Hans Journal of Medicinal Chemistry
Vol.
08
No.
02
(
2020
), Article ID:
35673
,
13
pages
10.12677/HJMCe.2020.82006
The Dual-Specificity Tyrosine Phosphorylation Regulated Kinase 1A and the Advances of Its Inhibitors
Yong Wang, Xiaozhen Jiao*
Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing
Email: *jiaoxz@imm.ac.cn
Received: Apr. 27th, 2020; accepted: May 14th, 2020; published: May 21st, 2020

ABSTRACT
Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A), a kind of serine/threonine protein kinase, is able to phosphorylate numerous substrates, playing an important role in various physiological activities and correlates to multiple diseases including nervous system disease, cancer, diabetes and so on. In particular, it is considered as an important target of curing neurodegenerative diseases. This passage briefly introduces the genetic structure, physiological function, related diseases and the advances of the inhibitors of DYRK1A.
Keywords:DYRK1A, Inhibitors, Neurodegenerative Diseases

双底物特异性酪氨酸磷酸化调节激酶1A 及其抑制剂的研究进展
王勇,焦晓臻*
中国医学科学院&北京协和医学院,药物研究所,北京
Email: *jiaoxz@imm.ac.cn
收稿日期:2020年4月27日;录用日期:2020年5月14日;发布日期:2020年5月21日

摘 要
双底物酪氨酸磷酸化调节激酶1A (Dual-specificity tyrosine phosphorylation-regulated kinase 1A, DYRK1A)是一种丝氨酸/苏氨酸蛋白激酶,作用底物广泛,参与人体内多种生理活动,与神经系统疾病、糖尿病、癌症等多种疾病相关,尤其被认为是神经系统退行性疾病的重要治疗靶点。本文介绍了DYRK1A的基因结构、生理功能、相关疾病及其抑制剂的研究进展。
关键词 :DYRK1A,抑制剂,神经退行性疾病

Copyright © 2020 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
双底物特异性酪氨酸磷酸化调节激酶(Dual-specificity tyrosine phosphorylation-regulated kinases, DYRKs)是一类在进化上高度保守的蛋白激酶,属于细胞周期依赖性蛋白激酶CMGC家族,具有磷酸化酪氨酸(仅自身)、丝氨酸、苏氨酸残基活性。在哺乳动物中,DYRK家族包括1A,1B,2,3和4五种亚型 [1]。双底物特异性酪氨酸磷酸化调节激酶1A (DYRK1A)是其中表达最多的蛋白激酶,在神经发育、细胞增殖与分化、肿瘤发生等生理和病理过程中发挥重要作用。
2. DYRK1A的结构和生理功能
2.1. DYRK1A的结构
DYRK1A的编码基因位于人类21号染色体上唐氏综合征的关键区域(Down Syndrome Critical Region, DSCR),蛋白全长为763个氨基酸,包括六个结构域(图1):两个核定位信号区(Nuclear Localization Signal, NLS),一个激酶功能区,一个碳端的PEST区域,一个多组氨酸束和一个丝/苏氨酸富集区域 [1] [2] (图1)。其中第319位和321位酪氨酸是其发挥完全催化作用的关键位点 [3]。
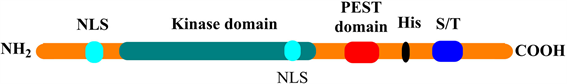
Figure 1. Protein structure of DYRK1A
图1. DYRK1A的蛋白结构
2.2. DYRK1A的分布与生理功能
DYRK1A在脑、心脏、肺、骨骼肌等器官及组织中均所表达 [4]。其中,在脑中DYRK1A在大脑海马中的表达最高,在嗅球,小脑皮层,脊髓,中脑和脑干的运动核中也有较高表达 [5],正常人的DYRK1A在胚胎时期表达量达到最高,随后维持在一个较低水平直到成年 [6]。
研究表明DYRK1A的表达与神经元基因转录,神经元分化以及脑发育紧密相关 [7]。DYRK1A通过自磷酸化其活化环上的酪氨酸残基,先形成一个瞬时的蛋白中间体,继而激发DYRK1A激酶的活性构象以磷酸化底物 [8]。其底物包括转录因子(CREB, NFAT, STAT3, FKHR, Gli1),剪接因子(cyclin L2, SF2, SF3),翻译因子(eIF2Bε),突触蛋白(dynamin I, amphiphysin I, synaptojanin I)和其它各种蛋白(糖原合成酶,caspase-9, Notch) [4] [9]。
其中,Tau蛋白具有维持细胞内微管稳定和聚合的作用 [10],目前已发现DYRK1A可磷酸化Tau蛋白中至少11个丝氨酸/苏氨酸残基位点,造成tau蛋白自聚集和纤维化,引起神经原纤维缠结(Neurofibrillary tangle, NTFs)和神经元微管的损伤 [11]。DYRK1A还可以通过磷酸化淀粉样前体蛋白(Amyloid precursor protein, APP)的第668位苏氨酸,促进β/γ分泌酶介导的APP切割,产生毒性β淀粉样蛋白(β-amyloid peptides, Aβ) [12],从而参与阿尔兹海默症(Alzheimer’s disease, AD)的发病过程。同时,Aβ的产生又会刺激DYRK1A的表达,相互形成正反馈机制 [13]。此外,有研究表明DYRK1A的表达水平可影响炎症相关的NFκB信号通路中IκBα蛋白水平,提示DYRK1A可能是调控炎症的重要蛋白,在神经系统中其过表达可能通过炎症机制引起神经退行性疾病 [14]。
3. DYRK1A激酶与疾病
一直以来,由于DYRK1A编码基因的特殊位置,其在神经系统中的作用受到了广泛关注,被认为在唐氏综合征(Down syndrome, DS)、AD和帕金森病(Parkinson’s disease, PD)等疾病的发病中起着关键的作用 [15] [16]。同时,因其作用底物的广泛性,也会影响体内其它病理生理过程,如参与癌症 [4] [17] 糖尿病 [18] [19] [20] 等疾病。下面主要介绍其与神经退行性疾病的关系。
3.1. DYRK1A与DS
DS由先天性21号染色体异常引起,致使患者脑体积减小、神经元大量丧失,进而导致严重的智能障碍并伴有多种脏器异常。研究表明,DS患者脑内DYRK1A的mRNA和蛋白水平比正常人高出约1.5倍 [21],且DS患者神经元缺陷、树突状萎缩、脊性发育不良、神经原纤维变性等多个神经发育异常进程均与DYRK1A的过度表达密切相关 [22] [23]。实验结果显示,DYRK1A过度表达的转基因小鼠明显表现出了神经发育迟缓,运动异常,突触可塑性改变,学习和记忆缺陷等问题 [13]。由于DYRK1A的过度表达,DS患者往往具有类似早期AD患者的病理表现 [22]。
3.2. DYRK1A与AD
AD是老年痴呆的主要类型,该病已成为目前老年人中仅次于肿瘤、心血管的第三大致命疾病。其病理学特征为大脑中出现由Aβ组成的淀粉样蛋白斑块和tau蛋白过度磷酸化导致的神经纤维缠结 [24]。研究显示,AD患者海马体中的DYRK1A表达远高于正常人,DYRK1A过度表达的转基因小鼠脑中Aβ的含量也显著高于正常小鼠 [12],毒性Aβ的聚集会损伤神经元,导致记忆缺失和老年痴呆 [25]。如前所述,DYRK1A的过度表达还会促进tau蛋白的过度磷酸化,引起神经原纤维缠结,进而导致神经死亡和痴呆的发生 [15]。
