Hans Journal of Ophthalmology
Vol.05 No.01(2016), Article ID:17233,7
pages
10.12677/HJO.2016.51005
Aqueous Humor Levels of Pigment Epithelium-Derived Factor and Oscillatory Potentials in Diabetic Retinopathy Patients
Jing Zang1*, Guoqi Guan2, Wenjuan Wang1, Jionglin Bao1, Lilun Chen1
1Department of Ophthalmology, The First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou Guangdong
2Jinan University, Guangzhou Guangdong

Received: Mar. 2nd 2016; accepted: Mar. 25th, 2016; published: Mar. 29th, 2016
Copyright © 2016 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



ABSTRACT
Objective: To investigate the relationship between pigment epithelium derived factor (PEDF) and oscillatory potentials (OPs) in various diabetic retinopathy and to provide an objective basis for the early diagnosis of diabetic retinopathy (diabetic retinopathy, DR) and the prediction of the progression of the disease. Methods: Aqueous humor samples were collected from 40 eyes of 40 type 2 diabetes patients, including 19 eyes without diabetic retinopathy and 21 eyes with diabetic (11 eyes with NPDR and 10 eyes with PDR), twenty aqueous samples from 20 cataract patients without other ocular or systemic diseases served as controls. The PEDF levels in aqueous humor were measured by enzyme-linked immunosorbent assay (ELISA). The total amplitude of OPs in the whole retina was detected by the Roland visual electrophysiological test instrument of the German CONSULT ROLAND company. Results: The PEDF and OPs level in eyes without diabetic retinopathy [(9.13 ± 4.43) ng/ml, 164.56 ± 37.41) μV, P < 0.01] was significantly lower than that in controls [(13.87 ± 0.36) ng/ml, (237.96 ± 35.96) μV]; the PEDF Ievel in eyes with NPDR [(7.00 ± 2.04) ng/ml, (79.80 ± 19.89) μV, P < 0.01] and PDR [(6.39 ± 1.66) ng/ml, (28.55 ± 17.47) μV, P < 0.01] was significantly lower than that in controls [(13.87 ± 0.36) ng/ml, (237.96 ± 35.96) μV]; the concentration of PEDF in the patients with type 2 diabetes mellitus was significantly lower than that in the NPDR and PDR group (P = 0.04, P = 0.02), while there was no significant difference between NPDR group and PDR group (P = 0.34). There were significant differences in different OPs group (P < 0.01). The PEDF concentration in the aqueous humor of the diabetic retinopathy was a typical positive correlation with the total amplitude of OPs (r = 0.905, P < 0.01). Conclusion: The concentration of PEDF in aqueous humor and the total amplitude of OPs were positively correlated with the diabetic retinopathy, which could provide an objective basis for the early diagnosis of diabetic retinopathy and the prediction of the progression of the disease.
Keywords:Diabetic Retinopathy, Pigment Epithelium Derived Factor, Oscillatory Potentials

各期糖尿病视网膜病变患者房水中PEDF与OPs的相关性分析
臧晶1*,管国奇2,王文娟1,鲍炯琳1,陈立伦1
1广东药学院附属第一医院,广东 广州
2暨南大学,广东 广州

收稿日期:2016年3月2日;录用日期:2016年3月25日;发布日期:2016年3月29日

摘 要
目的:探讨房水中色素上皮衍生因子(pigment epithelium derived factor, PEDF)与视网膜电图振荡电位(oscillatory potentials, OPs)在各期糖尿病性视网膜病变中的相关性研究分析,为糖尿病视网膜病变(diabetic retinopathy, DR)的早期诊断及病程进展预测提供客观的检测依据。方法:房水来自2型糖尿病患者40例40眼。单纯2型糖尿病患者组19例19只眼,非增殖期糖尿病性视网膜病变(non-proliferative diabetic retinopathy, NPDR)组患者11例11只眼,增殖期糖尿病视网膜病变(proliferative diabetic retinopathy, PDR)组患者10例10只眼,单纯老年性白内障患者20例20只眼作为对照组。用酶联免疫吸附法检測房水中PEDF的水平,用德国 ROLAND CONSULT公司生产的罗兰视觉电生理检查仪检测全视网膜电图中OPs的总振幅值。