Advances in Clinical Medicine
Vol.
12
No.
02
(
2022
), Article ID:
48780
,
10
pages
10.12677/ACM.2022.122161
早期胃癌内镜黏膜下剥离术C-2级切除的风险因素
王剑舒1,李晓宇1,张雪琳2,王占军1,刘建勋1,毛涛1*
1青岛大学附属医院消化内科,山东 青岛
2射阳县人民医院消化内科,江苏 盐城
收稿日期:2022年1月16日;录用日期:2022年2月9日;发布日期:2022年2月18日

摘要
目的:明确早期胃癌(Early gastric cancer, EGC)经内镜黏膜下剥离术(Endoscopic submucosal dissection, ESD)内镜治愈度(Endoscopic curability, eCura) C-2切除的风险因素。方法:回顾性收集2017年10月至2021年9月于青岛大学附属医院经ESD治疗的658个EGC病灶,根据病灶的治愈情况分为eCura C-2 ESD组与非eCura C-2 ESD组,分析两组的临床及病理特征。结果:65 (9.9%)个病灶经ESD eCura C-2切除。单因素分析提示男性患者,吸烟史,饮酒史,癌胚抗原 > 3.4 g/L,多发EGC,肿瘤位于贲门、胃底或胃体,未分化型组织学,混合组织学,以及病灶直径 > 20 mm均与ESD eCura C-2切除相关(P均 < 0.05)。多因素分析确认ESD eCura C-2切除的独立风险因素为肿瘤位于贲门(OR = 13.254, P < 0.001)、胃底或胃体(OR = 3.702, P < 0.001),未分化型组织学(OR = 8.918, P < 0.001),混合组织学(OR = 4.551, P < 0.001),病灶直径 > 20 mm (OR = 4.918, P < 0.001),多发EGC (OR = 2.871, P = 0.026),以及吸烟史(OR = 2.004, P = 0.036)。结论:具有上述ESD eCura C-2切除独立风险因素的EGC病灶应更加彻底地剥离。
关键词
早期胃癌,内镜黏膜下剥离术,内镜治愈度,非治愈性,手术

Risk Factors for Endoscopic Submucosal Dissection C-2 Resection in Early Gastric Cancer
Jianshu Wang1, Xiaoyu Li1, Xuelin Zhang2, Zhanjun Wang1, Jianxun Liu1, Tao Mao1*
1Department of Gastroenterology, The Affiliated Hospital of Qingdao University, Qingdao Shandong
2Department of Gastroenterology, Sheyang County People’s Hospital, Yancheng Jiangsu
Received: Jan. 16th, 2022; accepted: Feb. 9th, 2022; published: Feb. 18th, 2022

ABSTRACT
Objective: To clarify the risk factors for endoscopic curability (eCura) C-2 endoscopic submucosal dissection (ESD) in early gastric cancer (EGC). Methods: 658 EGCs who underwent ESD between October 2017 and September 2021 at the Affiliated Hospital of Qingdao University were retrospectively collected. All enrolled EGCs were divided into eCura C-2 ESD group and non-eCura C-2 ESD group according to the curability. The clinicopathological characteristics of the two groups were analyzed. Results: 65 (9.9%) lesions underwent eCura C-2 ESD. Univariate analysis revealed that male sex, smoking and drinking history, carcinoembryonic antigen level > 3.4 g/L, multiple EGCs, tumors located in cardia, fundus or corpora, undifferentiated histology, mixed histology and lesion size > 20 mm were associated with eCura C-2 ESD (All the above factors, P < 0.05). Multivariate analysis confirmed that tumors located in cardia (OR = 13.254, P < 0.001), fundus or corpora (OR = 3.702, P < 0.001), undifferentiated histology (OR = 8.918, P < 0.001), mixed histology (OR = 4.551, P < 0.001), lesion size > 20 mm (OR = 4.918, P < 0.001), multiple EGCs (OR = 2.871, P = 0.026) and smoking history (OR = 2.004, P = 0.036) were independent risk factors for eCura C-2 ESD. Conclusions: EGCs with independent risk factors for eCura C-2 ESD described above should be more thoroughly resected.
