et al. (2015) NF-κB Blockade in Hypothalamic Paraventricular Nucleus Inhibits High- Salt-Induced Hypertension through NLRP3 and Caspase-1. Cardiovascular Toxicology, 16, 345-354. https://doi.org/10.1007/s12012-015-9344-9
- 12. Krishnan, S.M., et al. (2014) IL-1β and IL-18: Inflammatory Markers or Mediators of Hypertension? British Journal of Pharmacology, 171, 5589-5602. https://doi.org/10.1111/bph.12876
- 13. Toshiro, F. (2014) Mechanism of Salt-Sensitive Hypertension: Focus on Adrenal and Sympathetic Nervous Systems. Journal of the American Society of Nephrology, 25, 1148-1155. https://doi.org/10.1681/ASN.2013121258
- 14. Lin, L., Phillips, W.E. and Jr., R.M. (2009) Intrarenal Angiotensin II Is Associated with Inflammation, Renal Damage, and Dysfunction in Dahl Salt-Sensitive Hypertension. Journal of the American Society of Hypertension, 3, 306-314. https://doi.org/10.1016/j.jash.2009.08.002
- 15. Wang, X., Ian, M.P. and Mehta, J.L. (2011) LOX-1 and Angiotensin Receptors, and Their Interplay. Cardiovascular Drugs and Therapy, 25, 401-417. https://doi.org/10.1007/s10557-011-6331-7
- 16. Gan, W., Ren, J., Li, T., et al. (2017) The SGK1 Inhibitor EMD638683, Prevents Angiotensin II-Induced Cardiac Inflammation and Fibrosis by Blocking NLRP3 Inflammasome Activation. Biochimica et Biophysica Acta, 1864, 1-10. https://doi.org/10.1016/j.bbadis.2017.10.001
- 17. Galkina, E. and Ley, K. (2008) Immune and Inflammatory Mechanisms of Atherosclerosis. Annual Review of Immunology, 27, 165-197. https://doi.org/10.1146/annurev.immunol.021908.132620
- 18. Cochain, C. and Zernecke, A. (2016) Protective and Pathogenic Roles of CD8+ T Cells in Atherosclerosis. Basic Research in Cardiology, 111, 71. https://doi.org/10.1007/s00395-016-0589-7
- 19. Lu, X. and Kakkar, V. (2014) Inflammasome and Atherogenesis. Current Pharmaceutical Design, 20, 108-124. https://doi.org/10.2174/13816128113199990586
- 20. Zheng, F., Xing, S., Gong, Z., et al. (2014) Silence of NLRP3 Suppresses Atherosclerosis and Stabilizes Plaques in Apolipoprotein E-Deficient Mice. Mediators of Inflammation, 2014, Article ID: 507208. https://doi.org/10.1155/2014/507208
- 21. Peter, D., Hajime, K., Rayner, K.J., et al. (2010) NLRP3 Inflammasomes Are Required for Atherogenesis and Activated by Cholesterol Crystals. Nature, 464, 1357-1361. https://doi.org/10.1038/nature08938
- 22. Hendrikx, T., Jeurissen, M.L., Van Gorp, P.J., et al. (2015) Bone Marrow-Specific Caspase-1/11 Deficiency Inhibits Atherosclerosis Development in Ldlr-/-Mice. Febs Journal, 282, 2327-2338. https://doi.org/10.1111/febs.13279
- 23. Amna, A., Dominique, C., Mahmood, D.F.D., et al. (2014) Anti-Inflammatory and Antiatherogenic Effects of the NLRP3 Inflammasome Inhibitor Arglabin in ApoE2.Ki Mice Fed a High-Fat Diet. Circulation, 6, 6-7.