结果:单纯2型糖尿病患者组、NPDR组及PDR组的房水中PEDF的浓度分别为(9.13 ± 4.43) ng/ml、(7.00 ± 2.04) ng/ml、(6.39 ± 1.66) ng/ml与对照组(13.87 ± 0.36) ng/ml相比,房水中PEDF浓度均明显降低(P < 0.01),单纯2型糖尿病患者组与NPDR组及PDR组相比,房水中PEDF浓度亦均明显降低(P = 0.04, P = 0.02),而NPDR组与PDR组相比无明显差异(P = 0.34);单纯2型糖尿病患者组、NPDR组及PDR组的OPs的总振幅值分别为(164.56 ± 37.41) μV、(79.80 ± 19.89) μV、(28.55 ± 17.47) μV与对照组(237.96 ± 35.96) μV相比,OPs的总振幅值均明显降低(P < 0.01),而且组间两两比较亦有明显差异(P < 0.01);各期糖尿病性视网膜病变房水中PEDF浓度变化与OPs的总振幅值呈典型的正相关(r = 0.905, P < 0.01)。结论:房水中PEDF的浓度与OPs总波幅在各期糖尿病性视网膜病变中呈正相关,可为糖尿病视网膜病变的早期诊断及病程进展预测提供客观的检测依据。
关键词 :糖尿病视网膜病变,PEDF,OPs

1. 引言
糖尿病视网膜病变(Diabetic retinopathy, DR)是目前糖尿病最常见和严重的并发症之一,已成为世界性的主要致盲性眼病之一 [1] ,目前尚无有效的根治方法,美国早期糖尿病性视网膜病变研究小组(Early Treatment Diabetic Retinopathy Study, ETDRS)的研究证明,早期发现、认真随访和干预,可以使90%的DR患者不发生严重的视力下降,是预防糖尿病盲的首要方法 [2] [3] ,国外研究显示早期DR中色素上皮衍生因子(pigment epithelium derived factor, PEDF)的改变有望成为DR早期诊断和预后的指标 [4] ,国内研究结果显示视网膜电图振荡电位(oscillatory potentials, OPs)总波幅是目前诊断早期糖尿病视网膜病变的最敏感指标,它不仅能早期发现病变,还能反映视网膜的进行性损害 [5] 。为了对其早期诊断、早期治疗,本研究对各期糖尿病视网膜病变患者房水中PEDF浓度变化与OPs总波幅进行相关性研究。
2. 资料与方法
2.1. 研究对象
2015年1月至2015年7月在我院眼科因老年性白内障行晶体超声乳化吸除 + 人工晶状体植人术的患者共60例,其中2型糖尿病患者40例,均符合WHO1999年2型糖尿病诊断标准 [6] ,糖尿病病程(7.3 ± 5.1)年,术前空腹血糖均控制在8.3 mmol/L以下,术前行眼底荧光素血管造影检测并分期分组,其中男16例,女24例,年龄51~76岁,平均65.1 ± 4.8岁。经眼部系统检查及眼底荧光造影确诊,根据2003年美国眼科学会制定的糖尿病性视网膜病变分期标准 [7] 有糖尿病视网膜病变(DR组) 20例,非增殖期糖尿病性视网膜病变(Non-proliferative diabetic retinopathy, NPDR)患者11例11眼,增殖期糖尿病性视网膜病变(Proliferative diabetic retinopathy, PDR)患者9例9眼。单纯2型糖尿病患者组19例19只眼。对照组为单纯性老年性白内障20例20眼,男11例11眼,女9例9眼;年龄51~76岁,平均年龄58.4 ± 12.8岁。研究组患者与对照组之间年龄差异无统计学意义(P < 0.05)。排除:并发其它严重全身性疾病者(肾功能不全、明显高血脂、血压控制不佳者);单眼或双眼屈光介质明显混浊,影响眼底观察者;眼底病变已做过治疗者;伴有其它明显眼底病变者。
2.2. OPs总波幅测定
术前受检者用0.5%托吡卡胺眼液充分扩瞳(≥7 mm),暗适应30 min后滴0.5%盐酸丙美卡因角膜表面麻醉剂,暗红光下安装电极,作用电极使用Jet角膜接触电极,参考电极和地电极使用金箔皮肤电极,分别置于眼眶颞侧和耳垂。受检者下颌置于Ganzfeld球的下颌托上,两眼注视正前方,用Ganzfeld全视野刺激球作闪光刺激器。OPs参数采用国际标准Max刺激闪光强度3.0 cd∙s/m,背景光亮度0 cd/m,闪光间隔15 s,放大器通频带宽100~300 Hz。双眼同时记录,结果由计算机自动处理分析并打印。操作由同一人完成。