Keywords:Early Gastric Cancer, Endoscopic Submucosal Dissection, Endoscopic Curability, Non-Curative, Surgery

Copyright © 2022 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
早期胃癌是指癌灶局限于黏膜层或黏膜下层而不论有无淋巴结转移的胃癌 [1]。近年来,内镜黏膜下剥离术(Endoscopic submucosal dissection, ESD)由于其微创、高效的特点而逐渐成为早期胃癌的主要治疗手段。然而,尽管内镜医师会尽可能地遵循ESD指征选取病灶,仍有相当数量的病灶在ESD后未达治愈性切除 [2] [3]。ESD的非治愈性切除在日本胃癌协会(Japanese Gastric Cancer Association, JGCA)发布的第5版日本胃癌治疗指南中被定义为内镜治愈度(Endoscopic curability, eCura) C级切除,并可细分为eCuraC-1和eCuraC-2 [4]。eCuraC-2切除后肿瘤复发的可能性较高,往往需要追加外科手术。但追加手术意味着额外的费用和风险,甚至会造成患者术后生存质量的下降以及不必要的医患纠纷。因此,很有必要在ESD前评估eCura C-2切除的可能性并及时采取措施以避免这一结局发生。本研究旨在回顾性分析经ESD治疗的早期胃癌的临床及病理特征,从而明确我国早期胃癌ESD eCura C-2切除的风险因素。
2. 方法
2.1. 患者及研究设计
本研究获得青岛大学附属医院伦理委员会的批准(QYFY WZLL 26256),其所有步骤依据世界医学协会赫尔辛基宣言进行。回顾性收集2017年10月至2021年9月于青岛大学附属医院接受ESD治疗的早期胃癌病灶。排除标准为:1) 资料缺失,2) 残胃病灶,3) 既往早期胃癌内镜治疗史。收集的病灶依据第5版JGCA指南中ESD的治愈性标准分为两组 [4]:一组为ESD eCura C-2切除的病灶(eCura C-2 ESD组),另一组为ESD eCura A、eCura B和eCura C-1切除的病灶(非eCura C-2 ESD组),分析两组病灶的临床及病理特征。
2.2. ESD操作及治愈性判定标准
所有的ESD操作均由本院经验丰富的内镜医师完成。首先,确认病灶并用针刀在病灶边缘外5~10 mm处标记;然后,向黏膜下层注射混有去甲肾上腺素的生理盐水抬举病灶;接着,用IT刀或Flush刀在标记周围做一个切口;最后,于黏膜下层仔细剥离病灶至切除。
根据第5版JGCA指南,ESD的治愈性标准分为eCura A、eCura B和eCura C,分别代表治愈性切除、扩大指征的治愈性切除和非治愈性切除 [4]。eCura A和eCura B的前提条件是病灶被整块切除、切缘阴性且没有脉管侵犯。满足上述条件后,如果病灶:1) 局限于黏膜层、分化型为主、无溃疡;2) 局限于黏膜层、分化型为主、有溃疡、直径 ≤ 30 mm,治愈性归为eCura A。如果病灶满足前提条件且1) 局限于黏膜层、未分化型为主、无溃疡、直径 ≤ 20 mm,或者2) 侵犯黏膜下层小于500 µm、分化型为主、直径 ≤ 30 mm,治愈性归为eCura B。若不符合上述任何一条标准,则治愈性归为eCura C。eCura C包括eCura C-1和eCura C-2。分化型癌仅由水平切缘阳性或分段切除导致的非治愈性切除归为eCura C-1,其余非治愈性切除归为eCura C-2。需要指出的是,对于病理类型为混合型的癌灶,eCura A标准1) 中未分化成分 > 20 mm与eCura B标准2) 中未分化成分侵入黏膜下层皆归为eCura C-2。
2.3. 资料收集及统计分析
根据JGCA指南ESD的治愈性判定标准,收集病灶组织学类型、组织学混合情况、病灶直径以及溃疡情况。根据内镜下病灶的直观特征,收集病灶部位、环周定位以及肉眼类型。根据本院内镜医师的临床经验,收集病灶数量以及患者的体质指数(Body mass index, BMI)水平、癌胚抗原水平。由于烟酒可促进恶性肿瘤的发生发展,因此收集患者的吸烟史、饮酒史。由于阿司匹林以及幽门螺杆菌可对胃黏膜造成一定影响,因此收集患者的阿司匹林服用史以及幽门螺杆菌感染情况。由于ESD的非治愈性切除也可由术者操作因素造成,因此收集ESD医师情况。最后,补充收集患者的人口统计学资料如年龄、性别以及胃癌家族史。高级内镜医师在本研究中的定义为取得副主任及以上职称的内镜医师。根据胃的解剖特点将病灶部位分为胃角或胃窦、胃底或胃体、贲门三部分并将病灶环周定位分为小弯、前壁、后壁、大弯四部分;根据巴黎内镜分类标准将病灶肉眼类型分为隆起型(0-I),表浅隆起型(0-IIa)、表浅平坦型(0-IIb)、表浅凹陷型(0-IIc)、凹陷型(0-III)五类 [5]。病灶的组织学依据其主要组织学类型以及日本胃癌分类标准分为分化型(包括分化良好的管状腺癌和乳头状腺癌)和未分化型(包括印戒细胞癌和低分化腺癌) [1]。
单因素分析采用卡方检验或Fisher精确性检验。将单因素分析中显著的风险因素纳入多因素分析。多因素分析采用二元Logistic回归。所有的统计学分析均由SPSS 26完成,各因素对ESD eCura C-2切除的风险程度采用比值比与95%可信区间表示。统计学显著的标准为双侧P值小于0.05。
3. 结果
