World Journal of Cancer Research
Vol.
14
No.
02
(
2024
), Article ID:
85062
,
11
pages
10.12677/wjcr.2024.142014
NDRG1基因在亚洲人与高加索人大肠癌 细胞株中恶性行为的比较
朱宇1,2*,王开昕3*,华海蓉1,王芳1,叶宏1#
1昆明医科大学基础医学院病理学与病理生理学系,云南 昆明
2河南应用技术职业学院护理学院,河南 郑州
3华中科技大学协和深圳医院病理科,深圳
收稿日期:2024年3月25日;录用日期:2024年4月15日;发布日期:2024年4月23日

摘要
目的:NDRG1在大肠癌中的作用至今仍存在争议,是否与种族有关未见报导,本研究旨在分析NDRG1基因在亚洲人与高加索人大肠癌细胞株中的作用。方法:利用细胞计数法测定细胞生长速度;通过RT-qPCR在mRNA水平比较NDRG1在亚洲人和高加索人大肠癌细胞中的表达;24-Transwell法检测亚洲人和高加索人大肠癌细胞侵袭和迁移能力;流式细胞术检测亚洲人和高加索人大肠癌细胞的大小、细胞周期、DNA含量及NDRG1蛋白含量等;裸鼠背侧皮下移植检测细胞的增殖速度。结果:1) Caco2细胞的体外增殖速度比SNU-C1细胞快,且差异有统计学意义(P < 0.05)。2) 两株细胞所扩增出来的序列均与NDRG1一致,但Caco2与SNU-C1的NDRG1的mRNA水平比值为0.4061,SNU-C1的NDRG1的mRNA水平为Caco2的2.46倍。3) Caco2细胞侵袭能力在24 h、48 h和72 h各时间点均高于SNU-C1,但24 h时间点两组细胞之间差异无统计学意义(P > 0.05),而48 h和72 h两个时间点两组细胞之间差异有统计学意义(P = 0.006, P = 0.000)。4) Caco2组除24 h组迁移能力比SNU-C1组低之外,48 h和72 h两个时间点迁移能力均高于SNU-C1组,且两组细胞之间差异有统计学意义(P = 0.038, P = 0.045, P = 0.012)。5) 经FSC分析发现Caco2平均体积明显大于SNU-C1,从SSC分析得到SNU-C1细胞表面的皱折度、细胞内亚细胞器、颗粒的数目等均大于Caco2。6) 两种细胞的细胞周期各间期之间差异无统计学意义(P > 0.05)。7) 两株细胞NDRG1蛋白含量为Caco2细胞平均值36.3,SNU-C1细胞平均值86.5。8) 两株细胞NDRG1的DNA含量为Caco2细胞平均值45,SNU-C1细胞平均值50.2。9) 裸鼠背侧皮下移植后Caco2细胞增殖速度快于SNU-C1细胞的,但差异无统计学意义(P > 0.05)。结论:在高加索人大肠癌细胞株Caco2与亚洲人大肠癌细胞株SNU-C1中NDRG1的mRNA水平存在差异,且在体外侵袭与迁移中的作用也存在差异。
关键词
大肠癌,Caco2,SNU-C1,NDRG1,侵袭,迁移

A Comparison of the Malignant Behavior of Asian and Caucasian Colorectal Cancer Cell Lines in Vitro and in Vivo
Yu Zhu1,2*, Kaixin Wang3*, Hairong Hua1, Fang Wang1, Hong Ye1#
1Department of Pathology and Pathophysiology, School of Basic Medicine, Kunming Medical University, Kunming Yunnan
2School of Nursing, Henan Vocational College of Applied Technology, Zhengzhou Henan
3Department of Pathology, Huazhong University of Science and Technology Union Shenzhen Hospital, Shenzhen
Received: Mar. 25th, 2024; accepted: Apr. 15th, 2024; published: Apr. 23rd, 2024

ABSTRACT
Objective: The role of NDRG1 in colorectal cancer is still controversial, and whether it is related to ethnicity has not been reported. This study aims to analyze the role of NDRG1 gene in Asian and Caucasian colorectal cancer cell lines. Methods: The cell growth rate was measured by cell counting method. The expression of NDRG1 in Asian