3.3. DYRK1A与PD
PD也是一种常见的神经退行性疾病,PD患者脑内往往有大量的多巴胺能神经元丢失和α-突触核蛋白(α-Synuclein, α-Syn)聚集现象。而DYRK1A被证实可磷酸化α-Syn [26],从而对多巴胺神经元产生神经毒性,导致多巴胺能神经元功能丢失,产生PD症状 [27]。在一项针对中国汉族人基因的调查中显示,DYRK1A中rs8126696Td等位基因与PD密切相关 [28],进一步的实验表明PD患者尤其是男性患者DYRK1A的rs8126696TT基因型表达明显高于正常对照组 [29]。
此外,大量研究显示DYRK1A与其它神经系统疾病如皮克病 [30] 亨廷顿症 [31] 等也有一定关系。
4. DYRK1A激酶抑制剂研究进展
研究表明DYRK1A与DS、AD、PD等神经系统疾病的发病密切相关,为治疗该类疾病的新靶点。因此,DYRK1A抑制剂的研发受到越来越多的药物学家的关注。愈来愈多的DYRK1A抑制剂被发现,其中有的已进入临床研究阶段,下面对基于天然产物的和基于计算机辅助药物分子设计及高通量筛选的DYRK1A抑制剂的研究进展进行详细介绍,化学结构分别见图2、图3。
4.1. 基于天然产物的DYRK1A抑制剂
4.1.1. 表没食子儿茶素没食子酸酯
表没食子儿茶素没食子酸酯(Epigallocatechin gallate, EGCG, 1)为在绿茶中含量较高的茶多酚物质。研究显示其为DYRK1A抑制剂 [32],它不仅可以在体外以非竞争性形式结合DYRK1A激酶,而且还可以通过激活ERK和PI3K通路增加星形胶质细胞NEP分泌,从而引发细胞外β淀粉样蛋白降解 [33]。另外,动物体内实验表明其可以在一定程度上改善DS模型小鼠和DS患者的学习记忆障碍 [34] [35]。EGCG针对DS适应症的II期临床研究结果显示,结合认知训练的EGCG给药组患者大脑的功能性连接和皮质兴奋性的正常化相较对照组均有所改善 [36]。
4.1.2. 肉叶芸香碱类化合物
肉叶芸香碱(Harmine, 2)是一种β-咔啉生物碱,其对DYRK1A具有较好的抑制活性,IC50值可达80 nmol,其可以抑制DYRK1A并干扰神经突形成 [37]。肉叶芸香碱虽然对DYRK1A有较高的选择性,但由于其兼具较强的单胺氧化酶A抑制作用从而引起致幻等副作用。为了提高天然产物Harmine对DYRK1A的选择性,Ruben等通过对其9位进行修饰得到了具有较高DYRK1A抑制作用的化合物3和4 [38]。Drung等根据计算机模拟结果,将9位氰乙基以脂肪长链取代也得到了保持一定活性且选择性有较大改善的化合物5 [39]。Kumar等通过对Harmine的1位进行修饰,得到了选择性有较大提升的化合物6 [40]。经过持续的研究,他们又于最近发现了9位以长链酰胺取代的化合物7,选择性和体内DYRK1A抑制活性均有所提升 [41]。
4.1.3. Meridianin类化合物
Meridianin D (8)是一种从海衣中提取的天然生物碱,结构为3位由氨基嘧啶取代的吲哚环衍生物,对多种蛋白激酶有抑制作用且具有抗肿瘤活性 [42] [43] [44],其对DYRK1A具有较好的抑制活性,IC50值为130 nM。Giraud等通过对Meridianin吡啶环上氨基对位的大量取代修饰得到了具有较高DYRK1A抑制活性的碘代9 [45]。Yadav等通过对Meridianin吲哚环上1位N取代的大量修饰得到了具有良好DYRK1A抑制活性和选择性的化合物10 [46]。
Yannick等基于对Meridianin的构效分析,加入苯环固定吲哚环和氨基嘧啶部分,又将吲哚环部分简化为吡啶作为基本骨架设计合成了一系列刚性结构的吡啶骈喹唑啉类化合物,其中化合物11对DYRK1A和CLK1均有较高的抑制活性 [47]。其同组的Wael对5位进行修饰仅能得到具有CDK5/GSK3抑制活性而DYRK1A抑制作用减弱的化合物 [48]。近期他们又研究2位氨基取代对DYRK1A抑制活性的影响,得到了DYRK1A抑制活性更高的化合物12和13 [49]。
4.1.4. Leucettamine B类化合物
Leucettamine B (14)是由一种从多孔动物海绵中提取得到的生物碱类化合物,Bazureau等发现其对DYRK1A具有一定的抑制活性,且选择性较好 [50]。通过对Leucettamine B进行结构修饰得到的化合物L41 (15)对DYRK1A表现了良好的抑制作用,其IC50值为28 nmol [50]。体外实验表明,L41可抑制谷氨酸酯介导的HT22细胞死亡和淀粉样前体蛋白引起的大鼠脑内细胞死亡以及降低炎症因子水平 [51],动物体内实验结果也显示其可有效减轻小鼠的记忆损伤和认知缺陷 [52]。
4.1.5. Acrifoline
Acrifoline (16)是由Jarhad等从Glycosmis chlorosperma的茎皮提取分离得到的吖啶酮生物碱类化合物,其对于DYRK1A选择性最高且抑制效果显著 [53]。分子对接结果显示,其作用方式可能是通过两个羟基分别与DYRK1A 203位谷氨酸及保守区188位赖氨酸残基和铰链区329位谷氨酸及241位亮氨酸残基形成氢键从而发挥抑制活性 [53]。
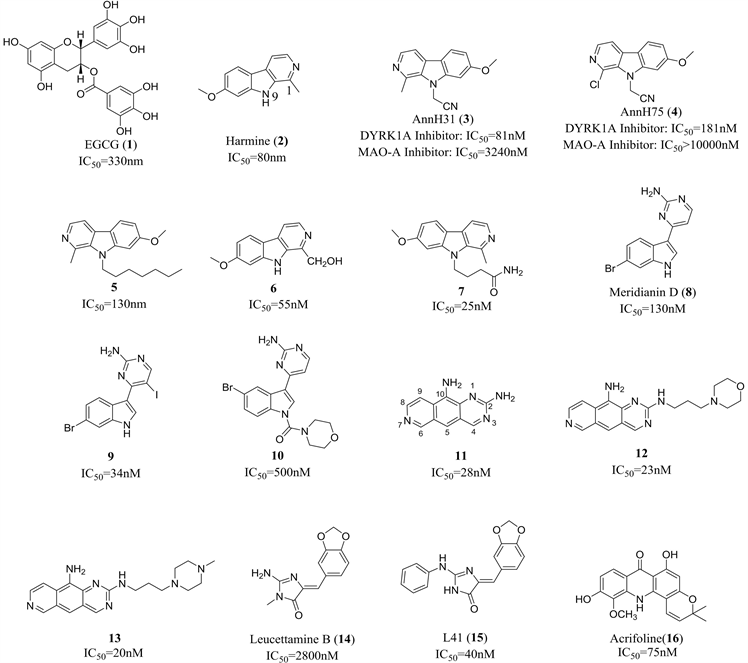
Figure 2. Inhibitors of DYRK1A from natural products
图2. 天然产物来源的DYRK1A抑制剂
4.2. 基于计算机辅助药物分子设计及高通量筛选的DYRK1A抑制剂
虽然大量基于天然产物的DYRK1A抑制剂表现出良好的活性,但其中很多化合物选择性较差,作用机制复杂,并可能产生一定的副作用。而结合计算机虚拟筛选和化合物库高通量筛选得到的很多人工合成的不同结构类型的化合物表现出了较高的活性与选择性。下面进行详细介绍。
4.2.1. 苯并噻唑类
Ogawa等通过改造CLK的抑制剂TG003得到了对DYRK1A抑制活性较好的苯并噻吩类化合物—INDY (17)分子 [54]。体外实验结果显示INDY不与单胺氧化酶作用,但可以有效抑制有爪蟾蜍胚胎中DRYK1A的过度表达 [54]。遗憾的是INDY抑制DYRK1A的同时对DYRK1B也产生了较高的抑制作用。随后,Masaki等根据DYRK1A/INDY的晶体复合物结构,以氧芴替换INDY中的苯酚结构得到了新型的DYRK1A抑制剂BINDY (18),其IC50值为25.1 nmol [55]。同课题组的Kii等通过筛选已合成的化合物分子库,也得到了活性较高且选择性较好的化合物FINDY (19),它可以抑制DYRK1A细胞内97位丝氨酸的自体磷酸化,但不影响酪氨酸的磷酸化,且不抑制DYRK家族其它亚型的蛋白激酶活性 [56]。
Sonamoto等采用CDC37-nanoKAZ双荧光检测的细胞检测的方法筛选得到了活性极高的ATP竞争型DYRK1A抑制剂CaNDY (20) [57]。
Salah等通过引入脲基限制构象,得到了化合物21,大大提高苯并噻嗪类化合物的选择性,其对DYRK1A的选择性是DYRK1B的16倍 [58]。
4.2.2. 吲哚类
Falke等通过对其现有化合物库的高通量筛选并经过结构优化得到具有高DYRK1A抑制活性的吲哚骈喹啉骨架类化合物22,但是由于其水溶性较差,细胞透过率较低导致其细胞内活性降低 [59]。为了改善其理化性质,该课题组简化分子中的刚性四元环母核为吲哚环,设计合成了化合物23,但遗憾的是其选择性有所下降且水溶性并未得到明显提升 [60]。近期他们又将碘原子以氯取代,并以环庚三烯酮替换苯环,得到了活性略有下降但水溶性稍有提升的化合物24 [61]。
4.2.3. 喹唑啉类
Rosse等设计合成了一系列噻唑喹唑啉类化合物,该类化合物显示出很强的DYRK1A抑制活性,其中以化合物EHT1610 (25)和EHT5372 (26)活性最为显著,后者的IC50值达到了0.22 nm [62],且在与诸多经典的DYRK1A抑制剂进行平行试验中EHT5372表现出了更高的选择性,能有效降低tau蛋白磷酸化,明显减少β淀粉样蛋白的生成 [63] [64]。
Fruit等设计合成的噻唑骈喹唑啉骨架化合物FC162 (27)也有明显的DYRK1A抑制活性且具有良好的血脑屏障透过性,能有效抑制tau蛋白磷酸化 [65]。