2.3. 房水标本采集
手术室无菌条件下用1 ml一次性注射器在角膜缘内1 mm行前房穿刺,针头不触及晶体、虹膜及角膜内皮,抽取瞳孔中央区未稀释房水0.2 ml,注入离心管中,置于冰上,放人−80℃低温冰箱保存。
2.4. 房水中PEDF浓度检测
通过酶联免疫吸附测定法(enzyme-linked immunosorbent assay, ELISA)检测,采用人类PEDF (Chemicon international公司)夹心ELISA试剂盒。标准溶液(100 μl)及待测样品稀释后(1:4)取100 μl (PEDF检测前先加人尿素至8 mol/L浓度,在冰上孵育1 h后再稀释)加入已包被单克隆抗体96孔板,37℃孵育1 h后,用洗涤液充分洗涤,每孔中加入稀释的链霉菌抗生物素蛋白–过氧化物酶结合物100 μl,置37℃孵育1 h,用洗涤液充分洗涤,每孔加人TMB/E底物100 μl,置室温反应5~10 min,每孔加入1滴终止液混匀,用分光光度计450 nm处测吸光度(D)值,用标准品溶液D值画出标准曲线,根据样品D值在该曲线图上查出相应PEDF含量。PEDF检测敏感度为0.9 ng/ml,检测范围0.9~62.5 ng/ml,检测中变异±5.3%,检测间变异±16.0%。
2.5. 统计学方法
采用SPSS 13.0统计软件进行统计学分析。各组患者房水中PEDF浓度及OPs总波幅用x ± s表示,比较各组间房水中PEDF浓度及OPs总波幅的比值用独立t检验检测,PEDF浓度与OPs总波幅相关性用Pearson相关性检验,P < 0.05为差异具有统计学意义。
3. 结果
单纯2型糖尿病患者组、NPDR组及PDR组的房水中PEDF的浓度分别为为(9.13 ± 4.43) ng/ml、(7.00 ± 2.04) ng/ml、(6.39 ± 1.66) ng/ml与对照组(13.87 ± 0.36) ng/ml相比,差异均有统计学意义(P < 0.01),单纯2型糖尿病患者组与NPDR组及PDR组相比,房水中PEDF浓度亦均明显降低,差异均有统计学意义(P = 0.04, P = 0.02),而NPDR组与PDR组相比无明显统计学差异(P = 0.34),见图1;单纯2型糖尿病患者组、NPDR组及PDR组的OPs的总振幅值分别为(164.56 ± 37.41) μV、(79.80 ± 19.89) μV、(28.55 ± 17.47) μV与对照组(237.96 ± 35.96)相比,差异均有统计学意义(P < 0.01),而且组间两两比较亦有明显差异(P < 0.01),见图2;各期糖尿病性视网膜病变房水中PEDF浓度变化与OPs的总振幅值呈典型的正相关(r = 0.905, P < 0.01),见图3。
4. 讨论
1) 由于伦理的原因,较难获得大量的较好的反映视网膜中PEDF水平的无糖尿病性视网膜病变的玻璃体标本。有研究发现在动物实验的房水中的PEDF浓度与光感受器基质及玻璃体中PEDF浓度具有相关性 [8] ,所以本研究选择了检测房水标本中PEDF的水平以便间接反映其在视网膜中的水平。DR的最早病理改变为视网膜微血管周细胞丧失及功能障碍。AGEs或活性氧族(reactive oxygen species, ROS)可抑制视网膜周细胞内PEDFm-RNA的表达,造成PEDF水平下降,而PEDF水平的下降可使氧化应激诱导的周细胞凋亡及功能障碍进一步加剧,从而促进DR的进展,微血管瘤形成,血管渗漏,内皮细胞增殖、移行、新生血管形成进入PDR期 [9] 。Ogata等的临床研究表明DR患者玻璃体中PEDF水平低于正常对照组,PDR患者的这种趋势更加明显 [10] 。本研究中2型糖尿病组、NPDR组及PDR组房水中PEDF水平低于正常对照组,DR组患者的这种表现更加明显,与既往报道结果相一致,差异有统计学意义,提示低水平的PEDF可能与DR的血管发生有关,伴随PEDF浓度的持续降低,糖尿病视网膜病变程度逐渐加重,且可导致活动性PDR的发生。