本研究收集了2017年10月至2021年9月于青岛大学附属医院诊断为早期胃癌并接受ESD治疗的642例患者并680个病灶。应用排除标准后,最终纳入634例患者并658个病灶。其中,65 (9.9%)个病灶经ESD eCura C-2切除(eCura C-2 ESD组),另外593 (90.1%)个病灶经ESD eCura A、eCura B或eCura C-1切除(非eCura C-2 ESD组) (图1)。
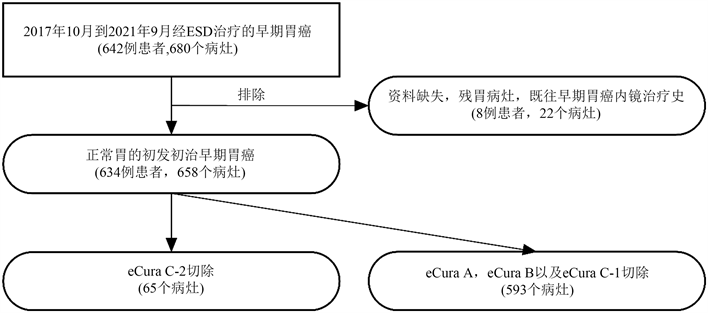
Figure 1. Flowchart for the patients included in this study
图1. 本研究纳入患者流程图
单因素分析提示男性患者(χ2 = 4.864, P = 0.027),吸烟史(χ2 = 4.373, P = 0.037),饮酒史(χ2 = 6.994, P = 0.008),癌胚抗原 > 3.4 g/L (χ2 = 4.909, P = 0.027),多发早期胃癌(P = 0.004),肿瘤位于贲门(P < 0.001)、胃底或胃体(χ2 = 24.025, P < 0.001),未分化型组织学(P < 0.001),混合组织学(χ2 = 83.094, P < 0.001),以及病灶直径 > 20 mm (χ2 = 46.725, P < 0.001)为早期胃癌ESD eCura C-2切除的显著风险因素。其它因素如年龄、阿司匹林服用史、胃癌家族史、BMI水平、ESD医师情况、病灶环周定位、肉眼类型、幽门螺杆菌感染情况以及溃疡情况则与早期胃癌ESD eCura C-2切除无明显关联(表1)。
Table 1. Univariate analysis of risk factors for eCura C-2 ESD [n (%)]
表1. ESD eCura C-2切除风险因素的单因素分析[例(%)]
注:“-”为无此值,发生于采用Fisher精确性检验的因素中。
多因素分析确认肿瘤位于贲门(OR = 13.254, P < 0.001)、胃底或胃体(OR = 3.702, P < 0.001),未分化型组织学(OR = 8.918, P < 0.001),混合组织学(OR = 4.551, P < 0.001),病灶直径 > 20 mm (OR = 4.918, P < 0.001),多发早期胃癌(OR = 2.871, P = 0.026),以及吸烟史(OR = 2.004, P = 0.036)是早期胃癌ESD eCura C-2切除的独立风险因素(表2)。
Table 2. Multivariate analysis of risk factors for eCura C-2 ESD
表2. ESD eCura C-2切除风险因素的多因素分析
4. 讨论
了解早期胃癌ESD eCura C-2切除的风险因素有助于改善ESD的治愈情况从而降低医患双方的负担。本研究共纳入658例经ESD切除的早期胃癌病灶,eCura C-2切除率为9.9%,略低于近期一项研究的报道(11.9%) [6]。我们的结果表明,肿瘤位于贲门、胃底或胃体,未分化型组织学,混合组织学,病灶直径 > 20 mm,多发早期胃癌,以及吸烟史均为早期胃癌ESD eCura C-2切除的独立风险因素。
本研究依据胃的解剖特点将胃分为贲门、胃底或胃体、胃角或胃窦三部分,前两者约占据胃的上2/3部,并与ESD eCura C-2切除显著相关。我们的结果在一定程度上得到了其他研究的证实 [7] - [13]。其实,近端胃癌的侵袭性要强于远端胃癌。有研究发现胃上2/3部胃癌的黏膜下层侵犯率更高 [14] [15],这或许是因为胃上部的胃壁及黏膜下层薄于胃下部,从而使肿瘤更易向深部浸润 [16] [17]。再者,胃的淋巴管分布具有“地域性”特点,相比于胃下部,胃上部的淋巴管相对密集,由此造成肿瘤更易发生脉管浸润 [16]。最后,鉴于胃的结构特点,胃上部特别是贲门及胃底的肿瘤往往需要折返胃镜进行操作,这会在一定程度上造成诊断与剥离的困难。
未分化型早期胃癌并非ESD的禁忌。然而,既往研究发现未分化型癌更易发生黏膜下浸润 [18] [19]。Kim等发现未分化型癌特别是印戒细胞癌具有沿着上皮下层波散的倾向 [20];在Shim等的研究中,未分化型癌是内镜下大小被低估的独立危险因素 [21]。因此,未分化型癌的实际体积可能要大于内镜下估计的体积,应在ESD前考虑切除失败的可能性并采取适当扩大剥离面积等措施以避免其发生。混合型癌是指混有未分化成分的分化型癌或混有分化成分的未分化型癌。我们的结果提示混合型早期胃癌显著影响ESD的治愈性。这或许是因为混合型癌的恶性程度较高。有研究发现混合型癌的恶性病理特征较多,其在外科手术标本中的淋巴结转移率高于单纯型癌 [22] - [27]。再者,内镜医师很难在ESD前准确诊断混合型早期胃癌。具体而言,由于内镜图像及活检部位的局限性,许多混合型癌可能在ESD前被诊断为单纯型癌并按此处理。
病灶大小在一些研究中被认为显著影响ESD的治愈性 [7] [11] [13] [28]。然而,另有研究报道病灶大小与ESD的治愈性无明显关联 [29]。本研究中,直径大于20 mm的病灶是ESD eCura C-2切除的独立风险因素。事实上,较大的病灶往往预示着较深的肿瘤浸润程度 [19]。并且Xu等发现大病灶中未分化型癌的占比更高,更易发生ESD切缘阳性 [6]。不仅如此,一项来自日本的研究发现,超声内镜对于早期胃癌浸润深度诊断的准确率随着病灶尺寸的增大而下降,较大尺寸病灶是降低超声内镜诊断准确率的显著危险因素 [30]。因此,结合上述研究,较大尺寸病灶可能更易发生ESD eCura C-2切除,在处理时需格外注意。