4.2.4. 苯并吡唑/咪唑类
Kobayashi等通过体外DS模型筛选得到了化合物ALGERNON (28),在进行309个激酶抑制试验后,进一步确认了其对DYRK1A激酶的高选择性抑制活性。一系列试验显示该化合物能有效抑制海马体内的tau蛋白磷酸化,促进神经干细胞生长,改善DS小鼠的认知障碍行为,并且不具有如EGCG明显的单胺氧化酶抑制作用 [66]。
Kumar等通过虚拟筛选200多万个类先导物分子,再由进一步的体外筛选找到先导化合物,经修饰优化后得到了活性和选择性较高的新型苯并咪唑类化合物29 [67]。
Samumed公司通过体外筛选得到具有Wnt通路阻断作用的DYRK1A抑制剂Lorecivivint (30) [68] [69],该化合物同时具有较高的CLK2抑制活性,能够诱导软骨细胞分化,促进软骨结构再生与修复及抑制炎症 [69],已进入骨关节炎的III期临床阶段。
4.2.5. 3-(2-噻吩)吡啶类
Engel课题组通过高通量筛选并优化先导物首先得到了2,4-双杂环取代噻吩类化合物31,其DYRK1A抑制活性与Harmine接近,但同时对于DYRK1B和Clk1均具有较高的抑制作用 [70]。为了提高其选择性,他们结合计算机分子模拟,以苯丙酰胺扩展了基本骨架并进行一系列修饰得到了DYRK1A抑制活性和选择性均极大提升的化合物32 [71]。随后他们以环丙酰胺替换苯丙酰胺部分得到了活性进一步提升的化合物33,该化合物具有良好的膜透过性,代谢稳定性且无细胞毒性 [72]。
4.2.6. 氮杂吲哚类
Dodd课题组结合计算机辅助设计和体外筛选,发现了活性极高的氮杂吲哚类化合物DANDY (34),其对DYRK1A的选择性高于DYRK1B和DYRK2的两倍 [73]。但考虑到化合物DANDY过多的羟基对其血脑屏障的透过性和代谢稳定性的影响,他们最近又设计合成了氟原子取代的化合物35,大分子质谱结果分析显示,化合物34进入大鼠脑内含量达到治疗量要求,且能明显改善大鼠在认知功能模型试验中的表现 [74]。

Figure 3. Synthesized inhibitors of DYRK1A
图3. 合成的DYRK1A抑制剂
4.2.7. 异喹啉类
Samumed公司通过理性设计得到了DYRK1A抑制活性极高的异喹啉类化合物SM07883 (36),临床前研究显示该化合物可显著抑制大鼠脑内Tau蛋白的磷酸化和聚集、神经炎症以及神经纤维缠结,化合物SM07883的膜透过性好且口服生物利用度高 [75],目前已进入治疗AD的I期临床研究阶段。
5. 结语
大量研究表明DYRK1A参与了人体多种生理功能调节,被视为多种疾病尤其是神经退行性疾病的重要治疗靶点,但其药物研发仍面临较多挑战。第一,DYRK1A的具体作用机制以及相关神经退行性疾病的发病机制仍有待进一步研究与确认。第二,虽然目前已有众多DYRK1A抑制剂展现了较好的生物活性,也有化合物处于临床研究阶段,但是如何提高其选择性仍是其抑制剂研发面临的挑战。第三,区别于抗肿瘤治疗,在神经系统疾病中,DYRK1A抑制剂透过血脑屏障的能力,长期用药带来的副作用对于患者生活质量的影响等问题均需得到关注。
基金项目
国家十三五重大专项子课题(2018ZX09711-001-005)。
文章引用
王 勇,焦晓臻. 双底物特异性酪氨酸磷酸化调节激酶1A及其抑制剂的研究进展
The Dual-Specificity Tyrosine Phosphorylation Regulated Kinase 1A and the Advances of Its Inhibitors[J]. 药物化学, 2020, 08(02): 38-50. https://doi.org/10.12677/HJMCe.2020.82006
参考文献
- 1. Aranda, S., Laguna, A. and de la Luna, S. (2011) DYRK Family of Protein Kinases: Evolutionary Relationships, Biochemical Properties, and Functional Roles. The FASEB Journal, 25, 449-462. https://doi.org/10.1096/fj.10-165837
- 2. Alvarez, M., Estivill, X. and de la Luna, S. (2003) DYRK1A Accumulates in Splicing Speckles through a Novel Targeting Signal and Induces Speckle Disassembly. Journal of Cell Science, 116, 3099-3107. https://doi.org/10.1242/jcs.00618
- 3. Sunke Himpel, P.P., Eirmbter, K., Czajkowska, H., Sayed, M., Packman, L.C., Blundell, T., Kentrup, H., Grotzinger, J., Joost, H.-G. and Becker, W. (2001) Identification of the Autophosphorylation Sites and Characterization of Their Effects in the Protein Kinase DYRK1A. The Biochemical Journal, 359, 497-507. https://doi.org/10.1042/0264-6021:3590497
- 4. Fernandez-Martinez, P., Zahonero, C. and Sanchez-Gomez, P. (2015) DYRK1A: The Double-Edged Kinase as a Protagonist in Cell Growth and Tumorigenesis. Molecular and Cellular Oncology, 2, e970048. https://doi.org/10.4161/23723548.2014.970048
- 5. Martı́, E., Altafaj, X., Dierssen, M., et al. (2003) Dyrk1A Expres-sion Pattern Supports Specific Roles of This Kinase in the Adult Central Nervous System. Brain Research, 964, 250-263. https://doi.org/10.1016/S0006-8993(02)04069-6
- 6. Michiyo Okui, T.I., Morita, K., Funakoshi, E., Ito, F., Ogita, K., Yoneda, Y., Kudoh, J. and Shimizu, N. (1999) High-Level Expression of the Mnb/DYRK1A Gene in Brain and Heart during Rat Early Development. Genomics, 62, 165-171. https://doi.org/10.1006/geno.1999.5998
- 7. Jerzy Wegiela, I.K., Nowickia, K., Frackowiaka, J., Dowjata, K., Silvermanb, W.P., Reisbergc, B., deLeonc, M., Wisniewskic, T., Adayevd, T., Chen-Hwangd, M.-C. and Hwangd, Y.-W. (2004) Cell Type- and Brain Structure-Specific Patterns of Distribution of Mini-brain Kinase in Human Brain. Brain Research, 1010, 69-80. https://doi.org/10.1016/j.brainres.2004.03.008
