Material Sciences
Vol.
09
No.
06
(
2019
), Article ID:
30806
,
11
pages
10.12677/MS.2019.96075
Synthesis of Thiol Functionalized Poly(3,4-Ethyldioxythiophene)/Au Nanocomposites and Its Application in Glucose Detection
Tursunnisahan Bakri1, Tursun Abdiryim2
1Key Laboratory of Petroleum and Gas Fine Chemicals, Educational Ministry of China, School of Chemistry and Chemical Engineering, Xinjiang University, Urumqi Xinjiang
2Key Laboratory of Functional Polymers, Xinjiang University, Urumqi Xinjiang

Received: May 28th, 2019; accepted: Jun. 11th, 2019; published: Jun. 18th, 2019

ABSTRACT
In this paper, thiol functionalized 3,4-ethylenedioxythiophene (EDOT-MeSH) was synthesized, and poly(EDOT-MeSH)/Au was prepared by suspension polymerization method through Au-S chemical adsorption in different concentrations of stabilizers condition. The structure and properties were characterized by a range of techniques such as Scanning Electron Microscope (SEM), Transmission Electron Microscope (TEM), Fourier Transform Infrared Spectroscopy (FTIR), X-Ray Diffraction (XRD) and Energy Dispersive X-ray spectrum (EDX). The electrocatalytic properties of glucose oxidation were studied using glass carbon electrode (GCE) modified with poly(EDOT-MeSH)/Au preparing under the different condition by cyclic voltammetry (CV) method. The results showed that the poly(EDOT-MeSH)/Au/Au modified electrode had good catalytic performance for glucose oxidation when the stabilizer concentration was 0.03 M. The linear detection range and detection limit are 0.2 - 17.0 mM and 0.059 mM, respectively. These results will further promote the development of these electrochemical sensors in biological small molecule sensors.
Keywords:Poly(EDOT-MeSH), Suspension Polymerization, Thiol, Au NPs, Glucose
基于巯基功能化聚(3,4-乙撑二氧噻吩)/Au纳米复合材料的合成及其在葡萄糖检测中的应用
吐尔孙尼萨汗·白克热1,吐尔逊·阿不都热依木2
1新疆大学化学化工学院,石油天然气教育部重点实验室,新疆 乌鲁木齐
2新疆大学,功能高分子重点实验室,新疆 乌鲁木齐