and Caucasian colorectal cancer cells was compared by Real-time Quantitative PCR. The difference of invasion and migration ability between Asian and Caucasian colorectal cancer cells was detected by 24-Transwell method. The differences of cell size, cell cycle, DNA content and NDRG1 protein content in Asian and Caucasian colorectal cancer cells were measured by flow cytometry. The cell proliferation rate was detected by dorsal subcutaneous transplantation in nude mice. Results: 1) The proliferation rate of Caco2 cells was faster than that of SNU-C1 cells (P < 0.05) in vitro. 2) The NDRG1 sequences detected by PCR were consistent in Caco2 and SNU-C1 cells. The mRNA expressions of Caco2 and SNU-C1 were detected by qPCR. The ratio of NDRG1 mRNA expression of Caco2 cells to that of SNU-C1 cells was 0.4061. The NDRG1 mRNA expression of SNU-C1 cells was 2.46 times of that of Caco2 cells. 3) Transwell method was used to detect the invasion and migration ability of the two cell lines. The invasion ability of Caco2 cells was higher than that of SNU-C1 cells at 24 h, 48 h and 72 h, However, there wasn't statistically significant at 24 h (P = 0.08). There were significant differences between the two groups at 48 h and 72 h (P = 0.006, P = 0.000). 4) The migration of Caco2 cells was lower than SNU-C1 cells at 24 h (P = 0.038) but was significantly higher than that of SNU-C1 at 48 h and 72 h (P = 0.045, P = 0.012). 5) Two cell sizes were measured by flow cytometry, and FSC analysis found that the average size of Caco2 was bigger than that of SNU-C1. SSC analysis found that SNU-C1 cell surface wrinkles, intracellular subcellular organelles, the number of particles were greater than Caco2. 6) The flow cytometry (FCM) showed no statistically significances in cell cycles (G1 interval P = 0.546, S interval P = 0.119, G2 interval P = 0.071). 7) The MEAN value of NDRG1 protein in Caco2 cells were 36.3 and 86.5 in SNU-C1 cells respectively. 8) The MEAN values of NDRG1 DNA contents in Caco2 cells were 45 and 50.2 in SNU-C1 cells respectively. 9) After dorsal subcutaneous transplantation in nude mice, the proliferation rate of Caco2 cells was faster than that of SNU-C1 cells, but the difference was not statistically significant (P > 0.05). Conclusions: There were differences in the mRNA levels of NDRG1 in Caucasian colorectal cancer cell line Caco2 and Asian colorectal cancer cell line SNU-C1, and there were also differences in the role of NDRG1 in vitro invasion and migration.