- 8. Pamela, G.S., Lochhead, A., Morrice, N. and Cleghon, V. (2005) Activation-Loop Autophosphorylation Is Mediated by a Novel Transitional Intermediate Form of DYRKs. Cell, 121, 925-936. https://doi.org/10.1016/j.cell.2005.03.034
- 9. Park, J., Song, W.J. and Chung, K.C. (2009) Function and Regulation of Dyrk1A: Towards Understanding Down Syndrome. Cellular and Mollecular Life Science, 66, 3235-3240. https://doi.org/10.1007/s00018-009-0123-2
- 10. Ramkumar, A., Jong, B.Y. and Ori-McKenney, K.M. (2018) ReMAP-ping the Microtubule Landscape How Phosphorylation Dictates the Activities of Microtubule-Associated Proteins. Deve-lopmental Dynamics, 247, 138-155. https://doi.org/10.1002/dvdy.24599
- 11. Azorsa, R.H.R.D.O., Frost, D., Hoovet, B.M., Brautigam, G.R., Dickey, C., Beaudry, C., Basu, G.D., Holz, D.R., Hernandez, J.A., Bisanz, K.M., Gwinn, L., Grover, A., Rogers, J., Reiman, E.M., Hut-ton, M., Stephan, D.A., Mousses, S. and Dunckley, T. (2010) High-Content siRNA Screening of the Kinome Identifies Ki-nases Involved in Alzheimer’s Disease-Related Tau Hyperphosphorylation. BMC Genomics, 11, 25. https://doi.org/10.1186/1471-2164-11-25
- 12. Ryoo, S.-R., Cho, H.-J., Lee, H.W., Jeong, H.K., Radnaabazar, C., Kim, Y.-S., Kim, M.-J., Son, M.-Y., Seo, H., Chung, S.-H. and Song, W.-J. (2008) Dual-Specificity Tyrosine(Y)-phosphorylation Regulated Kinase 1A-Mediated Phosphorylation of Amyloid Precursor Protein: Evidence for a Functional Link between Down Syndrome and Alzheimer’s Disease. Journal of Neurochemistry, 104, 1333-1344. https://doi.org/10.1111/j.1471-4159.2007.05075.x
- 13. Guedj, F., Pereira, P.L., Najas, S., et al. (2012) DYRK1A: A Master Regulatory Protein Controlling Brain Growth. Neurobiology of Disease, 46, 190-203. https://doi.org/10.1016/j.nbd.2012.01.007
- 14. Latour, A., Gu, Y., Kassis, N., et al. (2019) LPS-Induced Inflammation Abolishes the Effect of DYRK1A on IkB Stability in the Brain of Mice. Molecular Neurobiology, 56, 963-975. https://doi.org/10.1007/s12035-018-1113-x
- 15. Smith, B., Medda, F., Gokhale, V., et al. (2012) Recent Advances in the Design, Synthesis, and Biological Evaluation of Selective DYRK1A Inhibitors: A New Avenue for a Disease Modifying Treatment of Alzheimer’s? ACS Chemical Neuroscience, 3, 857-872. https://doi.org/10.1021/cn300094k
- 16. Wegiel, J., Gong, C.X. and Hwang, Y.W. (2011) The Role of DYRK1A in Neurodegenerative Diseases. The FEBS Journal, 278, 236-245. https://doi.org/10.1111/j.1742-4658.2010.07955.x
- 17. Uhl, K.L., Schultz, C.R., Geerts, D., et al. (2018) Harmine, a Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase (DYRK) Inhibitor Induces Caspase-Mediated Apoptosis in Neuroblastoma. Cancer Cell International, 18, 82. https://doi.org/10.1186/s12935-018-0574-3
- 18. Ackeifi, C., Swartz, E., Kumar, K., et al. (2020) Pharmacologic and Genetic Approaches Define Human Pancreatic Beta Cell Mitogenic Targets of DYRK1A Inhibitors. JCI Insight, 5, e132594. https://doi.org/10.1172/jci.insight.132594
- 19. Belgardt, B.F. and Lammert, E. (2016) DYRK1A: A Promising Drug Target for Islet Transplant-Based Diabetes Therapies. Diabetes, 65, 1496-1498. https://doi.org/10.2337/dbi16-0013
- 20. Courtney Ackeifi, P.W., Karakose, E., Fox Manning, J.E., González, B.J., Liu, H.T., Wilson, J., Swartz, E., Berrouet, C., Li, Y.S., Kumar, K., MacDonald, P.E., Sanchez, R., Thorens, B., DeVita, R., Ho-mann, D., Egli, D., Scott, D.K., Garcia-Ocaña, A. and Stewart, A.F. (2020) GLP-1 Receptor Agonists Synergize with DYRK1A Inhibitors to Potentiate Functional Human β Cell Regeneration. Science Translational Medicine, 12, eaaw9996. https://doi.org/10.1126/scitranslmed.aaw9996