收稿日期:2019年5月28日;录用日期:2019年6月11日;发布日期:2019年6月18日

摘 要
本文合成硫醇功能化的3,4-乙撑二氧噻吩(EDOT-MeSH),利用Au-S化学吸附通过悬浮聚合法分别在存在不同浓度稳定剂的体系中制备了poly(EDOT-MeSH)/Au并通过扫描电镜(SEM),透射电镜(TEM),傅里叶红外光谱(FT-IR),X-射线光谱(XRD)和能谱(EDX)等测试方法对结构进行表征。采用循环伏安法(CV),分别用在不同条件下制备的poly(EDOT-MeSH)/Au修饰的玻碳电极(GCE)对葡萄糖的氧化进行了电催化性能研究。结果表明,当稳定剂的浓度为0.03 M时制备的复合物poly(EDOT-MeSH)/Au修饰的电极对葡萄糖的氧化表现出良好的催化性能,线性检测范围和检出限分别为0.2~17.0 mM和0.059 mM。这些结果将进一步推动该类电化学传感器在生物小分子传感器中的发展。
关键词 :Poly(EDOT-MeSH),悬浮聚合,硫醇,Au NPs,葡萄糖

Copyright © 2019 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
在电化学传感器技术中,电极材料是影响传感器检测灵敏度和选择性的重要因素。目前,多种电极材料,从贵金属到碳,再到导电聚合物,都在生物小分子传感中得到了广泛的应用 [1]。在这些材料中,贵金属黄金(Au)、铂(Pt)和钯(Pd)由于其优异的电子、光学、催化和尺寸依赖性而被广泛应用于电极材料中 [2] [3]。尤其是Au纳米颗粒(Au NPs)作为“电子天线”由于高电子动力学,卓越的导电性、电催化性能和生物相容性等优点更受欢迎 [4] [5] [6]。此外,在化氧化还原反应过程中完全能恢复等独特的性能,Au NPs为电化学和生物传感器平台开起了新的篇章。但是Au NPs在制备和曾存过程中很容易倾向于团聚,在苛刻的条件下变惰性而限制实际应用 [7] [8] [9]。研究表明,导电聚合物、碳材料和一些金属氧化物等电活性材料由于优异的电导率和快速的电子动力学等优点是将Au NPs均匀分散的理想支撑材料 [10] [11] [12]。在导电聚合物中,由于噻吩环中Au NPs与S原子之间的强引力作用,PEDOT及其衍生物通常被用作Au NPs的基质 [13] [14] [15]。研究表明,用硫醇对分子进行功能化,利用-SH与Au之间的Au-S化学键是稳定Au NPs的有效方法 [16] [17]。此外,硫醇的S原子比噻吩环的S原子更容易与金表面结合。
本文中报道了以poly(EDOT-MeSH)为支撑材料,制备poly(EDOT-MeSH)/Au纳米复合材料并用poly(EDOT-MeSH)/Au修饰的电极对葡萄糖进行了电化学检测(图1)。研究发现,在聚合物单元中-SH的引入对金纳米粒子的稳定起着至关重要的作用。此外,电催化研究表明,poly(EDOT-MeSH)/Au修饰的电极在葡萄糖传感器领域中具有潜在的应用前景。

Figure 1. The preparation of modified electrode and detection of glucose
图1. 修饰电极的制备与葡萄糖的检测
2. 实验部分
2.1. 实验试剂及测试设备
3,4-二氧噻吩98%、3-氯-1,2-丙二醇99%、水合氯金酸(HAuCl4·4H2O)上海阿拉丁化学有限公司;对甲苯磺酸、柠檬酸钠分析纯天津致远化学试剂有限公司;D-(+)-葡萄糖分析纯天津致远化学试剂有限公司。
1H-NMR核磁共振测试:采用美国Varian公司Inova-400 MHz型核磁共振波谱仪(内标为Me4Si,溶剂为CDCl3);扫描电镜(SEM)分析:日本日立公司S-4800型场发射扫描电子显微镜(5 KV)。透射电镜(TEM)分析:日本HITACHI公司H-600型透射电子显微镜(100 KV)。红外光谱(FT-IR)分析:德国BRUKER EQUINOX-55型傅立叶红外光谱仪,扫描波数范围为400~4000 cm−1。X-射线粉末衍射(XRD)测试:德国BRUKER AXS D8型X射线衍射仪,Cu-Ka辐射(λ = 0.15418 nm)为辐射源,扫描范围为2θ = 10˚~80˚。电化学性能测试:上海辰华仪器有限公司电化学工作站CHI 660C化学工作站。
2.2. EDOT-MeSH的合成
EDOT-MeSH是通过三部反应来合成的(图2)。
