Material Sciences
Vol.
13
No.
04
(
2023
), Article ID:
64455
,
12
pages
10.12677/MS.2023.134035
生理响应型抗菌材料在创面修复中的应用
周倩,刘义*
四川轻化工大学化学工程学院,四川 自贡
收稿日期:2023年3月20日;录用日期:2023年4月18日;发布日期:2023年4月25日

摘要
细菌感染是创面修复过程中的主要难题之一,往往会导致强烈炎症反应使得缺损组织难以愈合,严重者甚至会引起败血症、脓毒血症等全身性并发症,威胁患者的生命。尽管抗生素的引入能够极大地降低创伤部位细菌感染的风险,然而其使用过程往往导致细菌多药耐药性及对机体的毒副作用。因此,临床医疗迫切需要开发能够避免细菌耐药性,并能够在感染创伤部位精准抑菌的新型抗菌材料。为解决上述问题,考虑到细菌感染创面具有区别于正常组织的特殊微环境,如较低的pH值、过表达的特殊酶和高浓度的过氧化氢等。生理微环境响应性被进一步引入到抗菌材料的设计过程中,以实现创伤部位的精准抑菌降低治疗过程对机体的毒副作用。本文基于细菌感染特殊微环境,总结了近年来用于创伤修复的生理响应型抗菌材料的研究及应用进展。
关键词
创伤修复,感染抑制,抗菌材料,生理环境响应

Applications of Antibacterial Materials with Physiological Responsiveness in Wound Repair
Qian Zhou, Yi Liu*
College of Chemical Engineering, Sichuan University of Science & Engineering, Zigong Sichuan
Received: Mar. 20th, 2023; accepted: Apr. 18th, 2023; published: Apr. 25th, 2023

ABSTRACT
One of the most serious issues in wound repair is bacterial infection. It frequently triggers severe inflammatory response, making the damaged tissue difficult to regenerate. Moreover, the bacterial infection can potentially develop systemic consequences like sepsis and sepsis, which threatened the patients’ lives. Although antibiotics can significantly reduce the level of bacterial infection at wound sites, the frequent usage of antibiotics leads to multidrug resistance and harmful side effects to the organism. Therefore, clinical medicine urgently requires the development of novel antibacterial materials, which are able to prevent the bacterial resistance and precisely inhibit microorganisms in infected wound sites. To address these issues, considering the wounds with bacterial infection has unique microenvironments compared to normal tissues, such as low pH, overexpressed particular enzymes, and high hydrogen peroxide concentrations. The physiological microenvironment responsiveness was further included to the fabrication process of antibacterial materials, which was expected to obtain accurate antibacterial inhibition at the wound site and limited the harmful side effects of the treatment on the organism. Based on the special microenvironment of bacterial infection, the research and application progress of physiologically responsive antibacterial materials for wound repair in recent years were reviewed.
Keywords:Wound Repair, Infection Inhibition, Antibacterial Materials, Physiological Responsiveness

Copyright © 2023 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
创面修复是一个复杂的过程,依赖于多种因素的相互作用 [1] [2] 。正常(急性)的伤口愈合通常可以分为四个重叠但截然不同的步骤,包括止血、炎症、增殖和修复,最后是创面重塑 [3] [4] 。从炎症期到增殖期有一个重要的转变——促炎性巨噬细胞向抗炎性巨噬细胞表型的转变。当转变受阻,伤口愈合会在炎症阶段停滞,导致慢性伤口发生 [5] (图1)。许多研究表明,此时伤口周围中性粒细胞和炎症介质不仅降解健康的胞外基质(ECM),而且产生有毒害的活性氧破坏细胞膜,促进炎症机制,致使炎症放大循环 [1] 。引发的强烈炎症反应,使得缺损组织难以愈合,长期暴露接触病原体,当细菌渗透皮肤损伤部位表面以下时,细菌感染发生 [1] [2] 。