- 21. Wieslaw, T.A., Dowjata, K., Kuchna, I., Nowicki, K., Palminiello, S., Hwang, Y.W. and Wegiel, J. (2007) Trisomy-Driven Overexpression of DYRK1A Kinase in the Brain of Subjects. Neu-roscience Letters, 413, 77-81. https://doi.org/10.1016/j.neulet.2006.11.026
- 22. Walter Becker, U.S. and Tejedor, F.J. (2014) DYRK1A_ A Potential Drug Target for Multiple Down Syndrome Neuropathologies. CNS & Neurological Disorders-Drug Targets, 13, 26-33. https://doi.org/10.2174/18715273113126660186
- 23. Feki, A. and Hibaoui, Y. (2018) DYRK1A Protein, a Promising Therapeutic Target to Improve Cognitive Deficits in Down Syndrome. Brain Sciences, 8, 187. https://doi.org/10.3390/brainsci8100187
- 24. Selkoe, D.J. (2002) Alzheimer’s Disease Is a Synaptic Failure. Science, 298, 789-791. https://doi.org/10.1126/science.1074069
- 25. Sakono, M. and Zako, T. (2010) Amyloid Oligomers: Formation and Toxicity of Abeta Oligomers. The FEBS Journal, 277, 1348-1358. https://doi.org/10.1111/j.1742-4658.2010.07568.x
- 26. Kim, E.J., Sung, J.Y., Lee, H.J., et al. (2006) Dyrk1A Phos-phorylates Alpha-Synuclein and Enhances Intracellular Inclusion Formation. The Journal of Biological Chemistry, 281, 33250-33257. https://doi.org/10.1074/jbc.M606147200
- 27. Cavallarin, N., Vicario, M. and Negro, A. (2010) The Role of Phosphorylation in Synucleinopathies: Focus on Parkinson’s Disease. CNS & Neurological Disorders-Drug Targets, 9, 471-481. https://doi.org/10.2174/187152710791556140
- 28. Fan, K., Tang, B.S., Wang, Y.Q., et al. (2016) The GBA, DYRK1A and MS4A6A Polymorphisms Influence the Age at Onset of Chinese Parkinson Patients. Neuroscience Letters, 621, 133-136. https://doi.org/10.1016/j.neulet.2016.04.014
- 29. Cen, L., Xiao, Y., Wei, L., et al. (2016) Association of DYRK1A Polymorphisms with Sporadic Parkinson’s Disease in Chinese Han Population. Neuroscience Letters, 632, 39-43. https://doi.org/10.1016/j.neulet.2016.08.022
- 30. Ferrer, I., Barrachina, M., Puig, B., et al. (2005) Constitutive Dyrk1A Is Abnormally Expressed in Alzheimer Disease, Down Syndrome, Pick Disease, and Related Transgenic Models. Neurobi-ology of Disease, 20, 392-400. https://doi.org/10.1016/j.nbd.2005.03.020
- 31. Kang, J.E., Choi, S.A., Park, J.B., et al. (2005) Regulation of the Proa-poptotic Activity of Huntingtin Interacting Protein 1 by Dyrk1 and Caspase-3 in Hippocampal Neuroprogenitor Cells. Journal of Neuroscience Research, 81, 62-72. https://doi.org/10.1002/jnr.20534
- 32. Bain, J., Plater, L., Elliott, M., et al. (2007) The Selectivity of Protein Kinase Inhibitors: A Further Update. Biochemical Journal, 408, 297-315. https://doi.org/10.1042/BJ20070797
- 33. Yamamoto, N., Shibata, M., Ishikuro, R., et al. (2017) Epigallocatechin Gallate Induces Extracellular Degradation of Amyloid Beta-Protein by Increasing Neprilysin Secretion from Astrocytes through Activation of ERK and PI3K Pathways. Neuroscience, 362, 70-78. https://doi.org/10.1016/j.neuroscience.2017.08.030
- 34. De la Torre, R., De Sola, S., Pons, M., et al. (2014) Epigallocatechin-3-Gallate, a DYRK1A Inhibitor, Rescues Cognitive Deficits in Down Syndrome Mouse Models and in Humans. Molecular Nutrition & Food Research, 58, 278-288. https://doi.org/10.1002/mnfr.201300325
- 35. De Toma, I., Ortega, M., Aloy, P., et al. (2019) DYRK1A Overexpression Alters Cognition and Neural-Related Proteomic Pathways in the Hippocampus That Are Rescued by Green Tea Extract and/or Environmental Enrichment. Frontiers in Molecular Neuroscience, 12, 272. https://doi.org/10.3389/fnmol.2019.00272