多发早期胃癌在临床中并不少见。尽管有研究报道多发早期胃癌的病理情况及预后结局与单发早期胃癌相当 [31] [32],但我们的结果提示多发早期胃癌相对较难达到ESD治愈性切除。一项来自韩国的大样本量研究发现多发早期胃癌相比于单发早期胃癌更易发生黏膜下浸润 [33]。而在另外一项纳入244例患者的外科手术研究中,16例多发早期胃癌患者中只有2 (12.5%)例符合内镜切除指征,黏膜下浸润为最常见的超指征原因 [34]。此外,在ESD的操作过程中,相邻病灶剥离后的创面会不可避免地对下一病灶的剥离造成一定影响,因而亦不除外此影响降低多发早期胃癌的治愈性。
我们进一步探讨了患者生活习惯对ESD治愈性的影响,这也是该研究的一个创新之处。吸烟被认为是胃癌的危险因素 [35] [36],既往研究报道具有吸烟史的早期胃癌患者更易在ESD后再发胃癌 [37] [38]。Jeon等通过分析内镜切除术前后肿瘤的病理情况发现吸烟是肿瘤“病理升级”的独立危险因素 [39]。Chang等通过评估ESD后生存结局发现吸烟可显著降低早期胃癌患者的远期生存率 [40]。上述研究提示吸烟促进胃癌的发生与发展。本研究中,具有吸烟史的患者更易发生ESD eCura C-2切除。一个可能的原因是尼古丁通过多种信号通路抑制胃癌细胞的凋亡并促进其侵袭,由此造成吸烟者的肿瘤实际扩散程度广于剥离前的预期 [41] [42]。
5. 结论
综上所述,肿瘤位于贲门、胃底或胃体,未分化型组织学,混合组织学,病灶直径 > 20 mm,多发早期胃癌,以及吸烟史是ESD eCura C-2切除的独立风险因素。我们建议对具有上述病灶特征的患者进行更加充分地沟通并对相应病灶进行更加彻底地切除。本研究的亮点在于其应用了最新的ESD治愈性评价标准并且是我国纳入样本量最多的早期胃癌ESD临床结局研究。然而,由于其单中心规模及回顾性设计,此研究仍有一定的局限性,在未来尚需进一步开展多中心、大规模、前瞻性研究。
致谢
所有作者感谢他们同事对本研究开展的支持。
基金项目
国家自然科学基金(81802777)。
文章引用
王剑舒,李晓宇,张雪琳,王占军,刘建勋,毛 涛. 早期胃癌内镜黏膜下剥离术C-2级切除的风险因素
Risk Factors for Endoscopic Submucosal Dissection C-2 Resection in Early Gastric Cancer[J]. 临床医学进展, 2022, 12(02): 1095-1104. https://doi.org/10.12677/ACM.2022.122161
参考文献
- 1. Japanese Gastric Cancer Association (2011) Japanese Classification of Gastric Carcinoma: 3rd English Edition. Gastric Cancer, 14, 101-112. https://doi.org/10.1007/s10120-011-0041-5
- 2. Akintoye, E., Obaitan, I., Muthusamy, A., et al. (2016) Endoscopic Submucosal Dissection of Gastric Tumors: A Systematic Review and Meta-Analysis. World Journal of Gastrointestinal Endoscopy, 8, 517-532. https://doi.org/10.4253/wjge.v8.i15.517
- 3. Choi, I.J., Lee, N.R., Kim, S.G., et al. (2016) Short-Term Outcomes of Endoscopic Submucosal Dissection in Patients with Early Gastric Cancer: A Prospective Multicenter Cohort Study. Gut and Liver, 10, 739-748. https://doi.org/10.5009/gnl15466
- 4. Japanese Gastric Cancer Association (2021) Japanese Gastric Cancer Treatment Guidelines 2018 (5th Edition). Gastric Cancer, 24, 1-21. https://doi.org/10.1007/s10120-020-01042-y
- 5. Participants in the Paris Workshop (2003) The Paris Endoscopic Classification of Superficial Neoplastic Lesions: Esophagus, Stomach, and Colon: November 30 to December 1, 2002. Gastrointestinal Endoscopy, 58, S3-S43. https://doi.org/10.1016/S0016-5107(03)02159-X