细菌感染可由伤口部位细菌细胞的积累和生长引发 [6] 。与伤口感染相关的常见致病菌包括金黄色葡萄球菌(S. aureus) [7] [8] ,耐甲氧西林金黄色葡萄球菌(MRSA),大肠杆菌(E. coli)、脓性链球菌(S. pyogenes),和铜绿假单胞菌(P. aeruginosa)等 [5] [8] [9] 。感染可以经过三个连续的阶段:污染、定植和感染 [9] [10] 。伤口部位的细菌感染导致伤口愈合延迟,在某些情况下,可能会导致严重的有害影响,危及生命 [6] 。
细菌感染引起的多种重大疾病从而导致的死亡率在全球范围内不断升高。抗生素和外科手术(从简单的切口引流到广泛的清创)是治疗严重细菌感染的两个关键组成部分 [11] [12] [13] ,而这些治疗方式都有一些优劣点 [14] [15] (表1)。如图2,随着抗生素的大量使用,细菌感染所导致的致病率、致死率不断上升,世界卫生组织已将细菌耐药性列为了重大公共安全问题 [16] [17] [18] [19] 。近年来已有大量的新型抗菌材料及药物被广泛制备及应用,而针对细菌特殊微环境的响应型药物精准控释体系的研究更为抗菌提供了新思路。
由于细菌感染和自身过激免疫反应的影响,创面细菌感染部位往往会有区别于正常细胞组织的特殊微环境,包括某些酶升高、酸性pH值、活性氧(ROS) [20] 增加,特别是过氧化氢(H2O2) [21] [22] (图3)。有效利用这些微环境可以更精准区分健康和感染部位,进行精准、高选择性治疗,避免杀菌剂的过度使用,减少细胞毒副作用。

Figure 1. Molecular and cellular mechanisms of normal skin repair (left) and molecular pathology of chronic wounds (right) [5]
图1. 正常皮肤修复的分子和细胞机制(左)及慢性创伤分子病理学研究(右) [5]
Table 1. Mechanism and advantages and disadvantages of traditional treatment methods and physiological response antibacterial materials
表1. 传统治疗手段与生理响应型抗菌材料的机制及优劣

Figure 2. Deaths number attributable to AMR every year by 2050 [19]
图2. 到2050年,每年可归因于抗生素耐药性的死亡人数 [19]
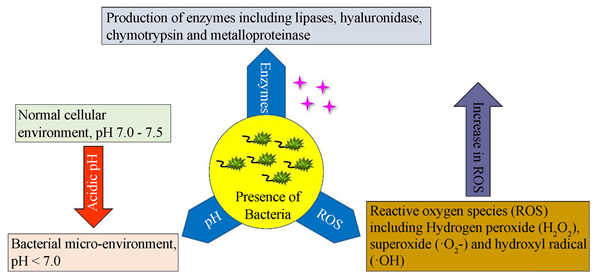
Figure 3. Changes in the microenvironment and the production of various substances in the presence of bacteria [21]
图3. 细菌存在时微环境的变化和各种物质的产生 [21]
2. 用于创面细菌感染治疗的酶响应性材料
细菌可以分泌多种酶类,这些酶可以分解特定的化学键(如酸和酶敏感键)或官能团,并且这种特性已被用于设计酶响应纳米系统 [23] 。酶响应纳米材料的典型机制大致可以分为两类:直接释放/激活或通过物理化学变化间接释放/激活 [22] 。细菌分泌的一系列可用于酶响应性材料触发点,最常用的酶包括脂肪酶和透明质酸酶。在细菌分泌的一系列酶中,脂肪酶和透明质酸酶常作为酶响应性材料的底物。报道显示,脂肪酶可分解酸酐和酯类官能团、透明质酸酶可以分解透明质酸盐和透明质酸 [21] 。
1) 据报道,明胶酶活性已被用作鉴定致病性金黄色葡萄球菌的指标 [24] [25] [26] 。受这一独特特性的启发,山东大学李永强和香港中文大学夏江课题组设计了一种多基序多肽(APP)。肽(APP)与金纳米团簇(AuNc)通过金硫醇键组装,形成明胶酶响应型抗菌光动力纳米复合材料(GRAPN)。体内、体外研究结果表明,GPAPN能对病原体在感染部位分泌的明胶酶产生反应。这可以赋予GRAPN选择性抗菌,减少对宿主细胞和健康菌群的毒性,并提高对病原体的治疗效果,促进伤口愈合 [27] 。
2) 西安交通大学陈鑫课题组将乙二胺四乙酸(EDTA)-Fe3+配合物与透明质酸(HA)动态配位交联,制备了HA-Fe-EDTA水凝胶(图4)。该水凝胶具有响应透明质酸酶及按需释放Fe3+和快速自愈功能,用于抑制感染和组织再生。凝胶到达细菌部位触发透明质酸酶响应使Fe3+按需、可控释放,进而被细菌还原为Fe2+后与H2O2反应生成毒性羟基自由基,破坏细菌蛋白和核酸。小鼠细菌感染模型研究结果表明,该凝胶具有优良的抗菌和促皮肤再生功能 [28] 。
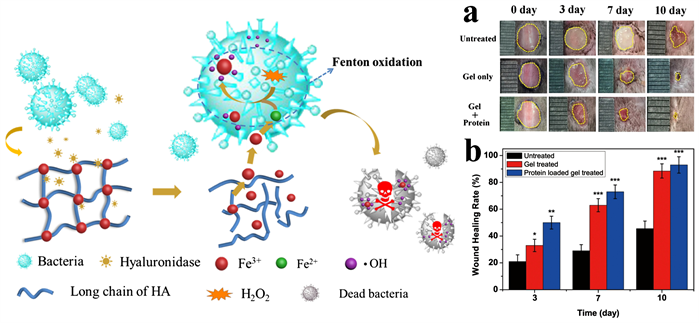
Figure 4. HA-Fe-EDTA hydrogel preparation with on-demand release and continuous release of biomolecules and promotes regeneration of infected skin [28]
图4. 具有按需释放的HA-Fe-EDTA水凝胶制备及生物分子的持续释放和促进感染皮肤再生 [28]
3) 抗菌肽(AMP)以其优良的抗菌性能以及较低的细菌耐药性而著称,然而,其在临床上的应用仍存在不足 [29] [30] [31] 。尽管细菌对抗菌肽的抗性并不常见,但有些微生物还是会利用细胞胞膜分子进行表面修饰、表达外排泵或通过内源性酶进行蛋白水解降低抗菌肽的抗菌活性 [32] 。为解决这些问题,浙江树人大学李兰娟和浙江大学毛峥伟课题组设计了一个具有明胶酶B响应性的多功能肽(BrEK),它由三个功能模块组成:抗菌肽Buforin 2b、明胶酶B反应性连接体和两性离子防污块。利用具有独特特性的金纳米棒(AuNRs)作为抗菌肽Buforin 2b的载体,Buforin 2b通过有效穿透细菌细胞膜并于细菌DNA结合而具有高抗性。但只是金纳米棒与Buforin 2b的简单组合,循环时间和潜在的毒性都是其需要补足的地方。为避免以上缺点,该课题组引入一个具有明胶酶B响应性的亲水防污层,该防污层自发地对感染微环境做出反应,并在表面暴露出能够附着在细菌上的AMPs,使纳米颗粒富集,增强光热效应;当在正常组织,具有明胶酶B响应性的亲水防污层保护BrEK,提高生物相容性和血液循环。该研究在体内外都有良好的稳定性和光热效应 [33] 。