- 36. De la Torre, S., Hernandez, G., Farré, M., Pujol, J., Rodriguez, J., Es-padaler, J.M., Langohr, K., Cuenca-Royo, A., Principe, A., et al. (2016) Safety and Efficacy of Cognitive Training plus Epi-gallocatechin-3-Gallate in Young Adults with Down’s Syndrome (TESDAD): A Double-Blind, Randomised, Place-bo-Controlled, Phase 2 Trial. The Lancet Neurology, 15, 810-810. https://doi.org/10.1016/S1474-4422(16)30034-5
- 37. Gockler, N., Jofre, G., Papadopoulos, C., et al. (2009) Harmine Specifically Inhibits Protein Kinase DYRK1A and Interferes with Neurite Formation. The FEBS Journal, 276, 6324-6337. https://doi.org/10.1111/j.1742-4658.2009.07346.x
- 38. Ruben, K., Wurzlbauer, A., Walte, A., et al. (2015) Selectivity Profiling and Biological Activity of Novel beta-Carbolines as Potent and Selective DYRK1 Kinase Inhibitors. PLoS ONE, 10, e0132453. https://doi.org/10.1371/journal.pone.0132453
- 39. Drung, B., Scholz, C., Barbosa, V.A., et al. (2014) Computational & Experimental Evaluation of the Structure/Activity Relationship of Beta-Carbolines as DYRK1A Inhibitors. Bioorganic Me-dicinal Chemistry Letters, 24, 4854-4860. https://doi.org/10.1016/j.bmcl.2014.08.054
- 40. Kumar, K., Wang, P., Sanchez, R., et al. (2018) Development of Ki-nase-Selective, Harmine-Based DYRK1A Inhibitors that Induce Pancreatic Human beta-Cell Proliferation. Journal of Medi-cinal Chemistry, 61, 7687-7699. https://doi.org/10.1021/acs.jmedchem.8b00658
- 41. Kunal Kumar, P.W., Wilson, J., Zlatanic, V., Berrouet, C., Khamrui, S., Secor, C., Swartz, E.A., Lazarus, M., Sanchez, R., Stewart, A.F., Garcia-Ocana, A. and DeVita, R.J. (2020) Synthesis and Biological Validation of a Harmine-Based, Central Nervous System (CNS)-Avoidant, Selective, Human β‑Cell Regenerative Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase A (DYRK1A) Inhibitor. Journal of Medicinal Chemistry, 63, 2986-3003. https://doi.org/10.1021/acs.jmedchem.9b01379
- 42. Gompel, M., Leost, M., De Kier Joffe, E.B., et al. (2004) Meridia-nins, a New Family of Protein Kinase Inhibitors Isolated from the Ascidian Aplidium meridianum. Bioorganic Medicinal Chemistry Letters, 14, 1703-1707. https://doi.org/10.1016/j.bmcl.2004.01.050
- 43. Llorach-Pares, L., Nonell-Canals, A., Sanchez-Martinez, M., et al. (2017) Computer-Aided Drug Design Applied to Marine Drug Discovery: Meridianins as Alzheimer’s Disease Therapeutic Agents. Marine Drugs, 15, 366. https://doi.org/10.3390/md15120366
- 44. Radwan, M.A. and El-Sherbiny, M. (2007) Synthesis and Antitumor Activity of Indolylpyrimidines: Marine Natural Product Meridianin D Analogues. Bioorganic Medicinal Chemistry, 15, 1206-1211. https://doi.org/10.1016/j.bmc.2006.11.023
- 45. Giraud, F., Alves, G., Debiton, E., et al. (2011) Synthesis, Protein Kinase Inhibitory Potencies, and in Vitro Antiproliferative Activities of Meridianin Derivatives. Journal of Medicinal Chemistry, 54, 4474-4489. https://doi.org/10.1021/jm200464w
- 46. Yadav, R.R., Sharma, S., Joshi, P., et al. (2015) Meridianin Derivatives as Potent Dyrk1A Inhibitors and Neuroprotective Agents. Bioorganic Medicinal Chemistry Letters, 25, 2948-2952. https://doi.org/10.1016/j.bmcl.2015.05.034
- 47. Esvan, Y.J., Zeinyeh, W., Boibessot, T., et al. (2016) Discovery of Py-rido[3,4-g]quinazoline Derivatives as CMGC Family Protein Kinase Inhibitors: Design, Synthesis, Inhibitory Potency and X-Ray Co-Crystal Structure. European Journal of Medicinal Chemistry, 118, 170-177. https://doi.org/10.1016/j.ejmech.2016.04.004
- 48. Zeinyeh, W., Esvan, Y.J., Nauton, L., et al. (2016) Synthesis and Preliminary in Vitro Kinase Inhibition Evaluation of New Diversely Substituted Pyrido[3,4-g]quinazoline Derivatives. Bio-organic & Medicinal Chemistry Letters, 26, 4327-4329. https://doi.org/10.1016/j.bmcl.2016.07.032