Keywords:Colorectal Cancer, Caco2, SNU-C1, NDRG1, Invasion, Migration

Copyright © 2024 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
结直肠癌(Colorectal cancer, CRC)为常见恶性肿瘤之一,世界上癌症相关死亡病例中CRC位列第三 [1] 。基于国家死亡率监测系统的多源数据,中国人群结直肠癌死亡人数估计从2005年的11.141万人增加到2020年的17.802万人 [2] 。
较多研究表明NDRG1是涉及肿瘤发生和发展的多功能蛋白,其功能在不同的肿瘤类型中亦不同 [3] ,譬如在乳腺癌 [4] 、前列腺癌 [5] 、胰腺癌 [6] [7] 、宫内膜癌 [8] 等中,NDRG1起到了抑制肿瘤转移的作用。然而,Cheng J等 [9] 通过143例肝移植后的肝细胞癌患者免疫组化染色显示,与NDRG1阴性表达相比,NDRG1阳性表达者预后较差,无论是无病生存期还是总生存期都较短(P < 0.001),多因素分析证实NDRG1是独立的预后价值(P < 0.001),同时在体外实验中,用对抗NDRG1的小干扰RNA作用于肝细胞癌的细胞可显著抑制其增殖、集落形成、侵袭和迁移能力;Yin X等 [10] 利用癌症基因组图谱、DNA甲基化交互式可视化数据库和单样本基因集富集分析等得出NDRG1和NDRG3高表达与生存预后差相关(P < 0.05);Villodre ES等 [11] 将侵袭性乳腺癌细胞注射到SCID/Beige小鼠尾静脉以研究体内NDRG1在肿瘤生长和脑转移中的作用,同时利用免疫组化法检测216位伴有淋巴结转移的乳腺癌患者NDRG1蛋白的表达得出:在小鼠中,NDRG1高表达的细胞产生更普遍的脑转移和更低的生存率等,体外实验显示在侵袭性乳腺癌细胞系中,沉默NDRG1导致迁移、侵袭和肿瘤启动细胞亚群减少,在异种移植物模型中,耗尽NDRG1抑制原发肿瘤生长和脑转移,在乳腺癌患者中,NDRG1高表达与较短的总生存期(风险比[HR] 2.27,95%可信区间[95% CI] 1.20至4.29,P = 0.009)和乳腺癌特异性生存期(HR 2.19, 95% CI 1.07至4.48,P = 0.03)相关,这表明此蛋白具有多效性。
NDRG1在大肠癌中的作用至今仍存在争议。Shah等 [12] 研究提示在美国大肠癌患者中,NDRG1的表达在原发性大肠癌组织中总是少于相邻的正常组织。Wang等 [13] 在中国大肠癌样本中的免组结果则与之相反;Koshiji等 [14] 在日本大肠癌患者,有淋巴管侵袭和门静脉侵袭、肿瘤侵袭深度深和Duke分期晚期时NDRG1蛋白表达高;故NDRG1在大肠癌中的作用或许与患者种族背景有关,关于此未见进一步报道。因此,本实验研究旨在了解NDRG1是否可成为不同种族背景大肠癌患者的生物学标记物,为其能否作为诱导分化治疗的靶点提供一定的实验依据。
2. 材料与方法
2.1. 材料
2.1.1. 细胞株
高加索人结肠癌细胞株Caco2,购自中国科学院昆明动物所昆明细胞库,此细胞株来源于72岁男性高加索人大肠腺癌。亚洲人结肠癌细胞株SNU-C1,购自ATCC (American Tissue Culture Colection,美国组织培养库),此细胞株来源于71岁男性亚洲人大肠腺癌。
2.1.2. 主要试剂
FBS、DMEM和1640购自GBICO;Matrigel购自BD;SYBR Green和CDNA反转录试剂盒购自Takara;TRIzol购自Invitrogen;抗体购自Alexa Fluor (Thermo Fisher);Triton购自SIGMA;引物及GADPH购自上海捷瑞生物等。
2.2. 方法
2.2.1. 细胞培养及传代
Caco2贴壁细胞及SNU-C1悬浮细胞的复苏、培养和传代按常规操作进行,为1:2~1:3传代。
2.2.2. NDRG1的检测
RNA的提取、浓度和纯度的测定、cDNA反转录和RT-qPCR扩增检测NDRG1均严格按照操作规程进行。并利用Primer软件设计NDRG1的引物:
3’端引物:ATGTCTCGGGAGATGCAGGATGTAG
5’端引物:CTAGCAGGAGACCTCCATGGACTTG
2.2.3. 侵袭及迁移实验