3. 用于创面细菌感染治疗的pH响应性材料
一个典型的健康细胞组织的微环境为中性至微碱性(pH = 7.0~7.5) [21] 。而在细菌感染部位,细菌厌氧代谢产生的乙酸、苹果酸、乳酸等使该部位酸度增加,显示酸性的微环境(pH = 4.5~6.5) [22] 。pH响应系统的设计采用了两种策略。其中一种是利用质子化/去质子化的官能团,这些官能团在低pH值下接受质子,或在高pH值下失去质子;另一种为利用pH响应性化学键,该化学键会在pH值变化的情况下断裂 [21] [22] [34] 。这种pH诱导的化学键断裂会通过直接溶解或破坏纳米载体的结构导致负载释放。
1) 密歇根理工大学Lee,Bruce P.课题组将本质杀菌的氯化儿茶酚(DMA-Cl)和苯硼酸(AAPBA)之间形成儿茶酚–硼酸连接,制备一种具有pH响应和可逆功能的新型抗菌水凝胶。该凝胶中的DMA-Cl可在酸性条件下(pH = 3)可逆解除绑定,释放ROS杀灭细菌;在碱性条件下(pH = 8.5)重新形成凝胶复合物,减少DMA-Cl在周围环境的暴露,提高生物安全性 [35] 。
2) 南洋理工大学Chan-Park,Mary B.和新加坡国立大学Kang,En-Tang课题组合成了一种阳离子性壳聚糖衍生物(CS-PLL-CA) (图5)。其在正常组织由于静电作用,可自组装成核内带相反电荷的肽和壳聚糖形成电晕的纳米胶束(NMs),这种“智能”细菌识别壳聚糖修饰纳米系统可通过pH响应转换抗菌,降低细胞毒性;一旦进入细菌感染部位,由于局部酸性,CS-PLL-CA的β-羧基酰胺自裂,导致NMs失去静电稳定性并爆发,溢出其阳离子段核心进行抗菌。体外研究结果显示高杀灭力和生物相容性 [34] 。
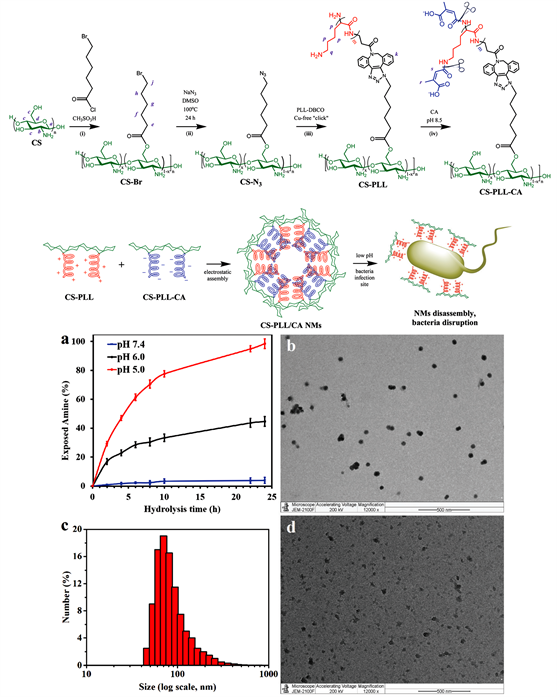
Figure 5. Synthesis of CS-PLL-CA, self-assembly of nanoparticles (NMs), and pH-responsive cleavage of NMs [34]
图5. CS-PLL-CA的合成,纳米微粒(NMs)的自组装,以及NMs的pH响应式裂解 [34]
3) 南北大学Sharker,Shazid Md课题组研究了单宁酸交联壳聚糖基纳米复合薄膜的制备方法,作为局部治疗贴片和贴膜或敷料应用于局部创面抗菌治疗。利用壳聚糖多糖主干存在的氨基可电离酸性pH值的特性,使相互连接,形成3D交联膜材料。此外,单宁酸的富羟基(-OH)部分可以形成氢键,可加固交联膜材料。结果显示,加入单宁酸后CT纳米复合膜具有优良的稳定性。装载局部抗生素(新霉素)后,CT纳米复合膜揭示了新霉素以pH依赖的方式持续释放,酸性介质显示出更高的药物释放,可用于区别于正常组织,在大鼠皮肤模型上显示出改善的伤口愈合 [36] 。
4) 湖北大学的梁继超课题组通过在Cu2O/Pt纳米酶和葡萄糖氧化酶(GOx)表面包覆磷酸钙(CaP)矿化层,形成了具有酸响应核壳结构的Cut-GOx-CaP纳米反应器。CaP矿化层在细菌感染部位酸性pH响应性释放GOx和Cu2O/Pt纳米酶,而中性条件稳定。研究表明,该纳米反应器在抗菌及促进糖尿病创面愈合具有良好性能 [37] 。
4. 用于创面细菌感染治疗的H2O2响应性材料
细胞可产生内源性活性氧(ROS),包括单线态氧(1O2)、超氧阴离子自由基(∙O−2)、羟基自由基(∙OH)和过氧化氢(H2O2) [38] [39] 。低水平ROS对机体正常生理过程至关重要,包括调节蛋白质和产生一些激素 [21] 。然而,当细菌感染发生时,由于细菌感染和自身过激免疫反应,ROS水平会升高,尤其是过氧化氢(H2O2)。有两个主要因素,一是机体对抗外源入侵病原体做出的免疫应答,发生高水平的氧化应激;二是细菌自身在感染周期中产生ROS。目前为止,ROS响应性材料的作用机制主要分为两类,其一是通过降解;另一类是通过疏水到亲水的转换,这两种机制只在ROS存在的情况下导致负载的可控释放 [21] [22] 。
1) Peng等利用甲壳素纳米纤维微球负载AgNPs和Fe3O4纳米颗粒制备了一种用于抗菌和伤口愈合的新型抗菌剂Ag-Fe3O4-NMs [40] 。该纳米纤维微球通过持续释放Ag+催化较低浓度H2O2形成羟基自由基(∙OH)。研究表面,该新型抗菌剂在体外显示低毒、低溶血的特点。
2) 南京邮电大学的宇文力辉课题组通过球磨和超声剥离制备FePS3 NSs,其可在酸性pH的细菌生物膜感染组织中裂解释放Fe2+和[P2S6]4− [41] (图6)。释放的Fe2+通过Fenton反应将H2O2转化成∙OH。值得关注的是,[P2S6]4−能加速铁的氧化还原循环,使生成的Fe3+还原为Fe2+,进一步增强Fenton活性。值得注意的是,其在中性条件具有ROS清除性能,有利于缓解炎症。实验结果表明,FePS3∙NSs具有微环境选择性的ROS调节特性,能够同时抗生物膜和抗炎治疗。
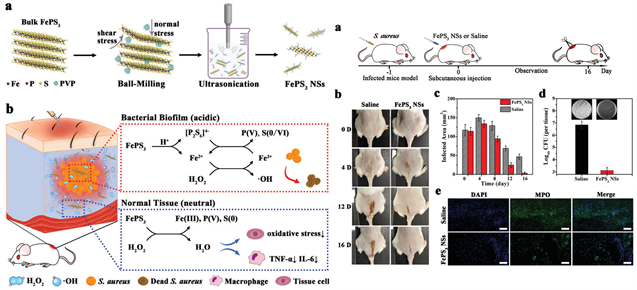
Figure 6. The acid responsiveness of FePS3 NSs and its self-enhancing Fenton anti-bacterial and neutral pH scavenging ROS relieves inflammation [41]
图6. FePS3 NSs的酸响应性及自我增强的Fenton抗菌和中性pH清除ROS缓解炎症性能 [41]
3) 皮肤创伤的自我修复是一个复杂、漫长和系统的过程,尤其是开放性伤口(如糖尿病足溃疡)会持续数月甚至数年 [42] [43] [44] [45] ,使用抗生素和反复频繁地换药更是会引起耐药和伤口反复损伤。为避免繁琐的换药和过度使用抗生素,促进伤口快速修复愈合,西安电子科技大学宁蓬勃、王忠良课题组和同济大学杨洋课题组构建了一种ROS响应的可降解水凝胶敷料(L-Arg@水凝胶)。该凝胶可使用低水平(100 × 10−6 M)家用消毒剂过氧化氢(H2O2)作为一个触发点,与L-Arg反应持续生成NO,用于细菌感染的开放性伤口的快速治疗 [46] 。