- 49. Tazarki, H., Zeinyeh, W., Esvan, Y.J., et al. (2019) New Pyrido[3,4-g]quinazoline Derivatives as CLK1 and DYRK1A Inhibitors: Syn-thesis, Biological Evaluation and Binding Mode Analysis. European Journal of Medicinal Chemistry, 166, 304-317. https://doi.org/10.1016/j.ejmech.2019.01.052
- 50. Debdab, M., Carreaux, F., Renault, S., et al. (2011) Leucettines, a Class of Potent Inhibitors of CDC2-Like Kinases and Dual Specificity, Tyrosine Phosphorylation Regulated Kinases Derived from the Marine Sponge Leucettamine B: Modulation of Alternative Pre-RNA Splicing. Journal of Medicinal Chemistry, 54, 4172-4186. https://doi.org/10.1021/jm200274d
- 51. Souchet, B., Audrain, M., Billard, J.M., et al. (2019) Inhibition of DYRK1A Proteolysis Modifies Its Kinase Specificity and Rescues Alzheimer Phenotype in APP/PS1 Mice. Acta Neuropathologica Communications, 7, 46. https://doi.org/10.1186/s40478-019-0678-6
- 52. Nguyen, T.L., Duchon, A., Manousopoulou, A., et al. (2018) Correc-tion of Cognitive Deficits in Mouse Models of Down Syndrome by a Pharmacological Inhibitor of DYRK1A. Disease Models & Mechanisms, 11, Article ID: 035634. https://doi.org/10.1242/dmm.035634
- 53. Beniddir, M.A., Le Borgne, E., Iorga, B.I., et al. (2014) Acridone Alkaloids from Glycosmis Chlorosperma as DYRK1A Inhibitors. Journal of Natural Products, 77, 1117-1122. https://doi.org/10.1021/np400856h
- 54. Ogawa, Y., Nonaka, Y., Goto, T., et al. (2010) Development of a Novel Selec-tive Inhibitor of the Down Syndrome-Related Kinase Dyrk1A. Nature Communication, 1, Article No. 86. https://doi.org/10.1038/ncomms1090
- 55. Masaki, S., Kii, I., Sumida, Y., et al. (2015) Design and Synthesis of a Potent Inhibitor of Class 1 DYRK Kinases as a Suppressor of Adipogenesis. Bioorganic Medicinal Chemistry, 23, 4434-4441. https://doi.org/10.1016/j.bmc.2015.06.018
- 56. Kii, I., Sumida, Y., Goto, T., et al. (2016) Selective Inhibition of the Kinase DYRK1A by Targeting Its Folding Process. Nature Communications, 7, Article No. 11391. https://doi.org/10.1038/ncomms11391
- 57. Sonamoto, R., Kii, I., Koike, Y., et al. (2015) Identification of a DYRK1A Inhibitor That Induces Degradation of the Target Kinase Using Co-Chaperone CDC37 Fused with Luciferase nanoKAZ. Scientific Reports, 5, Article No. 12728. https://doi.org/10.1038/srep12728
- 58. Salah, M., Abdel-Halim, M. and Engel, M. (2018) Design and Synthesis of Conformationally Constraint Dyrk1A Inhibitors by Creating an Intramolecular H-Bond Involving a Benzothiazole Core. Medchemcomm, 9, 1045-1053. https://doi.org/10.1039/C8MD00142A
- 59. Falke, H., Chaikuad, A., Becker, A., et al. (2015) 10-Iodo-11H-indolo[3,2-c] Quinoline-6-Carboxylic Acids Are Selective Inhibitors of DYRK1A. Journal of Medicinal Chemistry, 58, 3131-3143. https://doi.org/10.1021/jm501994d
- 60. Rosanna Meine, W.B., Falke, H., Preu, L., Loaëc, N., Meijer, L. and Kunick, C. (2018) Indole-3 Carbonitriles as DYRK1A Inhibitors by Fragment-Based Drug Design. Molecules, 23, 64. https://doi.org/10.3390/molecules23020064
- 61. Lechner, C., Flasshoff, M., Falke, H., et al. (2019) [b]-Annulated Halogen-Substituted Indoles as Potential DYRK1A Inhibitors. Molecules, 24, 4090. https://doi.org/10.3390/molecules24224090
- 62. Rosse, G. (2013) Tricyclic Pyrimidines as Inhibitors of DYRK1A/DYRK1B as Potential Treatment for Down’s Syndrome or Alzheimer’s Disease. Medicinal Chemistry Letters, 4, 502-503. https://doi.org/10.1021/ml400137s
- 63. Coutadeur, S., Benyamine, H., Delalonde, L., et al. (2015) A Novel DYRK1A (Dual Specificity Tyrosine Phosphorylation-Regulated Kinase 1A) Inhibitor for the Treatment of Alzheimer’s Disease: Effect on Tau and Amyloid Pathologies in Vitro. Journal of Neurochemistry, 133, 440-451. https://doi.org/10.1111/jnc.13018
