Advances in Clinical Medicine
Vol.
11
No.
06
(
2021
), Article ID:
43321
,
9
pages
10.12677/ACM.2021.116397
新生儿血清25(OH)D水平及其与钙、磷、 碱性磷酸酶的关系分析
王艳萍1*,李明2#,孙明明3,梁霞4,张雯5
1青岛市海慈医院,山东 青岛
2青岛市市立医院,山东 青岛
3青岛市城阳人民医院,山东 青岛
4青岛市海慈医院,山东 青岛
5河南中医药大学,河南 郑州
收稿日期:2021年5月17日;录用日期:2021年6月3日;发布日期:2021年6月23日

摘要
目的:探讨不同胎龄不同出生体重新生儿血清25(OH)D、钙、磷、碱性磷酸酶(ALP)水平,并对新生儿体内25(OH)D与钙、磷、钙磷乘积及ALP进行相关性分析,形成对本地区新生儿骨代谢的初步认识。方法:1) 选择2016年4月~2017年5月本院新生儿病室收治的新生儿共158例,检测其出生后3天内血清25(OH)D、钙、磷、ALP水平,比较不同胎龄、不同出生体重新生儿之间25(OH)D、钙、磷、ALP的差异。2) 分别将25(OH)D与钙、磷、钙磷乘积、ALP进行相关性分析,明确钙、磷、ALP对新生儿体内25(OH)D的影响。结果:1) 按胎龄分组,28~<33周组的新生儿血清25(OH)D、血钙显著低于另外两组,血清25(OH)D缺乏率高达57.16%,明显高于另外两组,差异有统计学意义(P < 0.05);不同组别之间血磷、血碱性磷酸酶差别无统计学意义。2) 按出生体质量分组,VLBW组的血清25(OH)D、血钙、碱性磷酸酶显著低于其余三组,差异有统计学意义(P < 0.05);VLBW组新生儿血清25(OH)D缺乏率高达50%,明显高于其余三组,差异有统计学意义(P < 0.05)。3) 新生儿体内25(OH)D含量与血钙浓度呈线性正相关(r = 0.16, P = 0.04)、与碱性磷酸酶含量呈线性负相关(r = −0.25, P = 0.001),体内25(OH)D含量越高,体内血钙水平也越高;另一角度,新生儿体内25(OH)D含量越低,ALP的水平却越高。结论:1) 新生儿体内25(OH)D含量与胎龄及出生体质量有关,胎龄越小、出生体质量越低的新生儿25(OH)D含量越少,日后发生代谢性骨病的可能性越大;2) 青岛地区新生儿体内25(OH)D普遍不足,需尽早补充维生素D,预防代谢性骨病的发生;3) 新生儿体内25(OH)D含量与钙含量呈正相关,与ALP水平呈负相关。
关键词
血清25(OH)D,新生儿,代谢性骨病

An Analysis of Serum 25(OH)D Level and Its Relationship with Calcium, Phosphorus and Alkaline Phosphatase in Newborns
Yanping Wang1*, Ming Li2#, Mingming Sun3, Xia Liang4, Wen Zhang5
1Qingdao Hiser Hospital, Qingdao Shandong
2Qingdao Municipal Hospital, Qingdao Shandong
3Chengyang People’s Hospital, Qingdao Shandong
4Qingdao Hiser Hospital, Qingdao Shandong
5Henan University of Traditional Chinese Medicine, Zhengzhou Henan
Received: May 17th, 2021; accepted: Jun. 3rd, 2021; published: Jun. 23rd, 2021

ABSTRACT
Objective: To study serum 25(OH)D, calcium, phosphorus and alkaline phosphatase (ALP) levels of newborns with different gestational age and birth weight, and, to analyze the correlation between 25(OH)D and calcium, phosphorus, calcium-phosphorus product and ALP in neonates. Methods: 1) We selected a total of 158 newborns hospitalized in the neonatal intensive care unit of our hospital from April 2016 to May 2017, and, measured serum levels of 25(OH)D, calcium, phosphorus and ALP in these newborns within 3 days after birth. We compared the differences of 25(OH)D, calcium, phosphorus and ALP among neonates of different gestational ages and birth weights. 