- 6. Xu, P., Wang, Y., Dang, Y., et al. (2020) Predictive Factors and Long-Term Outcomes of Early Gastric Carcinomas in Patients with Non-Curative Resection by Endoscopic Submucosal Dissection. Cancer Management and Research, 12, 8037-8046. https://doi.org/10.2147/CMAR.S263525
- 7. Hirasawa, K., Kokawa, A., Oka, H., et al. (2011) Risk Assessment Chart for Curability of Early Gastric Cancer with Endoscopic Submucosal Dissection. Gastrointestinal Endoscopy, 74, 1268-1275. https://doi.org/10.1016/j.gie.2011.07.067
- 8. Toyokawa, T., Inaba, T., Omote, S., et al. (2015) Risk Factors for Non-Curative Resection of Early Gastric Neoplasms with Endoscopic Submucosal Dissection: Analysis of 1,123 Lesions. Experimental and Therapeutic Medicine, 9, 1209-1214. https://doi.org/10.3892/etm.2015.2265
- 9. Ohnita, K., Isomoto, H., Yamaguchi, N., et al. (2009) Factors Related to the Curability of Early Gastric Cancer with Endoscopic Submucosal Dissection. Surgical Endoscopy, 23, 2713-2719. https://doi.org/10.1007/s00464-009-0473-8
- 10. Goto, A., Nishikawa, J., Okamoto, T., et al. (2013) Outcomes of Endoscopic Submucosal Dissection for Early Gastric Cancer and Factors Associated with Incomplete Resection. Hepatogastroenterology, 60, 46-53. https://doi.org/10.5754/hge12533
- 11. Kim, E.H., Park, J.C., Song, I.J., et al. (2017) Prediction Model for Non-Curative Resection of Endoscopic Submucosal Dissection in Patients with Early Gastric Cancer. Gastrointestinal Endoscopy, 85, 976-983. https://doi.org/10.1016/j.gie.2016.10.018
- 12. Numata, N., Oka, S., Tanaka, S., et al. (2015) Risk Factors and Management of Positive Horizontal Margin in Early Gastric Cancer Resected by En Bloc Endoscopic Submucosal Dissection. Gastric Cancer, 18, 332-338. https://doi.org/10.1007/s10120-014-0368-9
- 13. Fu, Q.Y., Cui, Y., Li, X.B., et al. (2016) Relevant Risk Factors for Positive Lateral Margin after En Bloc Endoscopic Submucosal Dissection for Early Gastric Adenocarcinoma. Journal of Digestive Diseases, 17, 244-251. https://doi.org/10.1111/1751-2980.12342
- 14. Kang, D.H., Choi, C.W., Kim, H.W., et al. (2017) Location Characteristics of Early Gastric Cancer Treated with Endoscopic Submucosal Dissection. Surgical Endoscopy, 31, 4673-4679. https://doi.org/10.1007/s00464-017-5534-9
- 15. Huang, Q., Fang, C., Shi, J., et al. (2015) Differences in Clinicopathology of Early Gastric Carcinoma between Proximal and Distal Location in 438 Chinese Patients. Scientific Reports, 5, Article No. 13439. https://doi.org/10.1038/srep13439
