Bioprocess
Vol.4 No.04(2014), Article
ID:14654,6
pages
DOI:10.12677/BP.2014.44012
Role and Mechanism of Protein Quality Control System in Enhancing the Thermotolerance of Saccharomyces cerevisiae
1The State Key Laboratory of Microbial Technology, Shandong University, Jinan
2The Taishan College of Shandong University, Jinan
3State Key Laboratory of Motor Vehicle Biofuel Technology, Nanyang
Email: wuhongyu93@163.com, *shenyu@sdu.edu.cn
Copyright © 2014 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received: Nov. 26th, 2014; revised: Dec. 8th, 2014; accepted: Dec. 17th, 2014
ABSTRACT
Increasing the fermentation temperature is considered to be beneficial to decrease the production cost of biochemicals such as ethanol. However, the high temperature leads to the unstable state of protein, which is harmful to the growth and metabolism of Saccharomyces cerevisiae. The protein quality control system of S. cerevisiae, composed by heat shock protein and ubiquitin-proteasome system, can release the heat shock stress by assisting the protein folding, refolding or degrading the misfolded protein. In this article, the regulatory mechanism of protein quality control system and its role to thermotolerance in Saccharomyces cerevisiae are reviewed.
Keywords:Saccharomyces cerevisiae, Heat Shock Stress, Thermotolerance, Heat Shock Protein, Ubiquitin Ligases

蛋白质质量控制系统在增强酿酒酵母耐热性中的作用及机制
吴宏宇1,2,吴显伟1,2,赵建志1,鲍晓明1,侯 进1,王林风3,高 楠3,沈 煜1*
1山东大学微生物技术国家重点实验室,济南
2山东大学泰山学堂,济南
3车用生物燃料技术国家重点实验室,南阳
Email: wuhongyu93@163.com, *shenyu@sdu.edu.cn
收稿日期:2014年11月26日;修回日期:2014年12月8日;录用日期:2014年12月17日

摘 要
提高发酵温度被认为有利于降低乙醇等产品的生产成本,但高温条件不利于酿酒酵母的生长与代谢,其中,高温引起的蛋白质不稳定是主要原因之一。酿酒酵母含有主要由热休克蛋白和泛素降解系统组成的复杂的蛋白质质量控制系统,通过辅助蛋白折叠、错误折叠蛋白修正以及降解,在不同层次缓解高温对酿酒酵母的胁迫。本文综述了蛋白质质量控制系统的调控机制以及该系统对酿酒酵母耐热性能的影响研究进展。