4) 为建立更高效、耐药率较低的抗菌方法,吉林大学徐家云和刘俊秋课题组通过侧修饰柱状 [5] 芳烃(BDMP5)的共价组装和甲基化修饰制备了带正电荷的聚合物胶囊,其内部封装Fe3O4纳米颗粒,外部吸附带负电荷的葡萄糖氧化酶(GOx),构建了一种级联催化纳米平台(GOx-NCs/Fe3O4)。GOx与葡萄糖产生的H2O2不仅弥补机体内源H2O2不足的问题,增强抗菌效率,且葡萄糖酸使pH的降低也有助后续的Fenton反应。聚合物胶囊可作为高效包覆Fenton催化剂和固定化GOx的合适替代品,用于建立级联催化纳米平台,在使用GOx和Fenton催化剂协同作用提高化学动力学(CDT)的效率和应用的同时,满足酶的固有特性并保持酶催化活性。体内外实验结果显示,该纳米平台对对哺乳动物细胞几乎没有细胞毒性,生成的∙OH对细菌的结构和形态造成了严重的破坏 [47] 。
5) 为克服细菌在创面大量繁殖引起的爆发性炎症对人类健康构成的影响 [48] [49] [50] ,南京大学孙卫斌课题组将聚乙二醇(PEG)和碳点CDs涂覆在氧化氯代铁纳米片(FeOCl NSs)上,合成了纳米复合材料FeOCl@PEG@CDs NCs,用于伤口愈合过程中精确的光热疗法(PTT)和化学动力学治疗(CDT)协同细菌感染治疗。FeOCl@PEG@CDs NCs基于一种新型FeOCl NSs的氧化还原循环使H2O2生成毒性羟基自由基用于化学动力学治疗;具有优良的生物安全性和高的光稳定性的CDs在808 nm激光下产生强PTT响应抗菌。FeOCl@PEG@CDs NCs集成了Fe(II)对∙OH生成的选择性(而非转化为Fe(IV)),以避免铁沉淀,提高Fenton效率。FeOCl@PEG@CDs NCs在体内和体外由于FeOCl的CDT效应和CDs的PTT效应共同作用,具有良好的抗菌效果 [51] 。
5. 用于创面细菌感染治疗的外源刺激响应性(热、光、磁)纳米材料
除利用细菌感染微环境进行响应抗菌外,外源刺激响应性聚合物粒子也是一类重要的智能抗菌材料。外源刺激响应抗菌材料对外界环境信号(如温度、光 [52] 、磁 [53] 等)非常敏感,能够使其物理和化学性质改变,以响应外界刺激并行使抗菌性能 [22] [39] [54] 。
1) 热响应材料可分为两类,一类是最低临界溶解温度(LCST)以上不溶于水,另一类是在最高临界溶液温度(UCST)以下不溶于水。聚乙烯(N-异丙基丙烯酰胺(PNIPAM)是最常用的热响应性聚合物,它可以通过调节水的溶解度在32℃附近可逆地从亲水性线圈状态转变为疏水性球状体。
浙江省人民医院Wu,Cui yun课题组以单宁酸/Fe3+(TA/Fe3+)胶层和聚(N-异丙基丙烯酰胺-甲基丙烯酸磺基甜菜素) (poly(NIPAM-co-SBMA))微凝胶为基础,采用一步共沉积法制备了一种简单高效的抗菌表面涂层,防止细菌附着和定植。其中TA/Fe3+复合层不仅对涂层表面具有良好的抗菌性能,且为微凝胶的锚固提供了良好的粘附层。两性离子聚合物SBMA起到抗粘附和抗定植作用。具有优异的热响应特性的NIPAM组件,可以通过降低温度,使聚合物涂层的粒径由收缩状态变为膨胀状态,去除表面几乎所有的死亡细菌和其他碎片,以此避免菌体沉积 [55] 。
2) 气体治疗如一氧化氮(NO)、硫化氢(H2S)和一氧化碳(CO)可以影响多种生理和病理生理过程,改变疾病状态 [56] [57] 。其挑战是有效的气体输送和可控的气体释放 [58] [59] ,与光响应抗菌材料的联用或许可以解决这一问题。光响应抗菌材料通常由可以将光输入转化为化学能量输出的发光团组成 [21] [22] ,由于光对于机体的非入侵性,与其他刺激相比,光可以以高精度瞬间施加。
中国科学技术大学胡进明课题组通过原子转移自由基聚合(ATRP)成功地合成了聚(环氧乙烷)-b-聚(4-((2-硝基-5-(((2-硝基苄基)氧基)甲氧基)苄基)(亚硝基)氨基)甲基丙烯酸苄酯(PEO-b-PNNBM)的两亲性二嵌段共聚物,该二嵌段共聚物在水溶液中可自组装成胶束纳米粒子。区别于研究得较多的仅能传送一个气体信号分子的纳米载体,该胶束在可见光的照射下,可以通过光催化分解,同时释放一氧化氮(NO)和甲醛(FA)两种气体信号分子(GSMs),表现出联合的抗菌性能。此外,光响应性分解使其在水溶液中具有相对稳定性,不会过早泄漏NO和FA。由于NO、FA都是内源性且形成胶束为负电位,其显出良好的生物相容性,无溶血现象 [56] 。
3) 利用载铁纳米材料进行Fenton反应通常催化效果低且缺乏可控性 [60] 。清华大学赵凌云课题组利用生物相容性聚乙烯吡咯烷酮(PVP)制备了功能化AIronNPs抗菌系统,并系统研究了作为一种远程策略的交变磁场(AMF)对非晶态铁纳米颗粒(AIronNPs)催化活性和抗菌效率的影响。结果显示,使用AMF作为AIronNPs的响应点,可以加速Fe2+的释放,AIronNPs在低浓度H2O2也可转化为强的毒性羟基自由基(∙OH)。此外,选用AMF作为AIronNPs 化学动力学的响应机制,可以提高组织的穿透能力,解除光照技术组织穿透深度差(≤1 cm)的限制。体外抗菌实验显示,相比AIronNPs单独作用的有限性,AIronNPs和AMF协同抗菌系统具有光谱抗菌能力,动物模型中,还可以促进肉芽组织的形成和伤口愈合 [61] 。
6. 总结及展望
能够对内源和外源性刺激反应的纳米系统在抗菌及创面修复领域正在被广泛研究,在未来将显示巨大的希望。感染部位由于细菌分泌代谢以及机体自身过度的免疫反应使得该部位表现出一种不同于正常组织的特殊微环境,如低pH、特异性酶高表达、高浓度的ROS,尤其是H2O2。具有这种特殊微环境响应的抗菌材料预计只在感染部位发挥作用。这种材料的优点是可以及时发现细菌感染部位,根据内源性刺激或外源刺激按需杀菌。这使得治疗更精准、更具选择性,减少对健康组织的伤害同时避免细菌耐药性,促进创面愈合。
虽然响应性材料系统有很大的前景,但实现临床应用还存在不足,一个因素是安全性问题。对于一些无机纳米材料,虽然与抗生素相比不会带来耐药性,但由于相关作用机制(如ROS、抗菌肽)的非特异性杀伤,可能会出现浓度依赖的毒副作用,对人类细胞和组织造成一定损害。类似的问题还包括稳定性降低、溶血和代谢毒性。因此,对于响应性纳米材料的应用,控制释药速度及局部或全身浓度是需要克服的挑战。即使如此,这些对刺激反应灵敏的纳米材料在对抗细菌耐药性方面显示出巨大的潜力,是治疗创面细菌感染的有希望的候选材料之一。
