Advances in Clinical Medicine
Vol.
13
No.
02
(
2023
), Article ID:
61038
,
5
pages
10.12677/ACM.2022.132165
小肠肠系膜韧带样纤维瘤病1例
张金亮,杨春*
山东第一医科大学第一附属医院,山东 济南
收稿日期:2023年1月1日;录用日期:2023年1月25日;发布日期:2023年2月3日

摘要
目的:小肠肠系膜韧带样纤维瘤病是一种罕见病,临床术前诊断通常容易误诊。本研究通过病理报道分析该疾病临床症状、影像特点及病理特征,回顾性分析并进行总结,旨在增加对该病的影像和临床认识,促进疾病的治疗及改善预后。方法:报道1例发生在腹腔内的小肠肠系膜处的韧带样纤维瘤病。结果:根据临床表现、CT检查及病理结果,诊断为小肠肠系膜韧带样纤维瘤病。结论:韧带样纤维瘤病发生率低,腹腔内发病者罕见,起病隐匿,肿瘤生长缓慢但腹腔内型一般体积较大,术前诊断通常容易误诊。
关键词
小肠肠系膜,韧带样纤维瘤病,1例

Mesenteric Desmoid-Type Fibromatosis of the Small Intestine
Jinliang Zhang, Chun Yang*
The First Affiliated Hospital of Shandong First Medical University, Jinan Shandong
Received: Jan. 1st, 2023; accepted: Jan. 25th, 2023; published: Feb. 3rd, 2023

ABSTRACT
Objective: Ligamentoid fibromatosis of small intestine mesentery is a rare disease, which is usually misdiagnosed before clinical operation. This study analyzed the clinical symptoms, imaging features, and pathological features of the disease through pathological reports, and made a retrospective analysis and summary, aiming to increase the imaging and clinical understanding of the disease, promote the treatment of the disease and improve the prognosis. Methods: A case of desmoid fibromatosis of small intestine mesentery in the abdominal cavity was reported. Results: According to the clinical manifestation, CT examination, and pathological results, it was diagnosed as small intestinal mesenteric ligament-like fibromatosis. Conclusion: The incidence of desmoid fibromatosis is low, the incidence in the abdominal cavity is rare, the onset is hidden, and the tumor grows slowly, but the intraperitoneal type is generally large, and the preoperative diagnosis is usually easy to be misdiagnosed.
Keywords:The Mesentery of Small Intestine, Desmoid-Type Fibromatosis, 1 Case

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
小肠肠系膜韧带样纤维瘤病是指发生在小肠肠系膜的韧带样纤维瘤病(desmoid-type fibromatosis, DTF),临床比较罕见,临床症状出现较晚且不典型,术前临床诊断容易误诊。本文报道1例小肠肠系膜韧带样纤维瘤病的临床、CT及病理学特点并复习相关文献,旨在增加对该病的影像和临床认识。现对我院收治的1例小肠肠系膜韧带样纤维瘤病报道如下。
2. 临床资料和结果
2.1. 一般资料及查体
患者男性,60岁。因上腹部疼痛不适2年余,加重1个月余入院。查体:右下腹触及一类圆形肿块,质地较硬,活动度差,伴有压痛,无反跳痛。实验室检查:血、尿常规及肾功能未见明显异常,便隐血试验阴性。
2.2. 辅助检查
2.2.1. 影像学检查及诊断
腹部CT平扫示右下腹腔内见一类圆形略低密度软组织肿块,密度大致均匀,边界较清晰,局部肠管受压移位,与肠管关系密切,局部肠管管壁增厚,肿块周围脂肪间隙模糊欠清;增强扫描示动脉期强化不明显,门脉期呈轻度强化,延迟期呈轻至中度的延迟性强化,平扫–动脉期–静脉期–延迟期平均CT值约为:39 Hu-42 Hu-48 Hu-59 Hu。如图1(a)~(d)所示。CT诊断:右下腹腹腔内肠系膜肿物,淋巴瘤可能。
2.2.2. 手术所见