2) We analyzed the correlation between 25(OH)D and calcium, phosphorus, calcium phosphorus product and ALP, and, found the relationship between 25(OH)D and calcium, phosphorus and ALP in neonates. Results: 1) According to the group of gestational age, serum 25(OH)D and serum calcium of newborns in the group of 28~<33 weeks were significantly lower than the other two groups, and the serum 25(OH)D deficiency rate was as high as 57.16%, significantly higher than the other two groups, with statistically significant differences (P < 0.05). There was no significant difference in blood phosphorus and alkaline phosphatase between different groups. 2) According to the group of birth weight, serum 25(OH)D, serum calcium and alkaline phosphatase in the very low birth weight group were significantly lower than those in the other three groups, with statistically significant differences (P < 0.05). The serum 25(OH)D deficiency rate of newborns in the very low birth weight group was as high as 50%, which was significantly higher than the other three groups, and the difference was statistically significant (P < 0.05). 3) There was a positive linear correlation between the content of 25(OH)D in neonates and serum calcium concentration (r = 0.16, P = 0.04) and the content of alkaline phosphatase (r = −0.25, P = 0.001). On the other hand, the lower the level of 25(OH)D, the higher the level of ALP. Conclusion: 1) The content of 25(OH)D in neonates is related to gestational age and birth weight. 2) There is generally insufficient 25(OH)D in newborns in Qingdao area, so vitamin D should be added as soon as possible to prevent the occurrence of metabolic bone disease. 3) The content of 25(OH)D in neonates was positively correlated with calcium content and negatively correlated with ALP level.
Keywords:Serum 25(OH)D, Neonate, Metabolic Bone Disease

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
新生儿佝偻病(Neonatal rickets)是由于维生素D和(或)钙磷缺乏引起钙磷代谢失常致使钙盐沉积障碍和(或)骨样组织过多积聚形成的代谢性骨病,严重影响新生儿的正常生长发育,亦常并发手足搐搦症、惊厥或喉痉挛,可危及生命或发生缺氧性脑损伤。胎儿的维生素D和钙磷水平依赖于母体转运(尤其是小于31周的胎儿);胎儿体内贮存的钙和磷80%是在胎龄28周以后完成的,因此早产儿维生素D和钙、磷贮存量往往不足,易发生此病。新生儿佝偻病在我国并不少见,尤其是在日照时间短、寒季较长、及膳食缺乏深海鱼类的东北、华北地区。青岛地区新生儿体内维生素D、钙、磷、ALP水平尚缺乏相关研究,本文旨在探讨青岛地区不同胎龄和不同出生体重新生儿维生素D、钙、磷、ALP水平,形成对本地区新生儿骨代谢的初步认识,为促进儿童健康成长提供帮助。