关键词
酿酒酵母,热胁迫,耐热性,热休克蛋白,泛素连接酶

1. 引言
酿酒酵母(Saccharomyces cerevisia)是一种真核模式微生物,广泛应用于食品及工业发酵生产。随着生物炼制理念的推广,利用酿酒酵母进行的生物乙醇、琥珀酸等化合物生产已实现大规模工业化生产。工业发酵一次上罐量巨大,微生物在发酵过程中会释放大量的热,造成高温环境。但酿酒酵母的最适发酵温度为25℃~33℃,维持适宜的发酵温度的降温过程花费较高。而提高酿酒酵母的发酵温度不仅可以降低能耗,而且还可以提高发酵速率,是改进工业发酵技术的另一途径。
当酿酒酵母生存环境受到胁迫时,例如渗透性变化、盐胁迫、饥饿以及热胁迫时,酿酒酵母许多细胞内活动都会受到影响[1] ,例如错误折叠蛋白的积累[2] 、氧化磷酸化的解偶联[3] 、细胞分裂的暂时停滞[4] 、质膜与细胞骨架结构的破坏以及细胞蛋白分泌途径的缺失[5] 等,这些都严重影响酵母细胞的生长及代谢。例如,当环境温度高于36℃~37℃时,酿酒酵母启动热休克响应(heat-shock response, HSR),通过表达热休克蛋白(heat-shock proteins, Hsps) [6] 、增加酵母细胞膜饱和脂肪酸[7] 和麦角固醇含量[8] 、以及增加细胞内海藻糖含量[9] 等措施缓解高温给酵母细胞带来的损害。其中,蛋白质质量控制系统在细胞遭遇包括热胁迫在内的多种胁迫时对细胞内蛋白质进行保护、修复以及降解再利用。该系统包括不依赖于ATP的小分子热休克蛋白(Small heat shock proteins, sHsps),依赖于ATP的较大的分子热休克蛋白,以及泛素介导的蛋白质降解系统[6] [10] 。本文综述了这些蛋白质质量控制系统在减少热胁迫条件下因蛋白错误折叠造成的细胞损伤中的作用(图1)及其分子机制。
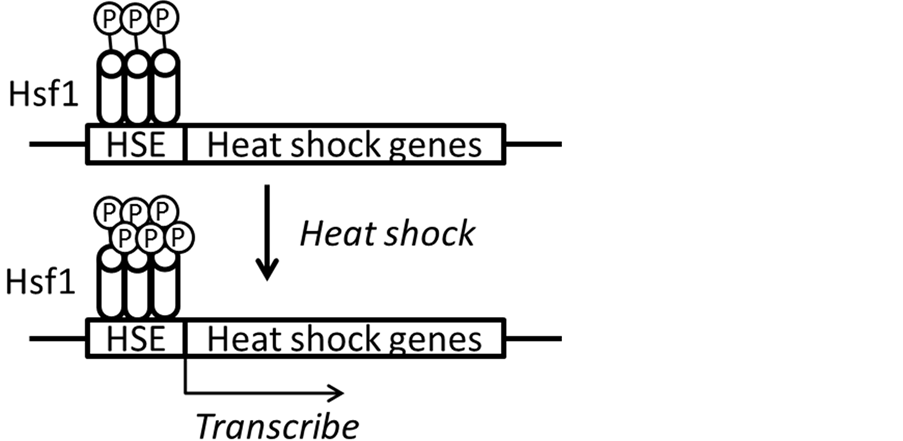
Figure 1. The mechanism of the transcription factor Hsf1 to regulate the transcription of heat shock protein
图1. 转录因子Hsf1调节热激蛋白转录的机制
2. 热休克系统在增强酿酒酵母耐热性中的作用及机制
酿酒酵母中,热休克转录因子(heat shock transcription factor) Hsf1调控几百个胁迫相关基因的表达,涉及细胞的多项功能,是十分重要的热胁迫响应转录因子[11] 。当周围环境升高,酿酒酵母启动热休克响应,Hsf1识别所调控基因启动子区域的热激元件(heat shock elements, HSEs)。典型的HSE由三个重复反向互补的NGAAN序列组成(NGAANNTTCNNGAAN),如HSP26,HSP104,SSA1启动子区的HSE。Hsf1以三聚体的形式和典型HSE结合,每个单体识别一个NGAAN序列。非典型的HSE的NGAAN序列间存在5 bp的间隔区,Hsf1不需要形成三聚体即可与之结合。在通常情况下,Hsf1处于本底水平的磷酸化状态,当细胞遇到热胁迫时,Hsf1被过度磷酸化,转化为活性构象,激活热休克蛋白的转录(图1) [12] [13] 。依赖于ATP的小分子热休克蛋白主要作为分子伴侣帮助蛋白折叠或者协助大分子热休克蛋白的作用。依赖于ATP的大分子热休克蛋白则起到多种保护、帮助折叠以及使凝集蛋白解聚[1] 、参与细胞壁重塑[14] 等作用,以减少高温对细胞的伤害。