- 16. Akashi, Y., Noguchi, T., Nagai, K., et al. (2011) Cytoarchitecture of the Lamina Muscularis Mucosae and Distribution of the Lymphatic Vessels in the Human Stomach. Medical Molecular Morphology, 44, 39-45. https://doi.org/10.1007/s00795-010-0503-6
- 17. Park, S., Chun, H.J., Kwon, Y.D., et al. (2008) Stretching Causes Extensive Changes of Gastric Submucosa: Is It Acceptable to Define 500 Microm as the Safe Margin? Gut and Liver, 2, 199-204. https://doi.org/10.5009/gnl.2008.2.3.199
- 18. Kim, J.H., Lee, Y.C., Kim, H., et al. (2009) Endoscopic Resection for Undifferentiated Early Gastric Cancer. Gastrointestinal Endoscopy, 69, e1-e9. https://doi.org/10.1016/j.gie.2008.10.040
- 19. Embaye, K.S., Zhang, C., Ghebrehiwet, M.A., et al. (2021) Clinico-Pathologic Determinants of Non-E-Curative Outcome Following En-Bloc Endoscopic Submucosal Dissection in Patients with Early Gastric Neoplasia. BMC Cancer, 21, Article No. 92. https://doi.org/10.1186/s12885-020-07762-9
- 20. Kim, H., Kim, J.H., Lee, Y.C., et al. (2015) Growth Patterns of Signet Ring Cell Carcinoma of the Stomach for Endoscopic Resection. Gut and Liver, 9, 720-726. https://doi.org/10.5009/gnl14203
- 21. Shim, C.N., Song, M.K., Kang, D.R., et al. (2014) Size Discrepancy between Endoscopic Size and Pathologic Size Is Not Negligible in Endoscopic Resection for Early Gastric Cancer. Surgical Endoscopy, 28, 2199-2207. https://doi.org/10.1007/s00464-014-3453-6
- 22. Takizawa, K., Ono, H., Kakushima, N., et al. (2013) Risk of Lymph Node Metastases from Intramucosal Gastric Cancer in Relation to Histological Types: How to Manage the Mixed Histological Type for Endoscopic Submucosal Dissection. Gastric Cancer, 16, 531-536. https://doi.org/10.1007/s10120-012-0220-z
- 23. Bang, C.S., Yang, Y.J., Lee, J.J., et al. (2020) Endoscopic Submucosal Dissection of Early Gastric Cancer with Mixed-Type Histology: A Systematic Review. Digestive Diseases and Sciences, 65, 276-291. https://doi.org/10.1007/s10620-019-05761-w
- 24. Lee, J.H., Choi, I.J., Han, H.S., et al. (2015) Risk of Lymph Node Metastasis in Differentiated Type Mucosal Early Gastric Cancer Mixed with Minor Undifferentiated Type Histology. Annals of Surgical Oncology, 22, 1813-1819. https://doi.org/10.1245/s10434-014-4167-7