分别收集Caco2和SNU-C1细胞。Caco2贴壁细胞倒出完全培养基,PBS洗一遍;SNU-C1悬浮细胞收集于离心管中,1000 rpm/min,离心5 min,弃上清,PBS洗一次,弃上清。之后,分别加入不含FBS的培养基,放入37℃恒温培养箱使细胞饥饿12~24 h。用不含FBS的培养基稀释Matrigel,稀释倍数为35倍,将稀释好的Matrigel均匀铺在Transwell小室的上室底部,24孔Transwell小室每孔铺胶100 μL,铺胶完成后,将Transwell小室置于37℃恒温培养箱孵育1 h,收集饥饿后的细胞用无FBS的培养基稀释至10 × 105吹打均匀,上室加入100 μL细胞悬液,下室加含20%FBS的完全培养基,再将上室慢慢放入下室中,后将小室放入37℃恒温培养箱中,分别在培养24 h、48 h、72 h时取出测穿出的细胞数。迁移实验不铺Matrigel,余同侵袭实验。每个实验均重复3次,结果一致。
Caco2细胞穿膜细胞数的计算方法为每个小室随机取上中下左右5个视野计数,每个小室底面的面积为0.33 cm2,所用显微镜目镜倍数为10×,视场为25 mm,物镜倍数为20×,利用公式计算出半径是25/20/2 = 0.625 mm,用200倍视野来观察,每个视野的面积为1.227 mm2,已知小室底面面积和每个视野的面积,算出整个Transwell小室底部面积约为一个视野面积的26.89倍,则一个小室细胞数量应为看到的5个视野的细胞平均数乘以倍数。
SNU-C1细胞穿膜细胞数的计数方法为小心吸取Transwell下室液体到流式EP管中,放入流式细胞仪,计穿入下室中的细胞数,计算平均值。
2.2.4. 流式细胞术测细胞周期等
严格按操作规程进行。
2.2.5. 裸鼠背侧皮下移植比较两株细胞的体内增殖速度
32只裸鼠,随机分为2组(Caco2组和SNU-C1组),行背侧皮下移植,接种细胞数为5 × 106/100μL,每5日用游标卡尺测肿瘤大小。
2.2.6. 统计学处理
应用SPSS 13.0统计软件包对所得数据进行统计分析。各组间比较采用单因素方差分析(球对称检验和选择校正);多重比较当方差具有齐性时采用LSD法、Duncan法,当方差不具有齐性时采用Tamhane法、Games-Howell法;皮下移植细胞体内增殖速度的比较采用T检验;P < 0.05为差异有统计学意义。
3 结果
3.1. Caco2细胞及SNU-C1细胞形态
Caco2细胞系来源于72岁高加索男性患者,细胞呈多边形极不规则贴壁生长,细胞生长速度快、密度大,细胞与细胞之间生长紧密,核分裂像多,传代后72 h可见细胞已贴壁,且生长旺盛(图1(A))。细胞生长至144 h,可见细胞生长极为旺盛,彼此间隙极小,细胞生长面积已达到90%左右(图1(B))。
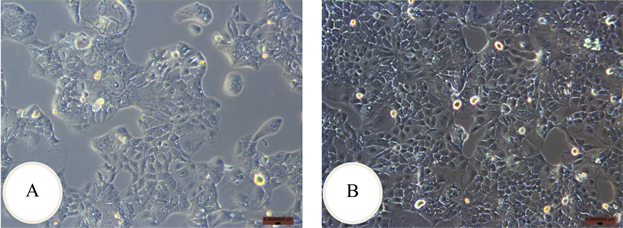
Figure 1. Morphology of Caco2 cell. A: cultured at 72 h; B: cultured at 144 h
图1. Caco2细胞的形态。A:培养72 h时;B:培养144 h时
SNU-C1细胞系来自于71岁亚洲男性患者,镜下可见传代后72 h时细胞悬浮抱团生长,细胞分裂像多,生长旺盛,且容易产生碎片(图2(A))。细胞长至144 h,细胞生长达到85%,镜下可见细胞成团生长且生长旺盛,分裂像多,碎片状物质也多(图2(B))。
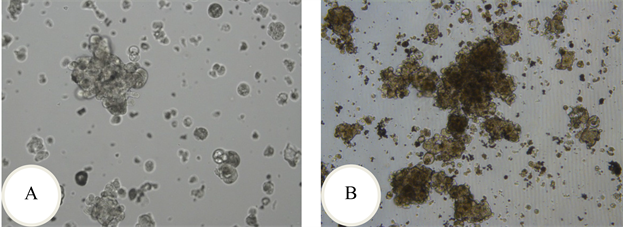
Figure 2. Morphology of SNU-C1 cell. A: cultured at 72 h; B: cultured at 144 h
图2. SNU-C1细胞的形态。A:培养72 h时;B:培养144 h时
3.2. Caco2及SNU-C1体外增殖速度比较
从Caco2与SNU-C1细胞各时间点细胞数来看,Caco2细胞的增殖速度比SNU-C1细胞快,且差异有统计学意义(P < 0.05),见表1。
Table 1. Number of Caco2 cells and SNU-C1 cells at each time point ( )
表1. SNU-C1细胞与Caco2细胞各时间点细胞数( )
检验水准α = 0.05。
3.3. RT-qPCR测序结果
利用PCR的方法分别将两种细胞扩增出目的基因,之后割胶回收,送昆明硕擎生物公司测序。结果分别于http://www.ncbi.nlm.nih.gov/nuccore/NM_001135242.1公布的NDRG1序列进行比较,运用DNAMAN软件对比,得出:两株细胞所扩增出来的序列均与NDRG1一致。
3.4. Caco2细胞和SNU-C1细胞中NDRG1的mRNA水平检测结果
利用qPCR法对Caco2与SNU-C1两株细胞NDRG1的mRNA水平进行检测,得出Caco2与SNU-C1的NDRG1的mRNA水平比值为0.4061,SNU-C1的NDRG1的mRNA水平为Caco2的2.46倍,见表2。