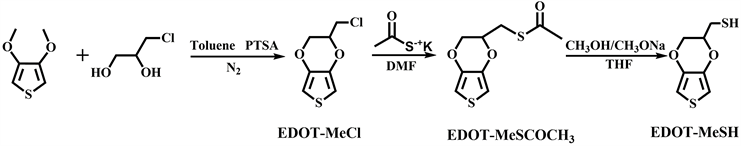
Figure 2. The synthetic route of EDOT-MeSH
图2. EDOT-MeSH的合成路线
第一步,在氮气保护下,将3,4-二甲氧基噻吩(1.64 g, 5.7 mmol)、3-氯-1,2-丙二醇(2.6 g, 12 mmol)、无水甲苯(28 mL)和对甲苯磺酸一水合物(0.16 g, 0.42 mmol)的混合物放入两口瓶中,加热至90℃磁力搅拌24 h。然后,加入2.6 g 3-氯-1,2-丙二醇(12 mmol),在90℃下搅拌5 h。反应结束后得到墨绿色油状物,用2 M KOH溶液和饱和食盐水洗涤,然后用无水MgSO4干燥。将甲苯旋蒸,进行柱层分析(体积比为正己烷:二氯甲烷 = 1:4),得到白色晶体EDOT-MeCl (产率为56%)。1HNMR (400 MHzCDCl3, δ, ppm): EDOT-MeCl, 6.36 (s, 2 H), 4.29 and 4.15 (m, 2 H), 4.37 (m, 1 H), 3.71 (m, 2 H)。
第二步,将EDOT-MeCl (1.20 g, 3.1 mmol)和硫代乙酸钾(1.08 g, 4.7 mmol)分别加入N, N-二甲基甲酰胺(DMF, 6 mL)溶液中,在50℃下磁力搅拌15小时。然后将反应混合物冷却到室温,用二氯甲烷(30 ml)萃取三次。有机相用无水MgSO4干燥,旋蒸二氯甲烷之后得到橙色的液体EDOT-MeSCOCH3 (产率为72%)。1HNMR (400 MHzCDCl3, δ, ppm): EDOT-MeSCOCH3, 6.34 (s, 2 H), 4.18 and 3.96 (m, 2 H), 4.25 (m, 1 H), 3.19 (m, 2 H), 2.38 (s, 3 H)。
第三步,将EDOT-MeSCOCH3 (1.0 g, 2.2 mmol)和甲醇钠/甲醇(1.2 M, 8.0 mL)分别加入蒸馏的四氢呋喃(THF) (80 mL)中,在室温条件下搅拌5 h。然后加入5 M HCl溶液,用二氯甲烷萃取,有机相用水洗三次,无水MgSO4干燥。旋蒸二氯甲烷之后得到棕红色液体EDOT-MeSH (产率为75%)。1HNMR (400 MHzCDCl3, δ, ppm): EDOT-MeSH, 6.34 (s, 2 H), 4.29 and 4.12 (m, 2 H), 4.22 (m, 1 H), 2.82 (m, 2 H), 1.67(s, 1 H)。
2.3. Poly(EDOT-MeSH)/Au的合成
首先,分别准备四个50 mL的单口瓶,分别倒入10 mL氯仿,再倒入10 mL HAuCl4溶液(上层是水相,下层是有机相),然后分别加入浓度为0.03,0.065,0.10和0.135 M的Na3C6H5O7·2H2O溶液。准确称取0.1 g EDOT,在少量的氯仿里面溶解,随后直接注入有机相里面,在室温条件下缓慢地搅拌,反应24 h。反应结束之后,分别用氯仿、异丙醇和蒸馏水交替洗涤,在烘箱里面60℃下烘干12 h。
2.4. 电化学性能测试
工作电极的制备:首先将玻碳电极(GCE)分别用粒径大小为1.0, 0.3和0.05 μm氧化铝抛光粉在鹿皮革上进行抛光,随后GCE分别用超纯水,浓硝酸:超纯水(v:v = 1:1),异丙醇:超纯水(v:v = 1:1)超声洗涤1分钟,用N2吹干GCE表面。最后,在GCE上滴加5 μL复合物溶液(浓度为1 mg/mL)在空气中自然晾干20分钟。
电化学测试在室温下使用电化学工作站CHI 660C进行测试采用三电极体系,Pt电极用作对电极,饱和甘汞电极(SCE)作为参比电极,poly(EDOT-MeSH)/Au修饰的GCE (d = 4 mm)作为工作电极,采用循环伏安法(CV)在50 mL 0.1 M NaOH缓冲液中检测葡萄糖。
3. 实验结果及讨论
3.1. 单体EDOT-MeSH的核磁氢谱(1H-NMR)分析