- 25. Seo, H.S., Lee, G.E., Kang, M.G., et al. (2019) Mixed Histology Is a Risk Factor for Lymph Node Metastasis in Early Gastric Cancer. Journal of Surgical Research, 236, 271-277. https://doi.org/10.1016/j.jss.2018.11.055
- 26. Zhao, B., Huang, R., Lu, H., et al. (2020) Risk of Lymph Node Metastasis and Prognostic Outcome in Early Gastric Cancer Patients with Mixed Histologic Type. Current Problems in Cancer, 44, Article ID: 100579. https://doi.org/10.1016/j.currproblcancer.2020.100579
- 27. Okagawa, Y., Sumiyoshi, T., Kondo, H., et al. (2021) Comparison of Clinicopathological Features and Long-Term Prognosis between Mixed Predominantly Differentiated-Type and Pure Differentiated-Type Early Gastric Cancer. BMC Cancer, 21, Article No. 235. https://doi.org/10.1186/s12885-021-07962-x
- 28. Ohara, Y., Toshikuni, N., Matsueda, K., et al. (2016) The Superficial Elevated and Depressed Lesion Type Is an Independent Factor Associated with Non-Curative Endoscopic Submucosal Dissection for Early Gastric Cancer. Surgical Endoscopy, 30, 4880-4888. https://doi.org/10.1007/s00464-016-4825-x
- 29. Isomoto, H., Shikuwa, S., Yamaguchi, N., et al. (2009) Endoscopic Submucosal Dissection for Early Gastric Cancer: A Large-Scale Feasibility Study. Gut, 58, 331-336. https://doi.org/10.1136/gut.2008.165381
- 30. Okada K, Fujisaki J, Kasuga A, et al. (2011) Endoscopic Ultrasonography Is Valuable for Identifying Early Gastric Cancers Meeting Expanded-Indication Criteria for Endoscopic Submucosal Dissection. Surgical Endoscopy, 25, 841-848. https://doi.org/10.1007/s00464-010-1279-4
- 31. Zhao, B., Mei, D., Luo, R., et al. (2020) Clinicopathological Features, Risk of Lymph Node Metastasis and Survival Outcome of Synchronous Multiple Early Gastric Cancer. Clinics and Research in Hepatology and Gastroenterology, 44, 939-946. https://doi.org/10.1016/j.clinre.2020.02.004
- 32. Isobe, T., Hashimoto, K., Kizaki, J., et al. (2013) Characteristics and Prognosis of Synchronous Multiple Early Gastric Cancer. World Journal of Gastroenterology, 19, 7154-7159. https://doi.org/10.3748/wjg.v19.i41.7154
- 33. Jeong, S.H., An, J., Kwon, K.A., et al. (2017) Predictive Risk Factors Associated with Synchronous Multiple Early Gastric Cancer. Medicine, 96, e7088. https://doi.org/10.1097/MD.0000000000007088