Table 2. Detection of NDRG1 mRNA in Caco2 and SNU-C1 cells
表2. Caco2与SNU-C1细胞NDRG1 mRNA检测结果
3.5. Transwell法检测Caco2与SNU-C1细胞的侵袭和迁移能力
侵袭实验结果用重复测量资料的方差分析:各时间点之间差异具有统计学意义P < 0.0005;时间和组间有交互作用P < 0.0005;用Bonferroni法作时间点间比较得出:24 h两组之间差异无统计学意义P = 0.083,48 h和72 h两组之间差异有统计学意义(P = 0.006, P = 0.000) (表3和图3(A))。
Table 3. Comparison of Caco2 and SNU-C1 cell invasion assay ( )
表3. Caco2与SNU-C1细胞侵袭实验比较( )
检验水准α = 0.05。
迁移实验结果用重复测量资料的方差分析:各时间点之间差异有统计学意义P < 0.0005;时间和组间有交互作用P = 0.006;用Bonferroni法作时间点间比较得出:24 h、48 h和72 h各时间点两组细胞之间差异有统计学意义(P = 0.038, P = 0.045, P = 0.012) (表4和图3(B))。
Table 4. Comparison of Caco2 and SNU-C1 cell migration assay ( )
表4. Caco2与SNU-C1细胞迁移实验比较( )
检验水准α = 0.05。
从上表看出,迁移能力在24 h时以SNU-C1的为高,但在48 h和72 h均以Caco2的为高,且均有统计学意义(P < 0.05)。

Figure 3. Caco2 cell invasion and migration experiment at 48 h. A: invasion experiment; B: migration experiment
图3. Caco2细胞侵袭和迁移实验第48 h时。A:侵袭实验;B:迁移实验
3.6. 细胞大小等的检测
将两组细胞固定后分别用流式细胞仪检测,经FSC分析发现Caco2细胞的平均体积明显大于SNU-C1细胞的;从SSC分析得出SNU-C1细胞表面的皱折度、细胞内亚细胞器、颗粒的数目等均大于Caco2细胞的。
3.7. 细胞周期的检测
两种细胞各间期之间差异无统计学意义(G1间期P = 0.546,S间期P = 0.119,G2间期P = 0.071),见表5。
Table 5. Comparison of Caco2 and SNU-C1 cell cycle
表5. Caco2与SNU-C1细胞周期的比较
检验水准α = 0.05。
3.8. DNA水平的检测
Caco2细胞MEAN值为45,SNU-C1细胞MEAN值为50.2。
3.9. 细胞蛋白的检测
Caco2细胞平均值为36.3,SNU-C1细胞平均值为86.5。
3.10. 裸鼠背侧皮下移植两株瘤细胞增殖速度的检测结果
裸鼠背侧皮下移植后,成瘤鼠Caco2组11只,SNU-C1组10只。从Caco2与SNU-C1细胞皮下移植后各时间点肿瘤的大小来看,Caco2细胞的体内增殖速度除了移植后第40天和第45天这两个时间点外均比SNU-C1细胞的快,除了移植后第15天(P = 0.027)和第20天(P = 0.037)差异有统计学意义外,其余时间点差异均无统计学意义(P > 0.05) (图4(A)、图4(B),图5(A)、图5(B)和表6)。
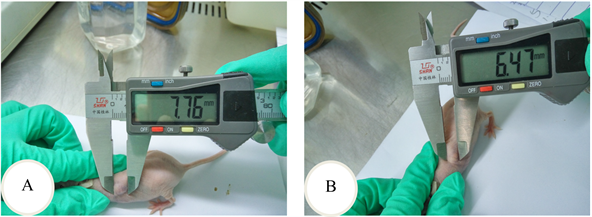
Figure 4. Tumor area was measured with vernier caliper. A: The long axis of tumor; B: The short axis of tumor
图4. 游标卡尺测肿瘤面积。A:肿瘤长径;B:肿瘤短径
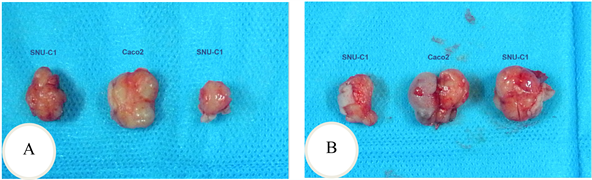
Figure 5. The implant tumor was dissected from the tumor bearing experiment. A: The 15th of the transplant; B: The 20th of the transplant
图5. 荷瘤实验剖出的种植瘤。A:移植第15天;B:移植第20天
Table 6. Areas of transplantation tumor after Caco2 and SNU-C1 cells subcutaneous transplantation in nude mice at respective point ( )
表6. Caco2与SNU-C1细胞裸鼠皮下移植后各时间点移植瘤的面积( )
检验水准α = 0.05。
4. 讨论