2. 资料与方法
2.1. 研究对象
选取2016年4月~2017年5月在青岛市海慈医院新生儿病室收治的新生儿共158例。入选标准:1) 无严重畸形;2) 生后0~3天入院,未补充维生素D。排除标准:1) 出生时有严重窒息、缺氧、酸中毒、DIC等疾病的患儿;2) 明确诊断有先天性甲状腺或甲状旁腺疾病的患儿。根据胎龄分为≥28周~<33周组,≥33周组~<37周组,≥37周~<42周组。根据出生体重分为极低出生体重组(VLBW, <1500 g),低出生体重组(LBW, ≥1500~2500 g),正常出生体重组(NBW, ≥2500~<4000 g),和巨大儿(≥4000 g)。
2.2. 研究方法
2.2.1. 检测方法
新生儿生后3天内常规留取用于血生化的静脉血2.5 ml,血标本离心后取肝肾功检测所需剩余血清,−80℃冷冻储存,3天内检测。血清钙、磷、碱性磷酸酶用自动生化仪由本院检验科进行自动检测。维生素D水平由药理基地进行检测。清晨喂奶前抽取新生儿静脉血1 ml送检。
2.2.2. 诊断标准
血清总25(OH)D ≥ 75 nmol/L为维生素D充足;50 nmol/L ≤ 25(OH)D < 75 nmol/L为维生素D不足;25(OH)D < 50 nmol/L为维生素D缺乏 [1]。
2.3. 统计学方法
应用SPSS17.0统计软件。计数资料用例数和百分比表示,组间比较用χ2检验。计量资料先进行描述性统计分析,符合正态分布资料以均值 ± 标准差( )表示,两组间比较采用t检验;多组定量资料的比较采用单因素方差分析。二变量相关分析根据变量为正态分布或偏态分布用Pearson或Spearman相关分析。P < 0.05差异有统计学意义。
3. 结果
3.1. 总体情况
全部新生儿中共有46例早产儿,其中28~<33周早产儿14例,33~<37周早产儿32例;全部新生儿出生体质量800 g~4400 g,平均(2953.81 ± 809.25) g,见表1。
Table 1. General situation of neonates in different gestational ages
表1. 不同胎龄新生儿的一般情况
3.2. 不同胎龄新生儿的血清25(OH)D、钙、磷、ALP水平分析
血清25(OH)D、血钙在不同胎龄间差异有统计学意义(P < 0.05),进一步两两分析发现,28~<33周组的新生儿血清25(OH)D、血钙显著低于另外两组。血清25(OH)D缺乏率在不同胎龄间差异有统计学意义(P < 0.05),28~<33周组的新生儿血清25(OH)D缺乏率高达57.16%,明显高于另外两组[33~<37周组(14.30%),37~<42周组(18.75%)],差异有统计学意义(P < 0.05);血磷、血碱性磷酸酶在不同胎龄间差异无统计学意义。不同组别新生儿血清25(OH)D水平普遍不足,其中28~<33周组不足率85.7%,33~<37周组不足率87.5%,37~<42周组不足率73.2%,见表2。
Table 2. Analysis of serum 25(OH)D, calcium, phosphorus and ALP levels in neonates of different gestational ages ( χ ¯ ± s )
表2. 不同胎龄新生儿的血清25(OH)D、钙、磷、ALP水平分析( )
3.3. 不同出生体质量新生儿的血清25(OH)D、钙、磷、ALP水平分析
结果表明,血清25(OH)D、血钙、碱性磷酸酶在不同出生体质量之间差异有统计学意义(P < 0.05),进一步两两比较发现,VLBW组的血清25(OH)D、血钙、碱性磷酸酶显著低于其余三组,差异有统计学意义(P < 0.05);血清25(OH)D缺乏率在不同体重组之间差异有统计学意义(P < 0.05),VLBW组新生儿血清25(OH)D缺乏率高达50%,明显高于其余三组[LBW组(25.00%),NBW组(13.20%),巨大儿组(25.00%)],见表3。不同出生体质量新生儿血清25(OH)D水平普遍不足,其中VLBW组不足率83.3%,LBW组不足率89.2%,NBW组不足率74.5%,巨大儿组不足率66.6%,见表3。
Table 3. Analysis of serum 25(OH)D, calcium, phosphorus and ALP levels in neonates of different birth weight ( χ ¯ ± s )
表3. 不同出生体质量新生儿的血清25(OH)D、钙、磷、ALP水平分析( )
3.4. 新生儿体内25(OH)D与钙、磷、钙磷乘积、ALP的关系分析
新生儿体内血清25(OH)D含量与血钙浓度呈线性正相关(r = 0.16, P = 0.04)、与碱性磷酸酶含量呈线性负相关(r = −0.25, P = 0.001),体内25(OH)D含量越高,体内血钙水平也越高;另一角度,新生儿体内,25(OH)D含量越低,ALP的水平却越高,见表4、图1~4。