图3(A)和图3(B)是两个中间体(EDOT-MeCl与EDOT-MeSCOCH3)和最终单体EDOT-MeSH在氘代氯仿(CDCl3)中的核磁共振氢谱(1HNMR)图。如图所示,在中间体EDOT-MeCl的氢谱中,化学位移(δ)为6.36 ppm的单峰是噻吩环上a和b位的-CH-的两个氢的峰。Hc收到Hd的影响在4.29和4.15 ppm处出两组多重峰。δ为4.37和3.71 ppm的两组多重峰分别是Hd和-CH2Cl上的两个氢(He)的峰。在第二个中间体EDOT-MeSCOCH3的氢谱图中,6.34 ppm处的单峰是噻吩环上=CH-S-CH=的Ha和Hb的共振峰。在4.18和3.96 ppm处的两组峰是Hc的共振峰。在4.25与3.19 ppm处的两组多重峰分别对应于Hd和He的共振峰。在2.38 ppm处的单峰归属于O=C-CH3上的α-位的氢(Hf)的共振峰。最终单体EDOT-MeSH的共振谱图(图3(C))中,δ为6.34 ppm处的单峰对应于噻吩环上的Ha和Hb的共振峰。在4.29和4.12 ppm处的两组多重峰归因于Hc的共振峰。在4.22和 2.82 ppm处的多重峰分别为Hd和He的共振峰。δ为1.67 ppm的单峰对应于-SH上的氢(Hf)。这个表征结果与文献报道结果是一致的 [18] [19]。
3.2. 红外光谱分析
图4是在不同浓度Na3C6H5O7·2H2O体系中制备的poly(EDOT-MeSH)/Au的FT-IR图。从图中可以看
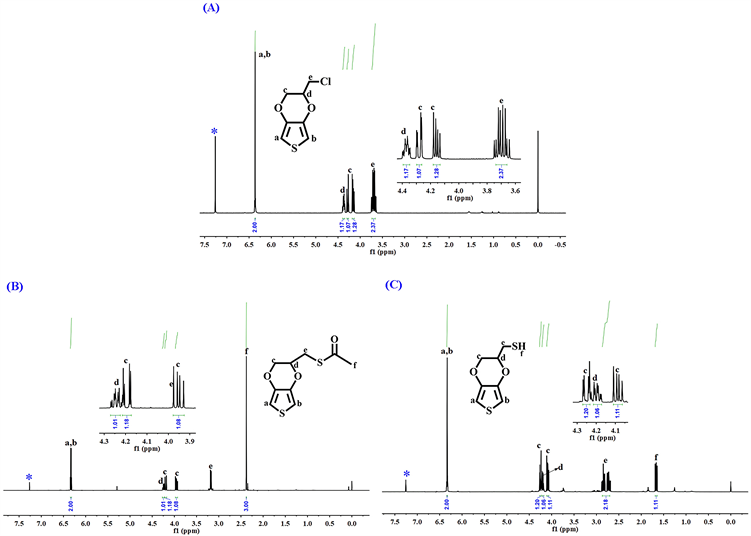
Figure 3. 1H-NMR spectrum of EDOT-MeSH
图3. EDOT-MeSH的核磁共振氢谱图

Figure 4. FT-IR spectra of poly(EDOT-MeSH)/Au (The concentration of Na3C6H5O7·2H2O were 0.03, 0.065, 0.1 and 0.135 M, respectively)
图4. Poly(EDOT-MeSH)/Au的红外光谱图(Na3C6H5O7·2H2O的浓度分别为0.03, 0.065, 0.1和0.135 M)
出,这四种复合物的红外光谱图基本上都一样。在~3117、~3012和~2930 cm−1处的振动峰,分别是由于脂肪酸长链中的C-H、C-H-C和C-H2-C的平面外变形振动造成的。在3200~3700 cm−1处的宽的峰是由-OH和S-H的振动引起的 [20]。在~971,~837和~684 cm−1处的峰是对应于噻吩环上的C-S键的拉伸振动峰。位于~1196,~1138和~1068 cm−1的振动峰是乙撑二氧基的C-O-C的弯曲振动峰。
3.3. XRD分析
图5为在不同浓度柠檬酸钠体系中制备的poly(EDOT-(MeSH)/Au的XRD图。在2θ = 38.36˚,44.38˚,64.72˚和77.77˚处的尖锐的衍射峰对应于Au的(111), (200), (220)和(311)晶面 [21] [22]。如图所示,所有的复合物的衍射峰基本上都一样,只是Au的衍射峰的强度有一点差别。三个XRD图中柠檬酸钠的浓度为0.03 M时,Au的衍射峰都相对强一些。但是,聚合物相应的衍射峰都看不到,可能是因为Au的衍射峰比较强,聚合物的峰都被Au的衍射峰掩盖。

Figure 5. XRD patterns of poly(EDOT-MeSH)/Au (The concentration of Na3C6H5O7·2H2O were 0.03, 0.065, 0.1 and 0.135 M, respectively)
图5. Poly(EDOT-MeSH)/Au的X-射线衍射图(Na3C6H5O7·2H2O的浓度分别为0.03, 0.065, 0.1和0.135 M)
3.4. 形貌分析
图6为存在不同浓度Na3C6H5O7·2H2O体系中制备的poly(EDOT-MeSH)/Au复合物的TEM图。如图所示,Na3C6H5O7·2H2O的浓度不一样,结构单元在液滴中通过自组装长出不同形貌的复合物。当Na3C6H5O7·2H2O的浓度分别为0.03 M时Poly(EDOT-MeSH)/Au呈现出空心球形貌(A),而浓度为0.065、0.1或0.135 M时长出片状(B、C和D)。
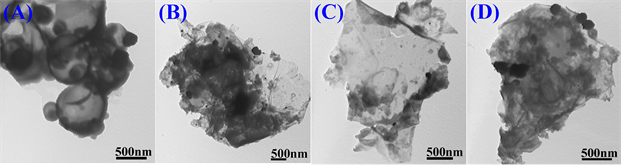
Figure 6. TEM images of poly(EDOT-MeSH)/Au (The concentration of Na3C6H5O7·2H2O were (A) 0.03, (B) 0.065, (C) 0.1 and (D) 0.135 M, respectively)
图6. Poly(EDOT-MeSH)/Au的透射电镜图(Na3C6H5O7·2H2O的浓度分别为(A) 0.03, (B) 0.065, (C) 0.1和 (D) 0.135 M)