70 kDa热休克蛋白Hsp70s是一类在进化中非常保守的蛋白家族,在热休克应答中起到帮助蛋白折叠,防止细胞凝集等功能。在酵母中的许多细胞器均发现了Hsp70s蛋白[15] ,细胞质中的Hsp70s包括Ssa,Ssb,Sse以及非典型的Ssz1(Stress Seventy sub-family A, B, C, E, Z)家族,最主要为Hsp70-Ssa1-4 [2] [16] 。超表达四种Ssa家族的蛋白均能够一定程度上提高酿酒酵母的耐热性[16] 。当细胞遭遇热胁迫,大量Hsp70s蛋白表达并与变性蛋白疏水区域结合,避免了变性蛋白的聚集,帮助初生肽链的重头折叠,在酿酒酵母中Hsp70s蛋白还辅助错误折叠蛋白的重新折叠,从而对酵母细胞起到保护作用。Hsp70s蛋白包括两个结构域:具有ATP催化活性的N-末端核苷酸结合结构域(NBD)以及C-末端底物结合结构域(SBD)。SBD又可分为两个次级结构域,一是底物催化结构域,二是C末端的可变结构域。对其作用机制的研究表明,Hsp70s的活性依赖于SBD和底物的瞬间结合,这种瞬间结合由N端结合核酸的状态调节。当ATP和NBD结合时,会引起Hsp70的构象变化,将SBD的底物结合口袋打开而处于和底物的高亲和状态;底物和SBD结合后,促使NBD的ATPase水解ATP,使SBD又恢复低亲和状态[17] 。
Hsp104属于Hsp100家族,在生物体中高度保守。Hsp104对酿酒酵母耐高温特性至关重要[18] 。同其他分子伴侣不同,Hsp104并不能防止热胁迫下蛋白的错误聚集,而是在Hsp70(Ssa1)的协助下使错误折叠的蛋白解凝集并重新折叠,恢复原始构象及功能[2] [19] [20] 。结构上,Hsp104含有两个ATP结合结构域(NBD1, NBD2),一个独特的中间螺旋结构域(coiled-coil middle domain),以及一个C末端结构域(C-terminal domain) [10] 。Hsp104需要与ATP结合并形成六聚体时才有活性[21] [22] 。在Hsp104不表达或Hsp104上ATP结合位点被突变掉之后,酿酒酵母中变性的蛋白无法重新折叠,使酵母耐热性丧失[23] 。目前的模型认为,Hsp104对蛋白进行解凝集前,Hsp70首先和凝集蛋白结合,松开凝集蛋白暴露在外的疏水区。之后,Hsp70通过蛋白质间的相互作用招募Hsp104。Hsp104和凝集蛋白的起始结合发生在NBD1核心中的Tyr残基,有时N-端其他区域也有参与但不是必须的。NBD1和NBD2核心中的Tyr残基抓住错误蛋白,在ATP水解的驱动下,穿过Hsp104孔道,完成蛋白的解凝集和重新折叠(图2) [10] [24] 。
小分子热休克蛋白(sHsp)是一类分子量在15 - 30 kDa的保守蛋白质,在细胞受到胁迫时增加蛋白的溶解度帮助蛋白质解折叠。酿酒酵母细胞质中包含两种sHsps,即Hsp26和Hsp42。Hsp42在通常状态下都具有分子伴侣的活性,而Hsp26则在热激条件下表达,这两种蛋白的存在能显著降低热胁迫条件下的蛋白凝集水平,提高Hsp104的解聚集效率[19] 。研究表明,sHsps能够形成多聚体并与有凝集倾向的蛋白形成复合物,从而实现对蛋白质的分选、集中[19] [25] ,继而由Hsp104和Hsp70完成重新折叠[26] [27] 。酵母蛋白聚集有两种位置倾向,近核质量控制(Juxta nuclear quality control compartment, JUNQ)和外围不可溶蛋白聚集(Insoluble protein deposit, IPOD)(图3)。Specht等的研究[28] 显示,HSP26缺失对热胁迫下细胞蛋白聚集状况没有显著影响,而HSP42缺失则导致外围不可溶蛋白聚集(IPOD)消失。进一步研究发现,Hsp42的N末端结构域(N-terminal domain, NTD)对于外围不可溶蛋白聚集是必须的,因而推测,Hsp42通过NTD完成对错误折叠蛋白的分选、定位。
此外,细胞器中的热休克蛋白也对酵母耐热性能有一定影响,例如Hsp78是酿酒酵母线粒体中的热休克蛋白,属于Hsp100s家族,对维持线粒体呼吸作用和线粒体基因组的完整性十分重要。Hsp78并不会对直接保护线粒体内的蛋白,在39℃时,基因HSP78的敲除对于酿酒酵母的生长没有太大的影响。但是,酿酒酵母线粒体中的蛋白合成是一个温度敏感的过程,在热胁迫下,酿酒酵母线粒体中的Hsp78与线粒体中的Hsp70s,包括Ssc1、Mdj1和Mge1,形成寡聚体,从而维持线粒体合成蛋白的能力[29] ,保持线粒体行使正常功能。