- 34. Fujiwara, S., Noguchi, T., Noguchi, T., et al. (2012) Radical Endoscopic Resection Is Unsuitable for Most Synchronous, Multiple and Early Gastric Cancers. Hepatogastroenterology, 59, 951-954.
- 35. Kumar, S., Metz, D.C., Ellenberg, S., et al. (2020) Risk Factors and Incidence of Gastric Cancer after Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology, 158, 527-536. https://doi.org/10.1053/j.gastro.2019.10.019
- 36. Praud, D., Rota, M., Pelucchi, C., et al. (2018) Cigarette Smoking and Gastric Cancer in the Stomach Cancer Pooling (StoP) Project. European Journal of Cancer Prevention, 27, 124-133. https://doi.org/10.1097/CEJ.0000000000000290
- 37. Ami, R., Hatta, W., Iijima, K., et al. (2017) Factors Associated with Metachronous Gastric Cancer Development after Endoscopic Submucosal Dissection for Early Gastric Cancer. Journal of Clinical Gastroenterology, 51, 494-499. https://doi.org/10.1097/MCG.0000000000000620
- 38. Brito-Gonçalves, G., Libânio, D., Marcos, P., et al. (2020) Clinicopathologic Characteristics of Patients with Gastric Superficial Neoplasia and Risk Factors for Multiple Lesions after Endoscopic Submucosal Dissection in a Western Country. GE Portuguese Journal of Gastroenterology, 27, 76-89. https://doi.org/10.1159/000501939
- 39. Jeon, J.W., Kim, S.J., Jang, J.Y., et al. (2021) Clinical Outcomes of Endoscopic Resection for Low-Grade Dysplasia and High-Grade Dysplasia on Gastric Pretreatment Biopsy: Korea ESD Study Group. Gut and Liver, 15, 225-231. https://doi.org/10.5009/gnl19275
- 40. Chang, J.W., Jung, D.H., Park, J.C., et al. (2020) Long-Term Outcomes and Prognostic Factors of Endoscopic Submucosal Dissection for Early Gastric Cancer in Patients Aged ≥ 75 Years. Cancers, 12, 3222. https://doi.org/10.3390/cancers12113222
- 41. Jia, Y., Sun, H., Wu, H., et al. (2016) Nicotine Inhibits Cisplatin-Induced Apoptosis via Regulating α5-nAChR/AKT Signaling in Human Gastric Cancer Cells. PLoS ONE, 11, e0149120. https://doi.org/10.1371/journal.pone.0149120
- 42. Kesh, K., Subramanian, L., Ghosh, N., et al. (2015) Association of MMP7-181A→G Promoter Polymorphism with Gastric Cancer Risk: Influence of Nicotine in Differential Allele-Specific Transcription via Increased Phosphorylation of cAMP-Response Element-Binding Protein (CREB). Journal of Biological Chemistry, 290, 14391-14406. https://doi.org/10.1074/jbc.M114.630129
NOTES
*通讯作者Email: maotaoqy@163.com