3.5. EDX分析
图7是加不同浓度柠檬酸钠时制备的poly(EDOT-MeSH)/Au的EDX图。即,柠檬酸钠的浓度分别为(A) 0.03,(B) 0.065,(C) 0.1和(D) 0.135 M。C, O和S来源于poly(EDOT-MeSH)的化学成分,Au主要来源于被柠檬酸钠和聚合物还原的Au NPs。当稳定剂柠檬酸钠的浓度为0.03 M时,在poly(EDOT-MeSH)上负载的Au NPs的含量相对高(图7(A))。聚合物上负载的Au NPs的含量可能是聚合物所形成的特殊形貌有关。即,如图6(A)所示,当柠檬酸钠的浓度为0.03 M时PEDOT/Au呈现出空心球状形貌,球的比表面积比片状形貌相对大,因此负载的Au NPs的量也多。
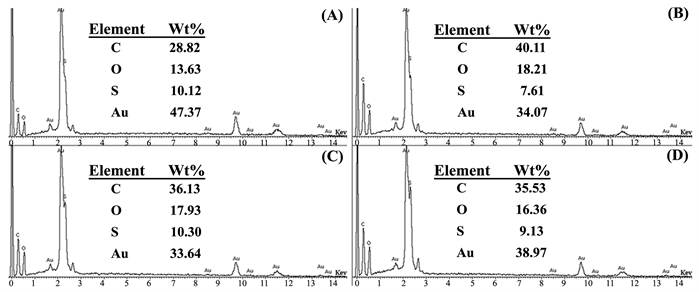
Figure 7. EDX spectra of poly(EDOT-MeSH)/Au (The concentration of Na3C6H5O7·2H2O were (A) 0.03, (B) 0.065, (C) 0.1 and (D) 0.135 M, respectively)
图7. Poly(EDOT-MeSH)/Au的能谱图(Na3C6H5O7·2H2O的浓度分别为 (A) 0.03, (B) 0.065, (C) 0.1和 (D) 0.135 M)
3.6. 电化学性能测试
图8分别为在不同浓度的Na3C6H5O7·2H2O体系中制备的Poly(EDOT-MeSH)/Au修饰的电极在含有5 mM葡萄糖的0.1 M NaOH溶液中的CV曲线图。如图所示,当Na3C6H5O7·2H2O的浓度0.03 M时Poly(EDOT-MeSH)/Au修饰的电极对葡萄糖氧化的电流信号相对强(图8(A)),说明该电极对于葡萄糖的氧化具有良好的催化性能。因此,当Na3C6H5O7·2H2O的浓度为0.03 M时制备出来的Poly(EDOT-MeSH)/Au修饰的电极被选为接下来的研究对象。

Figure 8. CV curves of poly(EDOT-MeSH)/Au modified electrode in 0.1 M NaOH solution containing 5 mM glucose (The concentration of Na3C6H5O7·2H2O were (A) 0.03, (B) 0.065, (C) 0.1 and (D) 0.135 M, respectively)
图8. Poly(EDOT-MeSH)/Au修饰的电极在含有5 mM葡萄糖的0.1 M NaOH溶液中的循环伏安法图(Na3C6H5O7·2H2O的浓度分别为 (A) 0.03, (B) 0.065, (C) 0.1和 (D) 0.135 M)
图9是Poly(EDOT-MeSH)/Au修饰的电极在0.1 M NaOH溶液中对葡萄糖氧化的电催化活性的CV图。在不存在葡萄糖的情况下(a),在+0.40和+0.09 V处观察到一对弱而不明显的氧化还原峰。在+0.45 V处的氧化峰对应于Au氧化成Au2O3,在+0.09处的还原峰来源于Au2O3的还原。加入了葡萄糖之后,在正扫描过程中,在−0.27和+0.15 V处出现了两个新的氧化峰(b)。在−0.27处的氧化峰来源于葡萄糖直接氧化成葡萄糖内酯,进一步氧化在+0.15 V处出峰。葡萄糖在金表面的氧化程度依赖于AuOH活性物质的量,在−0.27 V如此低的电位下AuOH的量非常有限,葡萄糖没有完全氧化。AuOH的量随着电势的增加而急剧增加,使葡萄糖内酯在+0.15 V处进一步氧化。在逆向扫描过程中,Au2O3的还原产生足够量的AuOH促进葡萄糖的氧化,在+0.06 V处出尖锐的氧化峰 [23] [24]。
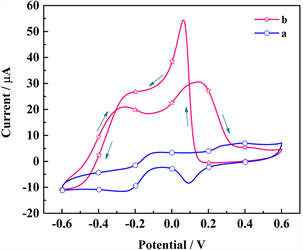
Figure 9. CV curves of poly(EDOT-MeSH)/Au modified electrode in 0.1 M NaOH solution containing 5 mM glucose (The concentration of Na3C6H5O7·2H2O was 0.03 M)
图9. Poly(EDOT-MeSH)/Au修饰的电极在含有5 mM葡萄糖的0.1 M NaOH溶液中的循环伏安法图(Na3C6H5O7·2H2O的浓度为0.03 M)