文章引用
周 倩,刘 义. 生理响应型抗菌材料在创面修复中的应用
Applications of Antibacterial Materials with Physiological Responsiveness in Wound Repair[J]. 材料科学, 2023, 13(04): 306-317. https://doi.org/10.12677/MS.2023.134035
参考文献
- 1. Ellis, S., Lin, E.J. and Tartar, D. (2018) Immunology of Wound Healing. Current Dermatology Reports, 7, 350-358. https://doi.org/10.1007/s13671-018-0234-9
- 2. Leong, C. and Gouliouris, T. (2021) Skin and Soft Tissue Infec-tions. Medicine, 49, 699-705. https://doi.org/10.1016/j.mpmed.2021.08.007
- 3. Gurtner, G., Werner, S., Barrandon, Y. and Longaker, M.T. (2008) Wound Repair and Regeneration. Nature, 453, 314-321. https://doi.org/10.1038/nature07039
- 4. Harrison, O. (2020) Poised for Tissue Repair. Science, 369, 152-153. https://doi.org/10.1126/science.abc5618
- 5. Eming, S.A., Martin, P. and Tomic-Canic, M. (2014) Wound Repair and Regeneration: Mechanisms, Signaling, and translation. Science Translational Medicine, 6, 265sr6. https://doi.org/10.1126/scitranslmed.3009337
- 6. Xu, C., Akakuru, O.U., Ma, X., et al. (2020) Nanoparti-cle-Based Wound Dressing: Recent Progress in the Detection and Therapy of Bacterial Infections. Bioconjugate Chemis-try, 31, 1708-1723. https://doi.org/10.1021/acs.bioconjchem.0c00297
- 7. Lee, A.S., de Lencastre, H., Garau, J., et al. (2018) Methicil-lin-Resistant Staphylococcus aureus. Nature Reviews Disease Primers, 4, Article No. 8033. https://doi.org/10.1038/nrdp.2018.33
- 8. Binte Mohamed Salleh, N.A., Tanaka, Y., Sutarlie, L. and Su, S. (2022) Detecting Bacterial Infections in Wounds: A Review of Biosensors and Wearable Sensors in Comparison with Conven-tional Laboratory Methods. Analyst, 147, 1756-1776. https://doi.org/10.1039/D2AN00157H
- 9. Brown, M.S., Ashley, B. and Koh, A. (2018) Wearable Technology for Chronic Wound Monitoring: Current Dressings, Advancements, and Future Prospects. Frontiers in Bioengineering and Biotechnology, 6, Article 47. https://doi.org/10.3389/fbioe.2018.00047
- 10. Parlet, C.P., Brown, M.M. and Horswill, A.R. (2019) Commensal Staphylococci Influence Staphylococcus aureus Skin Colonization and Disease. Trends in Microbiology, 27, 497-507. https://doi.org/10.1016/j.tim.2019.01.008
- 11. Cieplik, F., Deng, D.M., Crielaard, W., et al. (2018) Antimicrobial Photodynamic Therapy—What We Know and What We Don’t. Critical Reviews in Microbiology, 44, 571-589. https://doi.org/10.1080/1040841X.2018.1467876
- 12. Imani, I.M., Kim, B., Xiao, X., et al. (2023) Ultra-sound-Driven on-Demand Transient Triboelectric Nanogenerator for Subcutaneous Antibacterial Activity. Advanced Sci-ence, 10, e2204801. https://doi.org/10.1002/advs.202204801