结直肠癌是消化道常见恶性肿瘤之一,其发展过程涉及多种驱动因素和生物学转变。据世界卫生组织国际癌症研究机构(IARC) (International Agency for Research on Cancer)发布的资料显示,至2020年,在世界范围内大肠癌新发病例数和死亡病例数均居各种肿瘤的第五位;男性发病数和死亡数均居第三位,女性发病数居第二位、死亡数居第三位 [1] 。中国国家癌症中心2022年2月发布的资料显示,大肠癌新发病例数居各种肿瘤的第二(位),死亡病例数居第五位;男性发病数居第三位、死亡数居第五位,女性发病数居第三位,死亡数居第二位 [15] 。
van Belzen等 [16] 利用改良的差异显示法从HT-29 D4结肠癌细胞系中克隆的cDNA,人类基因组组织基因命名委员会将其正式命名为N-myc down-stream regulated gene 1(NDRG1),其位于人染色体8q24.2,长约60 kb,包含16个外显子和15个内含子。
NDRG1与细胞增殖、分化 [17] 、成人髓鞘化少突胶质细胞的形成 [18] 、血管生成 [19] 、缺氧感知 [20] 、卵泡发育 [21] 、凋亡、肿瘤发生、转移等许多的生理、病理状态有关 [22] [23] ,并可增强化疗药物(如西妥昔单抗)在结直肠癌细胞系中的敏感性 [24] ;在前列腺癌 [25] [26] 、乳腺癌 [27] [28] 、大肠癌 [12] 和黑色素瘤 [29] 病人中,NDRG1的低表达与高癌症分期和低生存率呈正相关关系。季等 [30] 的研究结果推测ERK通路活化后极可能是通过调低NDRG1的基因表达来起到促进侵袭转移、减低凋亡、抑制分化等一系列恶性表型;Chen等 [31] 的研究结果为上调NDRG1可通过靶向Wnt/β-catenin通路抑制结肠癌细胞增殖和EMT过程;Wei等 [32] 认为闭合蛋白-2 (Claudin-2)的缺失显著促进NDRG1的转录,导致CRC生长和转移在体内外终止;而小细胞肺癌患者NDRG1表达与生存和预后呈负相关 [33] ,Wang [13] 等的免疫组化结果则为NDRG1表达增高促进大肠癌转移;故此NDRG1在肿瘤中的作用尚不确定。
由于Shah等 [12] ,Wang等 [13] 和Koshiji等 [14] 的研究结果提示了NDRG1在大肠癌中的作用可能与患者种族背景有关,所以,本实验首先确定所用细胞株为Caco2 (来自72岁男性高加索人大肠腺癌)和SNU-C1 (来自71男性亚洲人大肠腺癌);结果显示SNU-C1细胞中NDRG1蛋白表达活跃、DNA含量更高,SNU-C1细胞侵袭能力在24 h、48 h和72 h各时间点均低于Caco2,但24 h时间点两组细胞之间差异无统计学意义,而48 h和72 h两个时间点两组细胞之间差异有统计学意义;SNU-C1组除24 h组迁移能力比Caco2组高之外,48 h和72 h两个时间点迁移能力均低于Caco2组,且两组细胞之间差异有统计学意义,提示同等条件下SNU-C1细胞的侵袭和迁移能力均弱于Caco2细胞。裸鼠背侧皮下移植结果显示两株细胞的体内增殖速度除移植后第40天和45天外,其余时间均为SNU-C1细胞株的慢,与体外实验的结果相呼应。
综上,本次实验结果不能说明NDRG1在大肠肿瘤侵袭转移中的作用与患者种族大背景有关,究其原因可能是实验中所用细胞株数量过少(仅用了各1株细胞)所致。
未来的研究方向为增加实验所用细胞株的数量,尽可能把现有大肠癌细胞株全部用来进行体外和体内检测,以获得更有力的实验数据,并利用通道抑制剂来观察NDRG1在亚洲人与高加索人大肠癌中是否通过同一信号通路发挥其作用,为临床治疗大肠癌方法的选择提供一定的实验依据。
致谢
感谢中国科学院动物研究所比较基因组学研究组为本研究提供的实验场地、技术指导、实验测试和实验试剂。感谢中国科学院动物研究所细胞库为本研究提供高加索人大肠癌细胞株Caco2。
基金项目
国家自然科学基金(81260361);云南省科技厅–昆明医科大学应用基础研究联合专项资金(202101AY070001-048);昆明医科大学硕士研究生创新基金项目(2016S49)。
文章引用
朱 宇,王开昕,华海蓉,王 芳,叶 宏. NDRG1基因在亚洲人与高加索人大肠癌细胞株中恶性行为的比较
A Comparison of the Malignant Behavior of Asian and Caucasian Colorectal Cancer Cell Lines in Vitro and in Vivo[J]. 世界肿瘤研究, 2024, 14(02): 97-107. https://doi.org/10.12677/wjcr.2024.142014
参考文献
- 1. Sung, H., Ferlay, J., Siegel, R.L., et al. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians, 71, 209-249. https://doi.org/10.3322/caac.21660
- 2. 时秀茹, 魏可, 吴雨伦, 等. MiR-372-5p促进结直肠癌细胞的转移: 通过靶向PTEN调控PI3K/AKT/CXCL12信号通路[J]. 南方医科大学学报, 2022, 42(8): 1191-1197.