Matsuoka等试验表明,在体外和动物模型DR早期,PEDF转录水平的改变先于血管内皮生长因子(vascular endothelial growth factor, VEGF),PEDF可能是引起VEGF改变的启动因素之一 [11] ,研究通过PEDF的治疗有希望更早的发现和阻断新生血管形成 [12] 。Boehm等的研究发现有视网膜病变的亚组PEDF含量明显低于无视网膜病变亚组,表明PEDF是房水血管生成的重要负调节剂,并且其在房水的低含量可强烈预示糖尿病患者发生视网膜病变的危险性,提示房水中PEDF的水平可作为预测DR发生、发展状况的一项指标 [13] 。PEDF是一种分泌型糖蛋白,属于丝氨酸蛋白酶抑制剂超家族,能够通过抑制还原型辅酶II氧化酶引起的氧化应激的产生防止在早期DR中血管通透性的增高及视网膜电流图a波及b波振幅的下降,提示PEDF可能对早期DR具有保护作用 [14] 。本研究中发现2型糖尿病组患者房水中PEDF水平不仅低于DR组,而且明显低于对照组,差异有统计学意义,提示PEDF水平的下降可能引起对早期DR保护作用的减弱,最终导致DR的发生、发展,进一步提示在糖尿病视网膜病变前期检测PEDF水平下降有可能早期发现病变,以便早期干预及治疗,防止或延缓往糖尿病视网膜病变发展。
2) OPs是视网膜电图的亚成分,起源于视网膜的内层,对视网膜血管性病变尤其是循环障碍特别敏感 [15] 。DR的微循环改变往往较早影响到视网膜的内层,因而,有报道发现当ERG a、b波尚正常时已
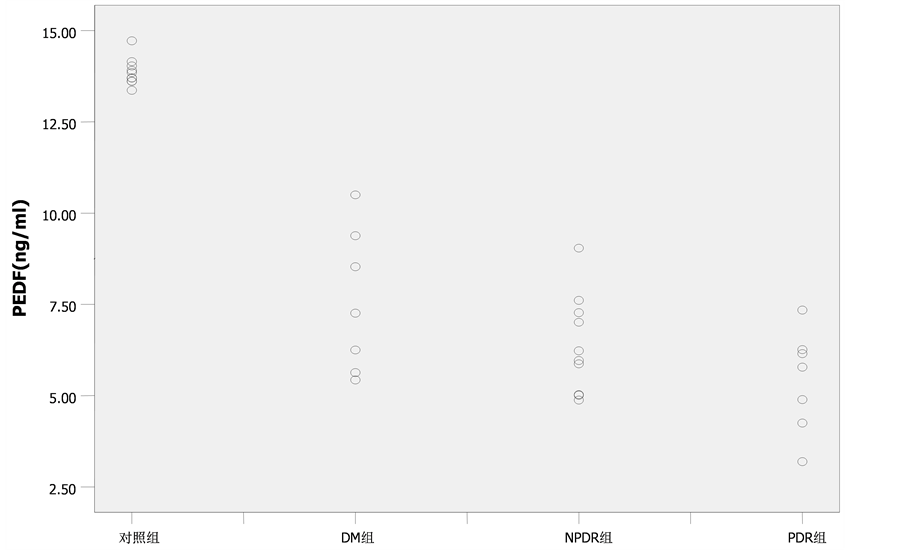
Figure 1. PEDF levels in aqueous humor in different groups
图1. 各组患者房水中PEDF水平

Figure 2. The total amplitude of OPs in different groups
图2. 各组患者OPs总波幅值
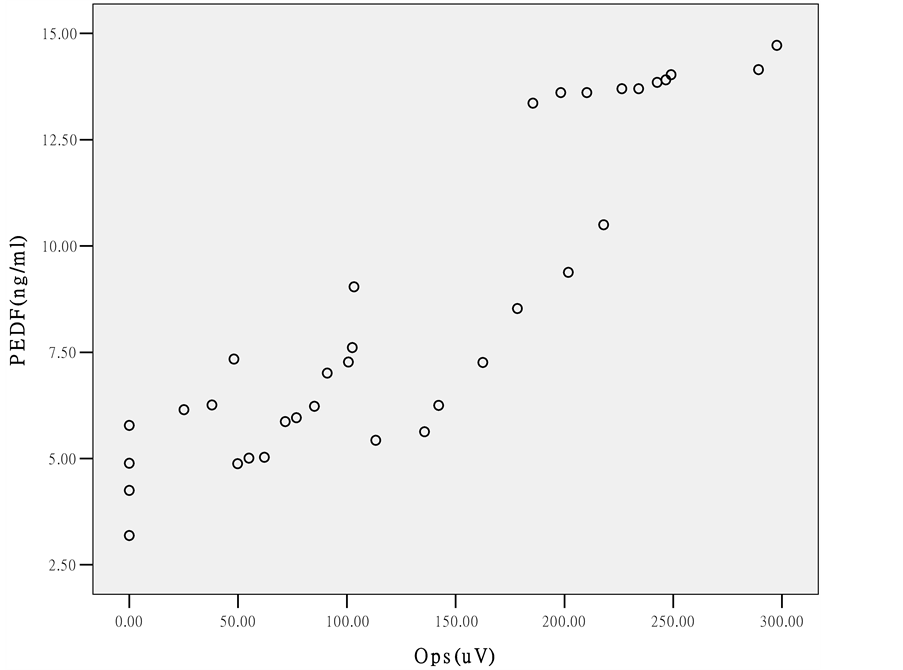
Figure 3. Correlation between PEDF levels in aqueous humor and OPs in different groups
图3. 各组患者房水中PEDF浓度与OPs总波幅的相关性
有OPs的缺失或明显降低 [16] 。Leozappa等认为OPs异常可作为DR进展的高危标志之一,还可以作为DR早期治疗的参考依据 [17] 。本研究中,观察发现单纯2型糖尿病的患者(DR前期)即有OPs总波幅的降低,并且随着糖尿病视网膜病变的加重,OPs总波幅的变化更加明显,说明在DR病程中,视网膜光感受细胞功能异常可先于血管病变,与既往报道结果相一致,本研究结果表明,OPs总波幅下降幅度可预测2型糖尿病人发生DR,或者DR发生、发展的可能。下降幅度越大,DR进展越快,视网膜功能损害越明显。
3) 本研究中我们还发现,房水中PEDF的浓度及OPs总波幅呈明显的正相关性,差异具有统计学意义,目前国内外未见相关报道,我们的研究结果表明当糖尿病病人眼底尚未出现改变时,房水中PEDF的浓度及OPs总波幅已降低。随着DR的发展,二者下降幅度越大。说明在临床上我们可以结合房水中PEDF和OPs的检测更客观地预测DR的发生,早期对DR作出诊断,发现视网膜的进行性损害,对DR的严重程度作出相应的估计,具有实际的临床应用价值。
文章引用
臧 晶,管国奇,王文娟,鲍炯琳,陈立伦. 各期糖尿病视网膜病变患者房水中PEDF与OPs的相关性分析
Aqueous Humor Levels of Pigment Epithelium-Derived Factor and Oscillatory Potentials in Diabetic Retinopathy Patients[J]. 眼科学, 2016, 05(01): 22-28. http://dx.doi.org/10.12677/HJO.2016.51005
参考文献 (References)
- 1. 霍鸣, 张海江, 吴昊, 等. 糖尿病视网膜病变的激光光凝治疗[J]. 国际眼科杂志, 2007, 7(1): 202-203.