于右下腹腔小肠肠系膜根部查见体积约8.5 * 7.5 * 5 cm的肿物,切面灰白,质韧,边界尚清,局部与小肠关系密切,似侵犯肠壁固有肌层,小肠肠管切除长度约36 cm,最大周长约4.5 cm,肠粘膜尚光滑,灰白色,质韧,距肿块浸润一端6.5 cm,另一端切线7 cm。于肿瘤周围肠系膜处查见淋巴结16个,大者长径约0.6 cm,灰白色,质韧。
2.2.3. 光镜所见及病理结果
光镜下见肿瘤由梭形纤维母细胞和胶原纤维构成,呈束状、编织状排列,如图2所示。病理诊断:小肠肠系膜韧带样纤维瘤病,体积8.5 * 7.5 * 5 cm,肿瘤侵犯肠壁固有肌层,未累及两端切线。肠系膜(16个)淋巴结呈反应性增生。免疫组化:B-catenin (核+),LEF-1 (−)、CD117 (−)、DOG1 (−)、CD34 (−)、SMA (−)、S-100 (−)。
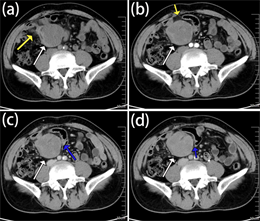
Figure 1. As indicated by the white arrow, (a) A kind of round slightly low-density mass was seen in the right lower abdominal cavity on the plain scan, the density was generally uniform, the boundary was clear, the local intestinal tube was pressed and displaced, closely related to the intestinal tube, the local intestinal tube wall was thickened; (b) The enhancement was not obvious in the arterial phase; (c) The portal phase of enhanced scanning showed mild enhancement (d) the delayed phase of enhanced scanning showed mild to moderate delayed enhancement. The yellow arrow indicated that the fat space around the focus was blurred. As pointed out by the blue arrow, the tumor pushed and pressed the local small intestinal tissue, which was closely related to the small intestine, and the local intestinal wall thickens
图1. 如图中白色箭头所指,(a) 平扫右下腹腔内见一类圆形略低密度肿块,密度大致均匀,边界较清晰,局部肠管受压移位,与肠管关系密切,局部肠管管壁增厚;(b) 增强扫描动脉期强化不明显;(c) 增强扫描门脉期呈轻度强化;(d) 增强扫描延迟期进一步强化,呈轻至中度的延迟性强化。如黄色箭头所指,病灶周围脂肪间隙模糊。如蓝色箭头所指,肿瘤推压局部小肠组织,与小肠关系密切,局部肠壁增厚
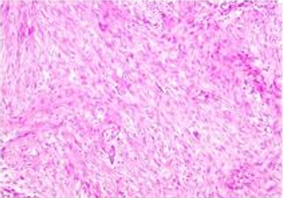
Figure 2. Pathological examination showed that the tumor was composed of proliferated fibroblasts and collagen fibers. The tumor cells were slender and long fusiform, arranged in bundles and weaves
图2. 病理学检查显示肿瘤由增生的纤维母细胞和胶原纤维构成。瘤细胞呈纤细的长梭形,呈束状、编织状排列
3. 讨论
韧带样纤维瘤病(desmoid-type fibromatosis, DTF)又称侵袭性纤维瘤病(aggressive fibromatosis, AF)、硬纤维瘤、韧带样瘤等,最早是在1948年由Stout提出的 [1]。DTF是一种无性纤维母细胞肿瘤性增生症,占所有肿瘤的0.03%,在所有软组织肿瘤中占比不到3% [2]。DTF是一种罕见的结缔组织良性肿瘤,起源于深层肌肉筋膜、腱膜、肌腱和瘢痕组织 [3] [4] [5],其特征是浸润性生长和没有转移潜力。虽然组织学上是良性的,即无坏死、核多形性和不典型有丝分裂,但具有局部侵袭性的特点,有压迫局部结构的倾向,如肠道、服盆腔神经和大血管、输尿管、膀胱、阴道等,因此被归为中度恶性肿瘤 [6] [7] [8]。发病部位多见于四肢及腹壁,腹腔内发病少见,尤其是肠系膜韧带样纤维瘤病少见。DTF的病因可能是创伤后、既往腹部手术史、激素因素等 [9],病灶可以零星发生,也可以出现在先天性综合征的背景下,如家族性腺瘤性息肉病等 [10]。然而,到目前为止,还没有相关文献表明髋关节假体和硬纤维瘤之间的联系 [11]。DTF发病年龄在16岁至60岁之间,高发病率年龄约为30岁 [9]。DTF女性发病率较高(男女比例为1:1.5~2.5) [12]。DTF临床上比较罕见,加上其临床病程的多变性,没有任何报道证明特定临床干预措施是有效的 [13]。DTF主要的治疗方法是根治性手术切除,安全切除范围为2~3 cm。然而,由于肿瘤边界不清,“真正的”根治性切除手术通常很难实现 [4] [14]。在一项有史以来病例数量最大的DTF调查中,大多数放射肿瘤学家认为放射疗法是一种值得和有用的侵袭性纤维瘤病的治疗适应症 [2]。本篇病例报告是一位60岁男性患者,在腹腔内小肠肠系膜处发现一类圆形肿块,术后病理证实为小肠肠系膜韧带样纤维瘤病。