3. 泛素–蛋白酶体降解系统在增强酿酒酵母耐热性中的作用及机制
若高温造成的蛋白凝集不能被上述系统修复,细胞还可以通过泛素–蛋白酶体降解系统将错误折叠蛋白降解[30] ,从而减轻细胞压力并再利用降解产生的氨基酸原料[31] 。泛素–蛋白酶体降解系统由泛素降解体系和蛋白酶体两部分组成。酵母泛素降解体系由E1(ubiquitin-actvating enzyme, Uba1),E2 (ubiquitin-conjugating enzymes, Ubc),E3(ubiquitin ligases)组成。JUNQ的错误折叠蛋白(图3)通常首先在ATP参与下,E1和泛素形成高能硫酯键,之后,活化的泛素被移至某个E2上,最后由E3介导E2上的泛素转移到其招募的靶蛋白上,以羧基末端与底物赖氨酸的ε-氨基连接,完成识别降解的过程[32] 。被泛素标记的蛋白质再被蛋白酶体特异性地识别并被迅速降解。26S蛋白酶体由20S的具有催化活性的核心颗粒(CP)和19S的调节颗粒(RP)组成,19S RP能够识别被泛素化的蛋白质并与之泛素结合,另外19S RP上还有一个允许底物进入催化核心颗粒的孔洞,19S RP能将蛋白质解折叠后输送给20SCP,完成蛋白质的降解[33] 。
Rsp5是酿酒酵母胞内十分重要的E3泛素连接酶,在细胞各个组分中发挥细胞通讯、蛋白分选以及错误蛋白降解的作用[34] 。Shahsavarani等对一株能在41℃下生长的酿酒酵母菌株(Htg+)的经典遗传学研究发现,有6个基因(该课题组将它们命名为HTG1-HTG6)对酿酒酵母耐高温的性状至关重要。序列分析

Figure 2. The protein disaggregation functions of Hsp104 and Hsp70 complex
图2. Hsp104和Hsp70复合物对蛋白的解凝集作用
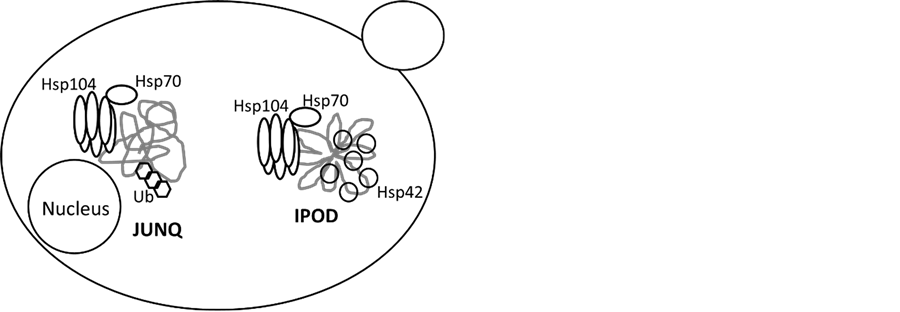
Figure 3. Two main kinds of protein deposition in S. cerevisiae
图3. 酿酒酵母中两种错误折叠蛋白聚集
显示,HTG6即为RSP5。过表达RSP5使该菌株中蛋白的泛素化水平明显上升,耐高温限度从41℃提高到43℃[35] 。此外,Rsp5在热胁迫环境下还能够帮助HSF1和MSN2/4的mRNA出核[36] ,对转录因子Hsf1和Msn2/4进行翻译后修饰,从而提高重要的胁迫应答转录因子Hsf1和Msn2/4的表达[37] ,进而提高热激蛋白的表达。另一方面,Shcherbik等发现在热胁迫条件下,RSP5突变的酿酒酵母菌株中18SrRNA,25SrRNA以及核糖体的含量呈下降趋势并且核糖体的沉降系数也相应减少,表明Rsp5对维持细胞质核糖体的完整性也具有重要作用[38] 。
4. 展望
热等胁迫条件下蛋白质的不稳定是影响细胞在该条件下生长代谢的关键因素之一。酿酒酵母体内有精细的蛋白质质量控制系统,通过小分子热激蛋白的协助折叠作用,Hsp104对凝集蛋白的解聚和再折叠,以及泛素介导的蛋白降解系统对无法修复的蛋白的分解再利用等一系列过程,在维持酿酒酵母热等胁迫条件下细胞稳定发挥着重要的作用。近来利用全转录工程方法对全局调控转录因子进行改造以获得耐胁迫酵母已初获成效[39] -[41] 。对这些非理性进化菌株的研究,以进一步揭示相关的耐热机制,并根据这些机制指导构建耐高温酿酒酵母,从而提高发酵速率,降低发酵成本是进一步努力的方向和研究热点。
基金项目
国家自然科学基金资助项目(J1103515),车用生物燃料技术国家重点实验室开放基金资助项目(2013004, KFKT2013002)。
参考文献 (References)
- [1] Verghese, J., Abrams, J., Wang, Y. and Morano, K.A. (2012) Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system. Microbiology and Molecular Biology Reviews, 76, 115-158.