Figure 1. Scatter diagram of 25(OH)D with calcium
图1. 25(OH)D与钙的散点图

Figure 2. Scatter diagram of 25(OH)D with phosphorus
图2. 25(OH)D与磷的散点图
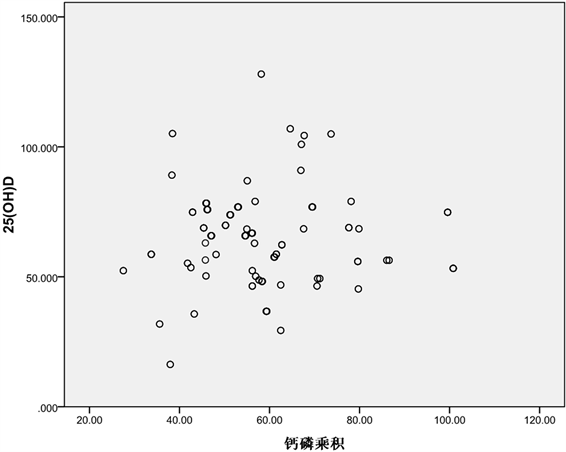
Figure 3. Scatter diagram of 25(OH)D with the calcium-phosphorus product
图3. 25(OH)D与钙磷乘积的散点图

Figure 4. Scatter diagram of 25(OH)D with ALP
图4. 25(OH)D与ALP的散点图
Table 4. Correlation analysis of serum 25(OH)D with serum calcium, calcium-phosphorus product, and ALP
表4. 血清25(OH)D与血钙、钙磷乘积及ALP相关性分析
4. 讨论
维生素D对新生儿及婴幼儿的健康起很重要的作用。在促进骨骼健康发育方面的作用已得到证实 [2] [3]。当维生素D缺乏或不足时,钙、磷经肠道吸收减少,血钙、血磷降低引起甲状旁腺素(PTH)分泌增加,加速旧骨吸收,释放钙、磷到细胞外液,以维持血浆钙的正常水平;同时刺激肾脏1α-羟化酶活性以促进1,25-(OH)2-D的合成,增加肾小管对钙的重吸收,而抑制对磷的重吸收,使得磷经肾脏被大量排出,血磷降低,钙磷乘积降低,骨样组织钙化过程发生障碍,成骨细胞代偿性增生,在干骺端造成骨样组织堆积,形成“串珠”及“手足镯”样改变。骨膜下骨样组织不能钙化,骨质疏松、软化,长骨负重后发生骨骼弯曲;颅骨骨样组织堆积、软化,形成方颅。近年来发现维生素D也可能与其他健康状况有关。例如,在儿童早期补充维生素D可以降低1型糖尿病和流感的风险 [4] [5]。尽管维生素D在健康方面发挥了积极作用,但它在人体中含量普遍不足 [6]。本研究发现,早产儿,尤其是28~<33周的早产儿血清25(OH)D、血钙水平显著偏低,血清25(OH)D缺乏率达57.16%,不足率高达85.7%,这与巴西Milene 等 [7] 的研究一致,其研究还发现,小于32周早产儿孕母体内血清25(OH)D水平明显低于足月儿母亲体内血清25(OH)D水平,因此考虑母亲体内25(OH)D水平影响新生儿体内25(OH)D的水平。Roth等 [8] [9] 研究认为,宫内生长受限的胎儿或宫外生长迟缓的婴儿普遍存在维生素D缺乏,母亲在怀孕中期至分娩前或哺乳期六个月内补充维生素D无肝肾功能损害,孕期可接受的上限剂量为4000 IU/天,因此,建议怀孕中期开始补充维生素D。但是,没有证据证明孕期维生素D缺乏会对婴儿产生直接不良后果,因此,孕期及哺乳期对维生素D的需求量具有不确定性,医学研究所推荐的膳食补充剂量 [10] (4200 IU/周)足以改善母体维生素D缺乏症,而不会导致维生素D过量。
本研究发现,新生儿体内25(OH)D含量与出生体质量有关,VLBW组的血清25(OH)D显著低于其余三组(P < 0.05),血钙也显著低于其余三组(P < 0.05);且VLBW组新生儿血清25(OH)D缺乏率高达50%,不足率83.3%,这与Munshi [11] 等的研究一致。Munshi等对186名极低出生体重儿(VLBW)和115名超低出生体重儿(ELBW)进行研究,根据美国儿科学会(AAP)推荐剂量,给早产儿口服维生素D3 400 IU/d补充治疗,在4、8和12周龄或出院前检查血清25(OH)D水平,发现大约80%的VLBW婴儿和ELBW婴儿在4周龄时25(OH)D水平仍不足,VLBW婴儿在8周龄和12周龄时其25(OH)D水平显著增加,而ELBW婴儿其25(OH)D水平直至12周龄时才明显增加,并且无一例出现肝肾功能异常。提示及早补充维生素D有助于改善VLBW和ELBW婴儿25(OH)D水平,同时也证实AAP建议是安全的。
本研究还分别将25(OH)D与钙、磷、钙磷乘积、ALP进行了相关性分析,结果发现,新生儿体内25(OH)D含量越高,体内血钙水平也较高。另外一个角度,新生儿体内25(OH)D含量越低,ALP的水平却越高。
新生儿以及生命早期的生存质量对患儿一生的健康及生活质量都具有相当大的价值和意义。随着新生儿救治技术的提高,新生儿存活率已大大改善,如何提高新生儿的存活质量也渐渐成为关注焦点。本研究已对新生儿骨代谢及骨健康做了很有价值的工作,我们还将在此基础上进行进一步研究,为提高我国儿童的健康水平提供更好的研究成果。
同意书
该试验经过伦理委员会批准,并签署知情同意书。
文章引用
王艳萍,李 明,孙明明,梁 霞,张 雯. 新生儿血清25(OH)D水平及其与钙、磷、碱性磷酸酶的关系分析
An Analysis of Serum 25(OH)D Level and Its Relationship with Calcium, Phosphorus and Alkaline Phosphatase in Newborns[J]. 临床医学进展, 2021, 11(06): 2744-2752. https://doi.org/10.12677/ACM.2021.116397