在我们的病例中,DTF的最终诊断是术后通过手术标本的组织学检查获得的。患者术后恢复良好,术后5个月内无不适,复查未见复发征象。DTF的罕见发生和不同的临床表现限制了影像科医生的术前诊断的能力。腹腔内DTF临床体征和影像学特征无特异性,大多数肠系膜纤维瘤位于肠系膜的根部,生长缓慢,临床症状明显时肿瘤体积多较大,多数为单发,偶尔可为多发,呈圆形或卵圆形病灶,周围无明显肿大的淋巴结及腹腔积液。CT平扫呈较均匀稍低软组织密度,增强扫描显示肿瘤体内部多数可见轻到中度的延迟性强化(镜下所见肿瘤内的纤维母细胞和大量变性的胶原纤维是延迟强化的病理基础)。
肠系膜DTF的鉴别诊断主要包括1) 胃肠道间质瘤,CD34和CD117染色为阳性,多数瘤体位于胃肠道腔外,也可向腔内生长,良性肿瘤可出现斑点状、环形或弧形钙化,肿瘤较大时多见低密度坏死、囊变区,增强实性部分呈明显、持续强化,坏死囊变区不强化,肿瘤可经蒂与胃肠道壁相连或局部壁增厚,有助于确定肿瘤起源;2) 胃肠道淋巴瘤,四十岁以上中老年多见,男多于女,多发生肠壁环形增厚,一般呈较均匀等密度,粘膜面可出血多发坏死,多伴有淋巴结增多增大,密度均匀,增强扫描呈不强化或轻度强化。3) 孤立性纤维瘤,可发生于身体的任何部位,包括肠系膜 [15] [16],多呈膨胀性生长,钙化少见,肿瘤较大时内可见钙化,肿瘤内可见多发坏死囊变区,增强扫描呈明显不均匀强化,富血管区和肿瘤细胞密集区明显强化,致密胶原纤维区强化相对较弱、呈延迟强化,坏死囊变区无强化。
本文考虑到小肠肠系膜韧带样纤维瘤病临床发病率低,术前诊断困难,误诊率高,对于疑似DTF的病例,应结合组织病理学检查。因此,本报道旨在通过罕见病例报道以及相关文献的复习来引起临床医师的重视。
文章引用
张金亮,杨 春. 小肠肠系膜韧带样纤维瘤病1例
Mesenteric Desmoid-Type Fibromatosis of the Small Intestine[J]. 临床医学进展, 2023, 13(02): 1198-1202. https://doi.org/10.12677/ACM.2022.132165
参考文献
- 1. P, S.A. (1948) Fibrosarcoma. The Malignant Tumor of Fibroblasts. Cancer, 1, 30-63. https://doi.org/10.1002/1097-0142(194805)1:1<30::AID-CNCR2820010104>3.0.CO;2-D
- 2. Micke, O., See-genschmiedt, M.H. and D. German Cooperative Group on Radiotherapy for Benign (2005) Radiation Therapy for Ag-gressive Fibromatosis (Desmoid Tumors): Results of a National Patterns of Care Study. International Journal of Radia-tion Oncology, Biology, Physics, 61, 882-891. https://doi.org/10.1016/j.ijrobp.2004.07.705
- 3. Enzinger, F.M. and Shiraki, M. (1967) Musculo-Aponeurotic Fibromatosis of the Shoulder Girdle (Extra-Abdominal Desmoid). Analysis of Thirty Cases Followed Up for Ten or More Years. Cancer, 20, 1131-1140. https://doi.org/10.1002/1097-0142(196707)20:7<1131::AID-CNCR2820200716>3.0.CO;2-8
- 4. Goy, B.W., Lee, S.P., Eilber, F., et al. (1997) The Role of Adjuvant Radiotherapy in the Treatment of Resectable Desmoid Tumors. Inter-national Journal of Radiation Oncology, Biology, Physics, 39, 659-665. https://doi.org/10.1016/S0360-3016(97)00334-9
- 5. Goy, B.W., Lee, S.P., Fu, Y.S., Selch, M.T. and Eilber, F. (1998) Treatment Results of Unresected or Partially Resected Desmoid Tumors. American Journal of Clinical Oncology, 21, 584-590. https://doi.org/10.1097/00000421-199812000-00011
- 6. Walczak, B.E. and Rose, P.S. (2013) Desmoid: The Role of Local Therapy in an Era of Systemic Options. Current Treatment Options in Oncology, 14, 465-473. https://doi.org/10.1007/s11864-013-0235-7