- [2] Lee, J., Kim, J.-H., Biter, A.B., Sielaff, B., Lee, S. and Tsai, F.T. (2013) Heat shock protein (Hsp) 70 is an activator of the Hsp104 motor. Proceedings of the National Academy of Sciences, 110, 8513-8518.
- [3] Patriarca, E.J. and Maresca, B. (1990) Acquired thermotolerance following heat shock protein synthesis prevents impairment of mitochondrial ATPase activity at elevated temperatures in Saccharomyces cerevisiae. Experimental Cell Research, 190, 57-64.
- [4] Shapiro, R.S. and Cowen, L.E. (2012) Uncovering cellular circuitry controlling temperature-dependent fungal morphogenesis. Virulence, 3, 400-404.
- [5] Hou, J., Österlund, T., Liu, Z., Petranovic, D. and Nielsen, J. (2013) Heat shock response improves heterologous protein secretion in Saccharomyces cerevisiae. Applied Microbiology and Biotechnology, 97, 3559-3568.
- [6] Morano, K.A., Grant, CM. and Moye-Rowley, W.S. (2012) The response to heat shock and oxidative stress in Saccharomycescerevisiae. Genetics, 190, 1157-1195.
- [7] Suutari, M., Liukkonen, K. and Laakso, S. (1990) Temperature adaptation in yeasts: The role of fatty acids. Journal of General Microbiology, 136, 1469-1474.
- [8] Swan, T.M. and Watson, K. (1998) Stress tolerance in a yeast sterol auxotroph: role of ergosterol, heat shock proteins and trehalose. FEMS Microbiology Letters, 169, 191-197.
- [9] Simola, M., Hänninen, A.L., Stranius, S.M. and Makarow, M. (2000) Trehalose is required for conformational repair of heat‐denatured proteins in the yeast endoplasmic reticulum but not for maintenance of membrane traffic functions after severe heat stress. Molecular Microbiology, 37, 42-53.