- 3. Deng, Z. and Richardson, D.R. (2023) The Myc Family and the Metastasis Suppressor NDRG1: Targeting Key Molecular Interactions with Innovative Therapeutics. Pharmacological Reviews, 75, 1007-1035. https://doi.org/10.1124/pharmrev.122.000795
- 4. Sun, J., Zhang, D.H., Bae, D.H., et al. (2013) Metastasis Suppressor, NDRG1, Mediates Its Activity through Signaling Pathways and Molecular Motors. Carcinogenesis, 34, 1943-1954. https://doi.org/10.1093/carcin/bgt163
- 5. Liu, R.L., Li, J., Teng, Z.G., et al. (2013) Overexpressed MicroRNA-182 Promotes Proliferation and Invasion in Prostate Cancer PC-3 Cells by Down-Regulating N-Myc Downstream Regulated Gene 1 (NDRG1). PLOS ONE, 8, E68982. https://doi.org/10.1371/journal.pone.0068982
- 6. Maruyama, Y., Ono, M., Kawahara, A., et al. (2006) Tumor Growth Suppression in Pancreatic Cancer by a Putative Metastasis Suppressor Gene Cap43/NDRG1/Drg-1 through Modulation of Angiogenesis. Cancer Research, 66, 6233-6242. https://doi.org/10.1158/0008-5472.CAN-06-0183
- 7. Geleta, B., Tout, F.S., Lim, S.C., et al. (2022) Targeting Wnt/Tenascin C-Mediated Cross Talk between Pancreatic Cancer Cells and Stellate Cells via Activation of the Metastasis Suppressor NDRG1. Journal of Biological Chemistry, 298, Article ID: 101608. https://doi.org/10.1016/j.jbc.2022.101608
- 8. Lv, X.H., Chen, J.W., Zhao, G., Feng, Z.Z., Yang, D.H., Sun, W.W., Fan, J.S. and Zhu, G.H. (2012) N-Myc Downstream-Regulated Gene 1/Cap43 May Function as Tumor Suppressor in Endometrial Cancer. Journal of Cancer Research and Clinical Oncology, 138, 1703-1715. https://doi.org/10.1007/s00432-012-1249-4
- 9. Cheng, J., Xie, H.Y., Xu, X., et al. (2011) NDRG1 as a Biomarker for Metastasis, Recurrence and of Poor Prognosis in Hepatocellular Carcinoma. Cancer Letters, 310, 35-45. https://doi.org/10.1016/j.canlet.2011.06.001
- 10. Yin, X., Yu, H., He, X.K., et al. (2022) Prognostic and Biological Role of the N-Myc Downstream-Regulated Gene Family in Hepatocellular Carcinoma. World Journal of Clinical Cases, 10, 2072-2086. https://doi.org/10.12998/wjcc.v10.i7.2072
- 11. Villodre, E.S., Hu, X.D., Eckhardt, B.L., et al. (2022) NDRG1 in Aggressive Breast Cancer Progression and Brain Metastasis. JNCI: Journal of the National Cancer Institute, 114, 579-591. https://doi.org/10.1093/jnci/djab222
- 12. Shah, M.A., Kemeny, N., Hummer, A., et al. (2005) Drg1 Expression in 131 Colorectal Liver Metastases: Correlation with Clinical Variables and Patient Outcomes. Clinical Cancer Research, 11, 3296-3302. https://doi.org/10.1158/1078-0432.CCR-04-2417
- 13. Wang, Z., Wang, F., Wang, W.Q., et al. (2004) Correlation of N-Myc Downstream-Regulated Gene 1 Overexpression with Progressive Growth of Colorectal Neoplasm. World Journal of Gastroenterology, 10, 550-554. https://doi.org/10.3748/wjg.10.550
- 14. Koshiji, M., Kumamoto, K., Morimura, K., et al. (2007) Correlation of N-Myc Downstream-Regulated Gene 1 Expression with Clinical Outcomes of Colorectal Cancer Patients of Different Race/Ethnicity. World Journal of Gastroenterology, 13, 2803-2810. https://doi.org/10.3748/wjg.v13.i20.2803
- 15. Xia, C., Dong, X., Li, H., et al. (2022) Cancer Statistics in China and United States, 2022: Profiles, Trends, and Determinants. Chinese Medical Journal, 135, 584-590. https://doi.org/10.1097/CM9.0000000000002108
- 16. Belzen, N.V., Dinjens, W.N., Diesveld, M.P., et al. (1997) A Novel Gene Which Is Up-Regulated during Colon Epithelial Cell Differentiation and Down-Regulated in Colorectal Neoplasms. Laboratory Investigation, 77, 85-92.