为系统的研究Poly(EDOT-MeSH)/Au修饰的电极对葡萄糖氧化的电催化性能,采用CV法在0.1 M NaOH溶液中对葡萄糖进行电化学检测。如图10所示,电流峰随着葡萄糖浓度的增加而线性增加,线性检测范围和线性方程分别为0.2~17.0 mM和y = 7.2623x + 6.3474 (R2 = 0.9947),最低检出限(LOD)为0.059 mM。
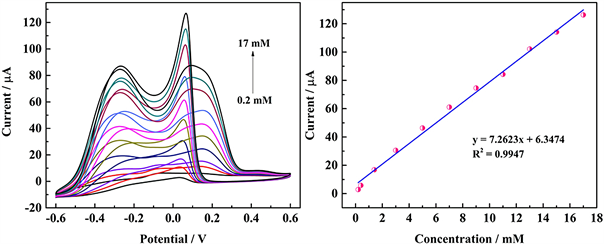
Figure 10. CV curves of poly(EDOT-MeSH)/Au modified electrode in 0.1 M NaOH solution (The concentration of Na3C6H5O7·2H2O was 0.03 M)
图10. Poly(EDOT-MeSH)/Au修饰的电极在0.1 M NaOH溶液中的循环伏安法图(Na3C6H5O7·2H2O的浓度为0.03 M)
3.7. 选择性
在复杂的生理体系中生物传感器的选择性非常重要,研究一些物质的干扰性是十分有意义的。本论文中以Na3C6H5O7·2H2O的浓度为0.03 M时制备的poly(EDOT-MeSH)/Au修饰的电极作为电化学葡萄糖传感器研究了体内存在的几种物质和粒子的干扰性。抗坏血酸(AA),尿酸(UA)以及K+, Zn2+, Fe3+, Mg2+等等离子是检测葡萄糖的主要干扰物质。图11是poly(EDOT-MeSH)/Au修饰的电极在含有5 mM葡萄糖的0.1 M NaOH溶液中,添加0.1 mM AA, 0.02 mM UA 和 0.1 mM K+, Zn2+, Fe3+, Mg2+等干扰离子前后的CV曲线电流峰的比值图。实验结果显示,RSD为5.03%,小于±10%。结果表明,上面提到的干扰物种的干扰性是可以忽略的。证明,poly(EDOT-MeSH)/Au的选择性好。
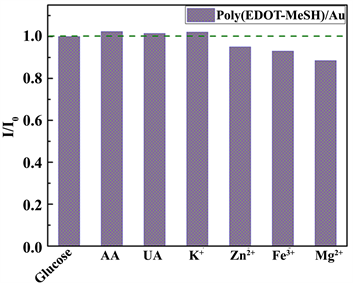
Figure 11. Interference of interfering species and ions on the poly(EDOT-MeSH)/Au modified electrode in 0.1 M NaOH solution containing 5 mM glucose (The concentration of Na3C6H5O7·2H2O was 0.03 M)
图11. 在含5 mM葡萄糖的0.1 M NaOH溶液中干扰物质和离子对poly(EDOT-MeSH)/Au修饰电极的干扰性(Na3C6H5O7·2H2O的浓度为0.03 M)
4. 结论
通过悬浮聚合法,在不同浓度Na3C6H5O7·2H2O的体系中制备了Poly(EDOT-MeSH)/Au复合物及研究了对葡萄糖氧化的电催化活性。研究表明,当加入0.03 M Na3C6H5O7·2H2O时制备的Poly(EDOT-MeSH)/ Au由于呈现出球状形貌,比表面积相对大,Poly(EDOT-MeSH)上的功能基团-SH与Au NPs形成Au-S化学键,能吸附更多的Au NPs并且将Au NPs均匀的分散于聚合物上而使活性点增多,对于葡萄糖的氧化表现出良好的电催化性能。线性检测范围为0.2~17.0 mM。
基金项目
感谢国家自然科学基金(No. 21564014)。
文章引用
吐尔孙尼萨汗•白克热,吐尔逊•阿不都热依木. 基于巯基功能化聚(3,4-乙撑二氧噻吩)/Au纳米复合材料的合成及其在葡萄糖检测中的应用
Synthesis of Thiol Functionalized Poly(3,4-Ethyldioxythiophene)/Au Nanocomposites and Its Application in Glucose Detection[J]. 材料科学, 2019, 09(06): 589-599. https://doi.org/10.12677/MS.2019.96075
参考文献
- 1. Wongkaew, N., Simsek, M., Griesche, C., et al. (2019) Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chemical Reviews, 119, 120-194.