- 7. Ogawa, N., Iseki, H., Tsunozaki, H., et al. (2014) In-tra-Abdominal Desmoid Tumor Difficult to Distinguish from a Gastrointestinal Stromal Tumor: Report of Two Cases. Surgery Today, 44, 2174-2179. https://doi.org/10.1007/s00595-013-0681-7
- 8. Mullen, J.T., Delaney, T.F., Kobayashi, W.K., et al. (2012) Des-moid Tumor: Analysis of Prognostic Factors and Outcomes in a Surgical Series. Annals of Surgical Oncology, 19, 4028-4035. https://doi.org/10.1245/s10434-012-2638-2
- 9. Aikaterini, M., Stylianos, G., Dimitrios, K., et al. (2010) Management of a Large Abdominal Wall Desmoid Tumor during Pregnancy. Case Report. Annali Italiani di Chi-rurgia, 81, 153-156.
- 10. Tayeb Tayeb, C., Parc, Y., Andre, T. and Lopez-Trabada Ataz, D. (2020) Familial Adenoma-tous Polyposis, Desmoid Tumors and Gardner Syndrome. Bulletin du Cancer, 107, 352-358. https://doi.org/10.1016/j.bulcan.2019.10.011
- 11. Gebhart, M., et al. (1999) Development of a Desmoid Tumor at the Site of a Total Hip Replacement. Acta Orthopaedica Belgica, 65, 230-234.
- 12. Reitamo, J.J., Scheinin, T.M. and Hayry, P. (1986) The Desmoid Syndrome. New Aspects in the Cause, Pathogenesis and Treatment of the Desmoid Tu-mor. The American Journal of Surgery, 151, 230-237. https://doi.org/10.1016/0002-9610(86)90076-0
- 13. Shields, C.J., Winter, D.C., Kirwan, W.O. and Redmond, H.P. (2001) Desmoid Tumours. European Journal of Surgical Oncology, 27, 701-706. https://doi.org/10.1053/ejso.2001.1169
- 14. Ballo, M.T., et al. (1999) Desmoid Tumor: Prognostic Factors and Outcome after Surgery, Radiation Therapy, or Combined Surgery and Radiation Therapy. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 17, 158-158. https://doi.org/10.1200/JCO.1999.17.1.158
- 15. Gaetano, M., Carmela, E., Maria, L., Giuseppe, V. and Paolo, G. (2008) Solitary Fibrous Tumour of the Kidney with Sarcomatous Overgrowth. Case Report and Review of the Literature. APMIS: Acta Pathologica, Microbiologica, et Immunologica Scandinavica, 116, 1020-1025. https://doi.org/10.1111/j.1600-0463.2008.01012.x
- 16. Liu, J.N., Liu, Z., Ji, P.Y., Zhang, H. and Guo, S.L. (2020) Solitary Fibrous Tumor of the Mesentery: A Case Report and Review of the Literature. Journal of International Medical Research, 48, No. 10. https://doi.org/10.1177/0300060520950111
NOTES
*通讯作者。