参考文献
- 1. Shin, Y.H., Shin, H.J. and Lee, Y.J. (2013) Vitamin D Status and Childhood Health. Korean Journal of Pediatrics, 56, 417-423. https://doi.org/10.3345/kjp.2013.56.10.417
- 2. Hossein-Nezhad, A. and Holick, M.F. (2012) Optimize Dietary Intake of Vitamin D: An Epigenetic Perspective. Current Opinion in Clinical Nutrition & Metabolic Care, 15, 567-579. https://doi.org/10.1097/MCO.0b013e3283594978
- 3. Holick, M.F. (2012) The D-Lightful Vitamin D for Child Health. Journal of Parenteral and Enteral Nutrition, 36, 9S-19S. https://doi.org/10.1177/0148607111430189
- 4. Holick, M.F., Binkley, N.C., Bischoff-Ferrari, H.A., et al. (2011) Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism, 96, 1911-1130. https://doi.org/10.1210/jc.2011-0385
- 5. Urashima, M., Segawa, T., Okazaki, M., et al. (2010) Randomized Trial of Vitamin D Supplementation to Prevent Seasonal Influenza A in Schoolchildren. The American Journal of Clinical Nutrition, 91, 1255-1260. https://doi.org/10.3945/ajcn.2009.29094
- 6. Holick, M.F. (2012) Vitamin D: Extraskeletal Health. Rheumatic Disease Clinics of North America, 38, 141-160. https://doi.org/10.1016/j.rdc.2012.03.013
- 7. Kassai, M.S., Cafeo, F.R., Affonso-Kaufman, F.A., et al. (2018) Vitamin D Plasma Concentrations in Pregnant Women and Their Preterm Newborns. BMC Pregnancy and Childbirth, 18, 412. https://doi.org/10.1186/s12884-018-2045-1
- 8. Roth, D.E., Morris, S.K., Zlotkin, S., et al. (2018) Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth. New England Journal of Medicine, 379, 535-546. https://doi.org/10.1056/NEJMoa1800927
- 9. Litonjua, A.A., Carey, V.J., Laranjo, N., et al. (2016) Effect of Prenatal Supplementation with Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA, 315, 362-370. https://doi.org/10.1001/jama.2015.18589
- 10. Ross, A.C., Taylor, C.L., Yaktine, A.L., et al. (2011) Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press, Washington DC.
- 11. Munshi, U.K., Graziano, P.D., Meunier, K., et al. (2018) Serum 25 Hydroxy Vitamin D Levels in Very Low Birth Weight Infants Receiving Oral Vitamin D Supplementation. Journal of Pediatric Gastroenterology and Nutrition, 66, 676-679. https://doi.org/10.1097/MPG.0000000000001831