- 2. Maduraiveeran, G., Sasidharan, M. and Ganesan, V. (2018) Electrochemical Sensor and Biosensor Platforms Based on Advanced Nanomaterials for Biological and Biomedical Applications. Biosensors & Bioelectronics, 103, 113. https://doi.org/10.1016/j.bios.2017.12.031
- 3. Chen, C., Xie, Q., Yang, D., et al. (2013) Recent Advances in Electrochemical Glucose Biosensors: A Review. RSC Advances, 3, 4473. https://doi.org/10.1039/c2ra22351a
- 4. Oko, D.N., Garbarino, S., Zhang, J., Xu, Z., Chaker, M., Ma, D., Guay, D. and Tavares, A.C. (2015) Dopamine and Ascorbic Acid Electro-Oxidation on Au, AuPt and Pt Nanoparticles Prepared by Pulse Laser Ablation in Water. Electrochimica Acta, 159, 174-183. https://doi.org/10.1016/j.electacta.2015.01.192
- 5. Thiagarajan, S. and Chen, S.M. (2007) Preparation and Characterization of PtAu Hybrid Film Modified Electrodes and Their Use in Simultaneous Determination of Dopamine, Ascorbicacid and Uric Acid. Talanta, 74, 212-222. https://doi.org/10.1016/j.talanta.2007.05.049
- 6. Yang, L., Huang, N., Lu, Q., Liu, M., Li, H., Zhang, Y. and Yao, S. (2015) A Quadruplet Electrochemical Platform for Ultrasensitive and Simultaneous Detection of Ascorbic Acid, Dopamine, Uric Acid and Acetaminophen Based on a Ferrocene Derivative Functional Au NPs/Carbon Dots Nanocomposite and Graphene. Analytica Chimica Acta, 903, 69-80. https://doi.org/10.1016/j.aca.2015.11.021
- 7. Giannetto, M., Mori, G., Terzi, F., Zanardi, C. and Seeber, R. (2011) Composite PEDOT/Au Nanoparticles Modified Electrodes for Determination of Mercury at Trace Levels by Anodicstripping Voltammetry. Electroanalysis, 23, 456-462. https://doi.org/10.1002/elan.201000469
- 8. Atta, N.F., Galal, A. and El-Ads, E.H. (2012) Gold Nanoparticles-Coated Poly(3,4-ethylenedioxythiophene) for the Selective Determination of Sub-Nano Concentrations of Dopamine in Presence of Sodium Dodecyl Sulfate. Electrochimica Acta, 69, 102-111. https://doi.org/10.1016/j.electacta.2012.02.082
- 9. Li, M., Wang, W., Chen, Z., Song, Z. and Luo, X. (2018) Electrochemical Determination of Paracetamol Based on Au@graphene Core-Shell Nanoparticles Doped Conducting Polymer PEDOT Nanocomposite. Sensors Actuators B, 260, 778-785. https://doi.org/10.1016/j.snb.2018.01.093
- 10. Wang, C., Du, J., Wang, H., Zou, C.E., Jiang, F., Yang, P. and Du, Y. (2014) A Facile Electrochemical Sensor Based on Reduced Graphene Oxide and Au Nanoplates Modified Glassy Carbon Electrode for Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. Sensors and Actuators B: Chemical, 204, 302-309. https://doi.org/10.1016/j.snb.2014.07.077
- 11. Hou, C., Liu, H., Zhang, D., Yang, C. and Zhang, M. (2016) Synthesis of ZnO Nanorods-Au Nanoparticles Hybrids via In-Situ Plasma Sputtering-Assisted Method for Simultaneous Electrochemical Sensing of Ascorbic acid and Uric Acid. Journal of Alloys and Compounds, 666, 178-184. https://doi.org/10.1016/j.jallcom.2016.01.092
- 12. Zhang, O., Wen, Y., Xu, J., Lu, L., Duan, X. and Yu, H. (2013) One-Step Synthesis of Poly(3,4-ethylenedioxythiophene)-Au Composites and Their Application for the Detection of Nitrite. Synthetic Metals, 164, 47-51. https://doi.org/10.1016/j.synthmet.2012.11.013
- 13. Mathiyarasu, J., Senthilkumar, S., Phani, K.L., et al. (2007) PEDOT-Au Nanocomposite Films for Electrochemical Sensing of Dopamine and Uric Acid. Journal of Nanoscience & Nanotech-nology, 7, 2206. https://doi.org/10.1166/jnn.2007.796