- 13. Wang, C., Xiao, Y., Zhu, W., et al. (2020) Pho-tosensitizer-Modified MnO2 Nanoparticles to Enhance Photodynamic Treatment of Abscesses and Boost Immune Protec-tion for Treated Mice. Small, 16, e2000589. https://doi.org/10.1002/smll.202000589
- 14. Levy, S.B. and Marshall, B. (2004) Antibacterial Resistance World-wide: Causes, Challenges and Responses. Nature Medicine, 10, S122-S129. https://doi.org/10.1038/nm1145
- 15. Czaplewski, L., Bax, R., Clokie, M., et al. (2016) Alternatives to Antibiot-ics—A Pipeline Portfolio Review. The Lancet Infectious Diseases, 16, 239-251. https://doi.org/10.1016/S1473-3099(15)00466-1
- 16. Klein, E.Y., Milkowska-Shibata, M., Tseng, K.K., et al. (2021) Assessment of WHO Antibiotic Consumption and Access Targets in 76 Countries, 2000-15: An Analysis of Pharmaceutical Sales Data. The Lancet Infectious Diseases, 21, 107-115. https://doi.org/10.1016/S1473-3099(20)30332-7
- 17. Browne, A.J., Chipeta, M.G., Haines-Woodhouse, G., et al. (2021) Global Antibiotic Consumption and Usage in Humans, 2000-18: A Spatial Modelling Study. The Lancet Plane-tary Health, 5, E893-E904. https://doi.org/10.1016/S2542-5196(21)00280-1
- 18. Lambert, M.-L., Suetens, C., Savey, A., et al. (2011) Clinical Outcomes of Health-Care-Associated Infections and Antimicrobial Resistance in Patients Admitted to European Inten-sive-Care Units: A Cohort Study. The Lancet Infectious Diseases, 11, 30-38. https://doi.org/10.1016/S1473-3099(10)70258-9
- 19. Sugden, R., Kelly, R. and Davies, S. (2016) Combatting An-timicrobial Resistance Globally. Nature Microbiology, 1, Article No. 16187. https://doi.org/10.1038/nmicrobiol.2016.187
- 20. Liang, C., Wang, X.D., Zhou, R.T., et al. (2019) Thermo- and Oxidation-Responsive Homopolypeptide: Synthesis, Stimuli-Responsive Property and Antimicrobial Activity. Polymer Chemistry, 10, 2190-2202. https://doi.org/10.1039/C8PY01735B
- 21. Quek, J.Y., Uroro, E., Goswami, N. and Vasilev, K. (2022) Design Principles for Bacteria-Responsive Antimicrobial Nanomaterials. Materials Today Chemistry, 23, Article ID: 100606. https://doi.org/10.1016/j.mtchem.2021.100606
- 22. Wang, X., Shan, M., Zhang, S., et al. (2022) Stimu-li-Responsive Antibacterial Materials: Molecular Structures, Design Principles, and Biomedical Applications. Advanced Science, 9, Article ID: 2104843. https://doi.org/10.1002/advs.202104843
- 23. Serena, T.E., Bayliff, S.W. and Brosnan, P.J. (2022) Bacterial Prote-ase Activity: A Prognostic Biomarker of Early Wound Infection. Journal of Wound Care, 31, 352-355. https://doi.org/10.12968/jowc.2022.31.4.352