- 24. Baldrighi, M., Mallat, Z. and Li, X. (2017) NLRP3 Inflammasome Pathways in Atherosclerosis. Atherosclerosis, 267, 127. https://doi.org/10.1016/j.atherosclerosis.2017.10.027
- 25. Lu, J., et al. (2011) Oxidative Stress and Lectin-Like ox-LDL-Receptor LOX-1 in Atherogenesis and Tumorigenesis. Antioxidants & Redox Signaling, 15, 2301-2333. https://doi.org/10.1089/ars.2010.3792
- 26. Nvk, P., Karathanasis, S.K., Ding, Z., et al. (2017) LOX-1 in Atherosclerosis and Myocardial Ischemia: Biology, Genetics, and Modulation. Journal of the American College of Cardiology, 69, 2759. https://doi.org/10.1016/j.jacc.2017.04.010
- 27. Sidhu, R.K. (2016) Association between Acute Myocardial Infarction and Periodontitis: A Review of the Literature. Journal of the International Academy of Periodontology, 18, 23.
- 28. Jung, M., Ma, Y., Iyer, R.P., et al. (2017) IL-10 Improves Cardiac Remodeling after Myocardial Infarction by Stimulating M2 Macrophage Polarization and Fibroblast Activation. Basic Research in Cardiology, 112, 33. https://doi.org/10.1007/s00395-017-0622-5
- 29. Rienks, M., Carai, P., Bitsch, N., et al. (2017) Sema3A Promotes the Resolution of Cardiac Inflammation after Myocardial Infarction. Basic Research in Cardiology, 112, 42. https://doi.org/10.1007/s00395-017-0630-5
- 30. Yellon, D. and Hausenloy, D. (2007) Myocardial Reperfusion Injury—Reply. New England Journal of Medicine, 357, 1121-1135. https://doi.org/10.1056/NEJMra071667
- 31. Abbate, A., Van Tassell, B.W., Seropian, I.M., et al. (2014) Interleukin-1β Modulation Using a Genetically Engineered Antibody Prevents Adverse Cardiac Remodelling Following Acute Myocardial Infarction in the Mouse. European Journal of Heart Failure, 12, 319-322. https://doi.org/10.1093/eurjhf/hfq017
- 32. Takahashi, M. (2011) Role of the Inflammasome in Myocardial Infarction. Trends in Cardiovascular Medicine, 21, 37-41. https://doi.org/10.1016/j.tcm.2012.02.002
- 33. Van Hout, G.P., Bosch, L., Ellenbroek, G.H., et al. (2016) The Selective NLRP3-Inflammasome Inhibitor MCC950 Reduces Infarct Size and Preserves Cardiac Function in a Pig Model of Myocardial Infarction. European Heart Journal, 38, 828. https://doi.org/10.1093/eurheartj/ehw247
- 34. Marchetti, C., Chojnacki, J., Toldo, S., et al. (2014) A Novel Pharmacologic Inhibitor of the NLRP3 Inflammasome Limits Myocardial Injury after Ischemia-Reperfusion in the Mouse. Journal of Cardiovascular Pharmacology, 63, 316. https://doi.org/10.1097/FJC.0000000000000053
- 35. Liu, Y., Lian, K., Zhang, L., et al. (2014) TXNIP Mediates NLRP3 Inflammasome Activation in Cardiac Microvascular Endothelial Cells as a Novel Mechanism in Myocardial Ischemia/Reperfusion Injury. Basic Research in Cardiology, 109, 415. https://doi.org/10.1007/s00395-014-0415-z
- 36. Masanori, K., Masafumi, T., Takeki, H., et al. (2011) Inflammasome Activation of Cardiac Fibroblasts Is Essential for Myocardial Ischemia/Reperfusion Injury. Circulation, 123, 594-604. https://doi.org/10.1161/CIRCULATIONAHA.110.982777
- 37. Yoshiyuki, I., Yoshikazu, Y. and Masafumi, T. (2013) Role of the Inflammasome in Inflammatory Responses and Subsequent Injury after Hepatic Ischemia-Reperfusion Injury. Hepatology, 58, 2212. https://doi.org/10.1002/hep.26480
NOTES
*通讯作者。