- 2. Cantrill, H.L. (1984) The Diabetic Retinopathy Study and the Early Treatment Diabetic Retinopathy Study. International Ophthalmology Clinics, 24, 13-29. http://dx.doi.org/10.1097/00004397-198402440-00004
- 3. Josifova, T., Schneider, U., Henrich, P.B. and Schrader, W. (2008) Eye Disorders in Diabetes: Potential Drug Targets. Infectious Disorders: Drug Targets, 8, 70-75. http://dx.doi.org/10.2174/187152608784746529
- 4. Boehm, B.O., Lang, G., Feldmann, B., Kurkhaus, A., Ro-singer, S., Volpert, O., et al. (2003) Proliferative Diabetic Retinopathy Is Associated with a Low Level of the Natural Ocular Anti-Angiogenic Agent Pigment Epithelium-Derived Factor (PEDF) in Aqueous Humor. A Pilot Study. Hor-mone and Metabolic Research, 35, 382-386. http://dx.doi.org/10.1055/s-2003-41362
- 5. 郭秀瑾, 王长龄, 张怡红, 等. 视网膜振荡电位在糖尿病性视网膜病变早期诊断的价值[J]. 河北医科大学学报, 2002(23): 14-18.
- 6. 叶任高, 陆再英. 内科学[M]. 6版. 北京: 人民卫生出版社, 2004, 5-797.
- 7. Sandri, F., Ancora, G., Lanzoni, A., et al. (2004) Prophylactic Nasal Continuous Positive Airways Pressure in Newborns of 28 - 31 Weeks Gestation: Multicentre Randomised Controlled Clinical Trial. Archives of Disease in Childhood—Fetal and Neonatal Edition, 89, F394-F398. http://dx.doi.org/10.1136/adc.2003.037010
- 8. Hentschel, R. and Jorch, G. (2002) Acute Side Effects of Surfac-tant Treatment. Journal of Perinatal Medicine, 30, 143-148.
- 9. Nam, D.H., Oh, J., Roh, J.H. and Huh, K. (2009) Different Expression of Vascular Endothelial Growth Factor and Pigment Epithelium-Derived Factor between Diabetic and Non-Diabetic Epiretinal Membranes. Ophthalmologica, 223, 188-191. http://dx.doi.org/10.1159/000198686
- 10. Ogata, N., Nishikawa, M., Nishimura, T., Mitsuma, Y. and Matsumura, M. (2002) Unbalanced Vitreous Levels of Pigment Epithelium-Derived Factor and Vascular Endothelial Growth Factor in Diabetic Retinopathy. American Journal of Ophthalmology, 134, 348-353. http://dx.doi.org/10.1016/S0002-9394(02)01568-4
- 11. Matsuoka, M., Ogata, N., Minamino, K. and Matsumura, M. (2007) Leukostasis and Pigment Epithelium-Derived Factor in Rat Models of Diabetic Retinopathy. Molecular Vision, 13, 1058-1065.
- 12. 陈海冰, 贾伟平, 陆俊茜, 包玉倩, 等. 2型糖尿病肾病患者血清色素上皮源因子水平的变化及意义[J]. 中华医学杂志, 2007, 87(18): 1230-1233.
- 13. Boehm, B.O., Lang, G., Volbert, O., Jehle, P.M., Kurkhaus, A., Rosinger, S., et al. (2003) Low Content of the Natural Ocular Anti-Angiogenic Agent Pigment Epithe-lium-Derived Factor (PEDF) in Aqueous Humor Predicts Progression of Diabetic Retinopathy. Diabetologia, 46, 394-400.
- 14. Yamagishi, S., Matsui, T., Nakamura, K., Takeuchi, M. and Imaizumi, T. (2006) Pigment Epithe-lium-Derived Factor (PEDF) Prevents Diabetes- or Advanced Glycation End Products (AGE)-Elicited Retinal Leukos-tasis. Microvascular Research, 72, 86-90.
- 15. Bearse Jr., M.A., Shimada, Y. and Sutter, E.E. (2000) Distribution of Oscillatory Components in the Central Retina. Documenta Ophthalmologica, 100, 185-205. http://dx.doi.org/10.1023/A:1002783719958
- 16. Kunikata, H., Nakagawa, Y. and Tamai, M. (2004) Evaluation of Visual Function and Prognosis for Patients with Proliferative Diabetic Retinopathy with the Low Vision Evaluator. The Tohoku Journal of Experimental Medicine, 204, 229-236. http://dx.doi.org/10.1620/tjem.204.229
- 17. Leozappa, M., Micelli Ferrari, T., Grossi, T., Pace, V., Rinaldi, M.L., Battista, D. and Micelli Ferrari, L. (2008) Prognostic Prediction Ability of Postoperative Multifocal ERG after Vitrectomy for Diabetic Macular Edema. European Journal of Ophthalmology, 18, 609-613.
*通讯作者。