- [10] Doyle, S.M., Genest, O. and Wickner, S. (2013) Protein rescue from aggregates by powerful molecular chaperone machines. Nature Reviews Molecular Cell Biology, 14, 617-629.
- [11] Jarolim, S., Ayer, A., Pillay, B., Gee, A.C., Phrakaysone, A., Perrone, G.G., Breitenbach, M. and Dawes, I.W. (2013) Saccharomyces cerevisiae genes involved in survival of heat shock. G3: Genes| Genomes| Genetics, 3, 2321-2333.
- [12] Hashikawa, N., Mizukami, Y., Imazu, H. and Sakurai, H. (2006) Mutated yeast heat shock transcription factor activates transcription independently of hyperphosphorylation. Journal of Biological Chemistry, 281, 3936-3942.
- [13] Yamamoto, A., Mizukami, Y. and Sakurai, H. (2005) Identification of a novel class of target genes and a novel type of binding sequence of heat shock transcription factor in Saccharomyces cerevisiae. Journal of Biological Chemistry, 280, 11911-11919.
- [14] Wanless, A.G., Lin, Y. and Weiss, E.L. (2014) Cell morphogenesis proteins are translationally controlled through UTRs by the Ndr/LATS target Ssd1. PLoS ONE, 9, e85212.
- [15] Craig, E.A., Gambill, B.D. and Nelson, R.J. (1993) Heat shock proteins: Molecular chaperones of protein biogenesis. Microbiological Reviews, 57, 402-414.
- [16] Hasin, N., Cusack, S.A., Ali, S.S., Fitzpatrick, D.A. and Jones, G.W. (2014) Global transcript and phenotypic analysis of yeast cells expressing Ssa1, Ssa2, Ssa3 or Ssa4 as sole source of cytosolic Hsp70-Ssa chaperone activity. BMC Genomics, 15, 194.
- [17] Vogel, M., Bukau, B. and Mayer, M.P. (2006) Allosteric regulation of Hsp70 chaperones by a proline switch. Molecular Cell, 21, 359-367.
- [18] Nakamoto, H., Fujita, K., Ohtaki, A., Watanabe, S., Narumi, S., Maruyama, T., Suenaga, E., Misono, T.S., Kumar, P.K., Goloubinoff, P. and Yoshikawa, H. (2014) Physical interaction between bacterial heat shock protein (Hsp) 90 and Hsp70 chaperones mediates their cooperative action to refold denatured proteins. Journal of Biological Chemistry, 289, 6110-6119.
- [19] Cashikar, A.G., Duennwald, M. and Lindquist, S.L. (2005) A chaperone pathway in protein disaggregation HSP26 alters the nature of protein aggregates to facilitate reactivation by HSP104. Journal of Biological Chemistry, 280, 23869-23875.
- [20] Tessarz, P., Mogk, A. and Bukau, B. (2008) Substrate threading through the central pore of the Hsp104 chaperone as a common mechanism for protein disaggregation and prion propagation. Molecular Microbiology, 68, 87-97.
- [21] Schirmer, E.C., Queitsch, C., Kowal, A.S., Parsell, D.A. and Lindquist, S. (1998) The ATPase activity of Hsp104, effects of environmental conditions and mutations. Journal of Biological Chemistry, 273, 15546-15552.
- [22] Schirmer, E.C., Ware, D.M., Queitsch, C., Kowal, A.S. and Lindquist, S.L. (2001) Subunit interactions influence the biochemical and biological properties of Hsp104. Proceedings of the National Academy of Sciences of the United States of America, 98, 914-919.
- [23] Hänninen, A.L., Simola, M., Saris, N. and Makarow, M. (1999) The cytoplasmic chaperone Hsp104 is required for conformational repair of heat-denatured proteins in the yeast endoplasmic reticulum. Molecular Biology of the Cell, 10, 3623-3632.
- [24] Wendler, P., Shorter, J., Snead, D., Plisson, C., Clare, D.K., Lindquist, S. and Saibil, H.R. (2009) Motor mechanism for protein threading through Hsp104. Molecular Cell, 34, 81-92.