- 14. Salsamendi, M., Marcilla, R., D?bbelin, M., et al. (2010) Simulta-neous Synthesis of Gold Nanoparticles and Conducting Poly(3,4-Ethylenedioxythiophene) towards Optoelectronic Nanocomposites. Physica Status Solidi, 205, 1451-1454. https://doi.org/10.1002/pssa.200778167
- 15. Yang, L., Zhang, J., Zhao, F., et al. (2016) Electrodeposition of Self-Assembled Poly(3,4-ethylenedioxythiophene) @gold Nanoparticles on Stainless Steel Wires for the Head-space Solid-Phase Microextraction and Gas Chromatographic Determination of Several Polycyclic Aromatic Hydrocarbons. Journal of Chromatography A, 1471, 80-86. https://doi.org/10.1016/j.chroma.2016.10.041
- 16. Rastgoolahrood, A., Martsinovich, N., Lischka, M., et al. (2016) From Au-Thiolate Chains to ThioetherSierpiński Triangles: The Versatile Surface Chemistry of 1,3,5-Tris(4-Mercaptophenyl)Benzene on Au(111). Acs Nano, 10, 10901-10911. https://doi.org/10.1021/acsnano.6b05470
- 17. Yang, L., Huang, N., Lu, Q., et al. (2016) A Quadruplet Electrochemical Platform for Ultrasensitive and Simultaneous Detection of Ascorbic Acid, Dopamine, Uric Acid and Acetaminophen Based on a Ferrocene Derivative Functional Au NPs/Carbon Dots Nanocomposite and Graphene. Analytica Chimica Acta, 903, 69-80. https://doi.org/10.1016/j.aca.2015.11.021
- 18. Goda, T., Toya, M., Matsumoto, A. and Miyahara, Y. (2015) Poly(3,4-ethylenedioxythiophene) Bearing Phosphorylcholine Groups for Metal-Free, Antibody-Free, and Low-Impedance Biosensors Specific for C-Reactive Protein. Acs Applied Materials & Interfaces, 7, 27440-27448. https://doi.org/10.1021/acsami.5b09325
- 19. Ali, A., Abdiryim, T., Huang, X., Jamal, R., Simayi, R. (2018) Hollow, Spherical, Poly(3,4-ethylenedioxythiophene)-Bearing Methanethiol as a Gold Stabilizer for High-Efficiency Electrochemical Sensors. Journal of the Electrochemical Society, 165, B335-B343. https://doi.org/10.1149/2.0021809jes
- 20. Yu, B., Wang, X., Xing, W., Yang, H., Wang, X., Song, L., Hu, Y. and Lo, S. (2013) Enhanced Thermal and Mechanical Properties of Functionalized Graphene/Thiol-Ene Systems by Photopolymerization Technology. Chemical Engineering Journal, 228, 318-326. https://doi.org/10.1016/j.cej.2013.04.093
- 21. Wang, H., Han, J., Lu, W., Zhang, J., Li, J. and Jiang, L. (2015) Facile Preparation of Gold Nanocages and Hollow Gold Nanospheres via Solvent Thermal Treatment and Their Surface Plasmon Resonance and Photo-thermal Properties. Journal of Colloid and Interface Science, 440, 236-244. https://doi.org/10.1016/j.jcis.2014.11.004
- 22. Hong, J.Y. and Huh, S. (2014) Facile Preparation of SERS-Active Nanostructured Au Spheres by Simple Reduction of AuCl4-Ions with EDOT. Journal of Colloid and Interface Science, 418, 360-365. https://doi.org/10.1016/j.jcis.2013.12.032
- 23. Zhong, S.L., Zhuang, J., Yang, D.P. and Tang, D. (2017) Eggshell Membrane-Templated Synthesis of 3D Hierarchical Porous Au Networks for Electrochemical Nonenzymatic Glucose Sensor. Biosensors and Bioelectronics, 96, 26-32. https://doi.org/10.1016/j.bios.2017.04.038
- 24. Han, L., Zhang, S., Han, L., Yang, D.P., Hou, C. and Liu, A. (2014) Porous Gold Cluster Film Prepared from Au@BSA Microspheres for Electrochemical Nonenzymatic Glucose Sensor. Electrochimica Acta, 138, 109-114. https://doi.org/10.1016/j.electacta.2014.06.095