- 24. Obreja, M., Miftode, E.G., Stoleriu, I., et al. (2022) Hepa-rin-Binding Protein (HBP), Neutrophil Gelatinase-Associated Lipocalin (NGAL) and S100 Calcium-Binding Protein B (S100B) Can Confirm Bacterial Meningitis and Inform Adequate Antibiotic Treatment. Antibiotics, 11, Article No. 824. https://doi.org/10.3390/antibiotics11060824
- 25. Wang, X., Wang, J., Qiu, L., et al. (2022) Gelatinase-Responsive Photothermal Nanotherapy Based on Au Nanostars Functionalized with Antimicrobial Peptides for Treating Staphylo-coccus aureus Infections. ACS Applied Nano Materials, 5, 8324-8333. https://doi.org/10.1021/acsanm.2c01390
- 26. Chang, Y.-H., Chiang, C.-Y., Fu, E. and Chiu, H.-C. (2022) Staphy-lococcus aureus Enhances Gelatinase Activities in Monocytic U937 Cells and in Human Gingival Fibroblasts. Journal of Dental Sciences, 17, 1321-1328. https://doi.org/10.1016/j.jds.2022.04.014
- 27. Qiu, L., Wang, C., Lei, X., et al. (2021) Gelatinase-Responsive Re-lease of an Antibacterial Photodynamic Peptide against Staphylococcus aureus. Biomaterials Science, 9, 3433-3444. https://doi.org/10.1039/D0BM02201B
- 28. Tian, R., Qiu, X., Yuan, P., et al. (2018) Fabrication of Self-Healing Hydrogels with on-Demand Antimicrobial Activity and Sustained Biomolecule Release for Infected Skin Regeneration. ACS Applied Materials & Interfaces, 10, 17018-17027. https://doi.org/10.1021/acsami.8b01740
- 29. Zhang, C. and Yang, M. (2022) Antimicrobial Peptides: From Design to Clinical Application. Antibiotics, 11, Article No. 349. https://doi.org/10.3390/antibiotics11030349
- 30. Li, X., Zuo, S., Wang, B., Zhang, Y. and Wang, Y. (2022) Anti-microbial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules, 27, Article No. 2675. https://doi.org/10.3390/molecules27092675
- 31. Stacy, A. and Belkaid, Y. (2019) Microbial Guardians of Skin Health. Science, 363, 227-228. https://doi.org/10.1126/science.aat4326
- 32. Chai, D., Liu, W., Hao, X., et al. (2020) Mussel-Inspired Synthesis of Magnetic N-Halamine Nanoparticles for Antibacterial Recycling. Colloid and Interface Science Communications, 39, Ar-ticle ID: 100320. https://doi.org/10.1016/j.colcom.2020.100320
- 33. Sheng, G.P., Ni, J.L., Xing, K.R., et al. (2021) Infection Mi-croenvironment-Responsive Multifunctional Peptide Coated Gold Nanorods for Bimodal Antibacterial Applications. Colloid and Interface Science Communications, 41, Article ID: 100379. https://doi.org/10.1016/j.colcom.2021.100379
- 34. Pranantyo, D., Kang, E.-T. and Chan-Park, M.B. (2021) Smart Nanomicelles with Bacterial Infection-Responsive Disassembly for Selective Antimicrobial Applications. Biomaterials Science, 9, 1627-1638. https://doi.org/10.1039/D0BM01382J