- [25] Haslbeck, M., Braun, N., Stromer, T., Richter, B., Model, N., Weinkauf, S. and Buchner, J. (2004) Hsp42 is the general small heat shock protein in the cytosol of Saccharomyces cerevisiae. The EMBO Journal, 23, 638-649.
- [26] Ehrnsperger, M., Gräber, S., Gaestel, M. and Buchner, J. (1997) Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. The EMBO Journal, 16, 221-229.
- [27] Haslbeck, M. (2002) sHsps and their role in the chaperone network. Cellular and Molecular Life Sciences CMLS, 59, 1649-1657.
- [28] Specht, S., Miller, S.B., Mogk, A. and Bukau, B. (2011) Hsp42 is required for sequestration of protein aggregates into deposition sites in Saccharomyces cerevisiae. The Journal of Cell Biology, 195, 617-629.
- [29] Krzewska, J., Langer, T. and Liberek, K. (2001) Mitochondrial Hsp78, a member of the Clp/Hsp100 family in Saccharomyces cerevisiae, cooperates with Hsp70 in protein refolding. FEBS Letters, 489, 92-96.
- [30] Zattas, D. and Hochstrasser, M. (2014) Ubiquitin-dependent protein degradation at the yeast endoplasmic reticulum and nuclear envelope. Critical Reviews in Biochemistry and Molecular Biology, 1-17.
- [31] Theodoraki, M.A., Nillegoda, N.B., Saini, J. and Caplan, A.J. (2012) A network of ubiquitin ligases is important for the dynamics of misfolded protein aggregates in yeast. Journal of Biological Chemistry, 287, 23911-23922.
- [32] Amm, I., Sommer, T. and Wolf, D.H. (2014) Protein quality control and elimination of protein waste: The role of the ubiquitin-proteasome system. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1843, 182-196.
- [33] Glickman, M.H. and Ciechanover, A. (2002) The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiological Reviews, 82, 373-428.
- [34] Krsmanović, T. and Kölling, R. (2004) The HECT E3 ubiquitin ligase Rsp5 is important for ubiquitin homeostasis in yeast. FEBS Letters, 577, 215-219.
- [35] Shahsavarani, H., Sugiyama, M., Kaneko, Y., Chuenchit, B. and Harashima, S. (2012) Superior thermotolerance of Saccharomyces cerevisiae for efficient bioethanol fermentation can be achieved by overexpression of RSP5 ubiquitin ligase. Biotechnology Advances, 30, 1289-1300.
- [36] Haitani, Y. and Takagi, H. (2008) Rsp5 is required for the nuclear export of mRNA of HSF1 and MSN2/4 under stress conditions in Saccharomyces cerevisiae. Genes to Cells, 13, 105-116.
- [37] Haitani, Y., Shimoi, H. and Takagi, H. (2006) Rsp5 regulates expression of stress proteins via post-translational modification of Hsf1 and Msn4 in Saccharomyces cerevisiae. FEBS Letters, 580, 3433-3438.
- [38] Shcherbik, N. and Pestov, D.G. (2011) The ubiquitin ligase Rsp5 is required for ribosome stability in Saccharomyces cerevisiae. RNA, 17, 1422-1428.
- [39] Alper, H., Moxley, J., Nevoigt, E., Fink, G.R. and Stephanopoulos, G. (2006) Engineering yeast transcription machinery for improved ethanol tolerance and production. Science, 314, 1565-1568.
- [40] Morano, K.A., Grant, C.M. and Moye-Rowley, W.S. (2012) The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics, 190, 1157-1195.
- [41] Noguchi, C., Watanabe, D., Zhou, Y., Akao, T. and Shimoi, H. (2012) Association of constitutive hyperphosphorylation of Hsf1p with a defective ethanol stress response in Saccharomyces cerevisiae sake yeast strains. Applied and Environmental Microbiology, 78, 385-392.

NOTES
*通讯作者。