- 17. Shi, X.L., Cen, Y.Z., Shan, L.Y., et al. (2022) N-Myc Downstream Regulated Gene 1 Suppresses Osteoblast Differentiation through Inactivating Wnt/β-Catenin Signaling. Stem Cell Research & Therapy, 13, Article No. 53. https://doi.org/10.1186/s13287-022-02714-5
- 18. Marechal, D., Dansu, D.K., Castro, K., et al. (2022) N-Myc Downstream Regulated Family Member 1 (NDRG1) Is Enriched in Myelinating Oligodendrocytes and Impacts Myelin Degradation in Response to Demyelination. Glia, 70, 321-336. https://doi.org/10.1002/glia.24108
- 19. Watari, K., Shibata, T., Fujita, H., et al. (2020) NDRG1 Activates VEGF-A-Induced Angiogenesis through PLCγ1/ ERK Signaling in Mouse Vascular Endothelial Cells. Communications Biology, 3, Article No. 107. https://doi.org/10.1038/s42003-020-0829-0
- 20. Le, N., Hufford, T.M., Park, J.S., et al. (2021) Differential Expression and Hypoxia-Mediated Regulation of the N-Myc Downstream Regulated Gene Family. FASEB Journal, 35, E21961. https://doi.org/10.1096/fj.202100443R
- 21. Nishigaki, A., Tsubokura, H., Ishida, M., et al. (2022) NDRG1 Is Expressed in Human Granulosa Cells: An Implicative Role of NDRG1 in the Ovary. Reproductive Medicine and Biology, 21, E12437. https://doi.org/10.1002/rmb2.12437
- 22. 王震, 王国英, 王芳. 分化相关基因NDRG1与肿瘤[J]. 中华病理学杂志, 2003, 32(2): 162-164.
- 23. Ghafouri Fard, S., Teshnizi, S.A., Hussen, B.M., et al. (2023) A Review on the Role of NDRG1 in Different Cancers. Molecular Biology Reports, 50, 6251-6264. https://doi.org/10.1007/s11033-023-08540-z
- 24. Yang, G., Huang, L., Jia, H.T., et al. (2021) NDRG1 Enhances the Sensitivity of Cetuximab by Modulating EGFR Trafficking in Colorectal Cancer. Oncogene, 40, 5993-6006. https://doi.org/10.1038/s41388-021-01962-8
- 25. Bandyopadhyay, S., Pai, S.K., Gross, S.C., et al. (2003) The Drg-1 Gene Suppresses Tumor Metastasis in Prostate Cancer. Cancer Research, 63, 1731-1736.
- 26. Lim, S.C., Geleta, B., Maleki, S., et al. (2021) The Metastasis Suppressor NDRG1 Directly Regulates Androgen Receptor Signaling in Prostate Cancer. Journal of Biological Chemistry, 297, Article ID: 101414. https://doi.org/10.1016/j.jbc.2021.101414
- 27. Abascal, M.F., Elia, A., Alvarez, M., et al. (2022) Progesterone Receptor Isoform Ratio Dictates Antiprogestin/Pro-gestin Effects on Breast Cancer Growth and Metastases: A Role for NDRG1. International Journal of Cancer, 150, 1481-1496. https://doi.org/10.1002/ijc.33913
- 28. Mishra, A., Bonello, M., Byron, A., et al. (2020) Evaluation of Gene Expression Data from Cybrids and Tumours Highlights Elevated NDRG1-Driven Proliferation in Triple-Negative Breast Cancer. Breast Cancer (Auckl), 14, 1-8. https://doi.org/10.1177/1178223420934447
- 29. Ercin, M.E., Bozdoğan, Ö., Çavuşoğlu, T., et al. (2019) Hypoxic Gene Signature of Primary and Metastatic Melanoma Cell Lines: Focusing on HIF-1βAnd NDRG-1. Balkan Medical Journal, 37, 15-23. https://doi.org/10.4274/balkanmedj.galenos.2019.2019.3.145
- 30. 季语祝, 王芳, 高倩, 等. MEK/ERK对HT-29结肠癌细胞分化、侵袭迁移及NDRG1基因表达的影响[J]. 肿瘤防治研究, 2012, 39(4): 403-407.
- 31. Chen, Z.Q., Sun, J., Li, T., et al. (2018) Iron Chelator-Induced Up-Regulation of Ndrg1 Inhibits Proliferation and EMT Process by Targeting Wnt/β-Catenin Pathway in Colon Cancer Cells. Biochemical and Biophysical Research Communications, 506, 114-121. https://doi.org/10.1016/j.bbrc.2018.10.054
- 32. Wei, M.T., Zhang, Y.G., Yang, X.Y., et al. (2021) Claudin-2 Promotes Colorectal Cancer Growth and Metastasis by Suppressing NDRG1 Transcription. Clinical and Translational Medicine, 11, E667. https://doi.org/10.1002/ctm2.667
- 33. Wang, Y.F., Zhou, Y., Tao, F., et al. (2017) N-Myc Downstream Regulated Gene 1(NDRG1) Promotes the Stem-Like Properties of Lung Cancer Cells through Stabilized C-Myc. Cancer Letters, 401, 53-62. https://doi.org/10.1016/j.canlet.2017.04.031
NOTES
*共同第一作者。
#通讯作者。