- 64. Chaikuad, A., Diharce, J., Schroder, M., et al. (2016) An Unusual Binding Model of the Methyl 9-Anilinothiazolo [5,4-f]quinazoline-2-carbimidates (EHT 1610 and EHT 5372) Confers High Selectivity for Dual-Specificity Tyrosine Phosphorylation-Regulated Kinases. Journal of Medicinal Chemistry, 59, 10315-10321. https://doi.org/10.1021/acs.jmedchem.6b01083
- 65. Fruit, C., Couly, F., Bhansali, R., et al. (2019) Biological Charac-terization of 8-Cyclopropyl-2-(pyridin-3-yl)thiazo- lo[5,4-f]quinazolin-9(8H)-one, a Promising Inhibitor of DYRK1A. Pharmaceuticals (Basel), 12, 185. https://doi.org/10.3390/ph12040185
- 66. Nakano-Kobayashi, A., Awaya, T., Kii, I., et al. (2017) Prenatal Neurogenesis Induction Therapy Normalizes Brain Structure and Function in Down Syndrome Mice. Proceedings of the National Academy of Sciences of the United States of America, 114, 10268-10273. https://doi.org/10.1073/pnas.1704143114
- 67. Kumar, K., Man-Un Ung, P., Wang, P., et al. (2018) Novel Selective Thiadiazine DYRK1A Inhibitor Lead Scaffold with Human Pancreatic Beta-Cell Proliferation Activity. European Journal of Medicinal Chemistry, 157, 1005-1016. https://doi.org/10.1016/j.ejmech.2018.08.007
- 68. Deshmukh, V., Hu, H., Barroga, C., et al. (2018) A Small-Molecule Inhibitor of the Wnt Pathway (SM04690) as a Potential Disease Modifying Agent for the Treatment of Osteoarthritis of the Knee. Osteoarthritis Cartilage, 26, 18-27. https://doi.org/10.1016/j.joca.2017.08.015
- 69. Deshmukh, V., O’Green, A.L., Bossard, C., et al. (2019) Modulation of the Wnt Pathway through Inhibition of CLK2 and DYRK1A by Lorecivivint as a Novel, Potentially Disease-Modifying Ap-proach for Knee Osteoarthritis Treatment. Osteoarthritis Cartilage, 27, 1347-1360. https://doi.org/10.1016/j.joca.2019.05.006
- 70. Schmitt, C., Kail, D., Mariano, M., et al. (2014) Design and Synthesis of a Library of Lead-Like 2,4-Bisheterocyclic Substituted Thiophenes as Selective Dyrk/Clk Inhibitors. PLoS ONE, 9, e87851. https://doi.org/10.1371/journal.pone.0087851
- 71. Sarah, M.A.-H., Darwish, S., Salah, M., Abadi, A.H. and Becker, W. (2018) Matthias Engel Development of Novel 2,4-bispyridyl Thiophene-Based Compounds as Highly Potent and Selective Dyrk1A Inhibitors. Part I: Benzamide and Benzylamide Derivatives. European Journal of Medicinal Chemistry, 157, 1031-1050. https://doi.org/10.1016/j.ejmech.2018.07.050
- 72. Darwish, S.S., Abdel-Halim, M., ElHady, A.K., et al. (2018) De-velopment of Novel Amide-Derivatized 2,4-bispyridyl Thiophenes as Highly Potent and Selective Dyrk1A Inhibitors. Part II: Identification of the Cyclopropylamide Moiety as a Key Modification. European Journal of Medicinal Chemistry, 158, 270-285. https://doi.org/10.1016/j.ejmech.2018.08.097
- 73. Gourdain, S., Dairou, J., Denhez, C., et al. (2013) Development of DANDYs, New 3,5-diaryl-7-azaindoles Demonstrating Potent DYRK1A Kinase Inhibitory Activity. Journal of Medicinal Chemistry, 56, 9569-9585. https://doi.org/10.1021/jm401049v
- 74. Neumann, F., Gourdain, S., Albac, C., et al. (2018) DYRK1A Inhibition and Cognitive Rescue in a Down Syndrome Mouse Model Are Induced by New Fluoro-DANDY Derivatives. Science Reports, 8, Article No. 2859. https://doi.org/10.1038/s41598-018-20984-z
- 75. Melchior, B., Mittapalli, G. K., Lai, C., et al. (2019) Tau Pathology Reduction with SM07883, a Novel, Potent, and Selective Oral DYRK1A Inhibitor: A Potential Therapeutic for Alzheimer’s Disease. Aging Cell, 18, 1-14. https://doi.org/10.1111/acel.13000
附录
缩略词对照表