- 35. Liu, B., Li, J., Zhang, Z., Roland, J.D. and Lee, B.P. (2022) pH Re-sponsive Antibacterial Hydrogel Utilizing Catechol–Boronate Complexation Chemistry. Chemical Engineering Journal, 441, Article ID: 135808. https://doi.org/10.1016/j.cej.2022.135808
- 36. Chowdhury, F., Ahmed, S., Rahman, M., et al. (2022) Chronic Wound-Dressing Chitosan-Polyphenolic Patch for Ph Responsive Local Antibacterial Activity. Materials Today Com-munications, 31, Article ID: 103310. https://doi.org/10.1016/j.mtcomm.2022.103310
- 37. Wang, T., Dong, D., Chen, T., et al. (2022) Acidi-ty-Responsive Cascade Nanoreactor Based on Metal-Nanozyme and Glucose Oxidase Combination for Starving and Photothermal-Enhanced Chemodynamic Antibacterial Therapy. Chemical Engineering Journal, 446, Article ID: 137172. https://doi.org/10.1016/j.cej.2022.137172
- 38. Fan, Z.Y. and Xu, H.P. (2020) Recent Progress in the Biological Applications of Reactive Oxygen Species-Responsive Polymers. Polymer Reviews, 60, 114-143. https://doi.org/10.1080/15583724.2019.1641515
- 39. Cheeseman, S., Christofferson, A.J., Kariuki, R., et al. (2020) Antimicrobial Metal Nanomaterials: From Passive to Stimuli-Activated Applications. Advanced Science, 7, Article ID: 1902913. https://doi.org/10.1002/advs.201902913
- 40. Yu, N.X., Cai, T.M., Sun, Y., et al. (2018) A Novel Anti-bacterial Agent Based on AgNPs and Fe3O4 Loaded Chitin Microspheres with Peroxidase-Like Activity for Synergistic Antibacterial Activity and Wound-Healing. International Journal of Pharmaceutics, 552, 277-287. https://doi.org/10.1016/j.ijpharm.2018.10.002
- 41. Li, Y.Q., Xiu, W.J., Yang, K.L., et al. (2021) A Multifunctional Fenton Nanoagent for Microenvironment-Selective Anti-Biofilm and Anti-Inflammatory Therapy. Materials Horizons, 8, 1264-1271. https://doi.org/10.1039/D0MH01921F
- 42. Rai, V., Moellmer, R. and Agrawal, D.K. (2022) Clinically Relevant Experimental Rodent Models of Diabetic Foot Ulcer. Molecular and Cellular Biochemistry, 477, 1239-1247. https://doi.org/10.1007/s11010-022-04372-w
- 43. Kandregula, B., Narisepalli, S., Chitkara, D. and Mittal, A. (2022) Exploration of Lipid-Based Nanocarriers as Drug Delivery Systems in Diabetic Foot Ulcer. Molecular Pharma-ceutics, 19, 1977-1998. https://doi.org/10.1021/acs.molpharmaceut.1c00970
- 44. Sharma, H., Sharma, S., Krishnan, A., et al. (2022) The Efficacy of Inflammatory Markers in Diagnosing Infected Diabetic Foot Ulcers and Diabetic Foot Osteomyelitis: Sys-tematic Review and Meta-Analysis. PLOS ONE, 17, e0267412. https://doi.org/10.1371/journal.pone.0267412
- 45. Pouget, C., Dunyach-Remy, C., Pantel, A., et al. (2021) Alter-native Approaches for the Management of Diabetic Foot Ulcers. Frontiers in Microbiology, 12, Article 47618. https://doi.org/10.3389/fmicb.2021.747618
- 46. Yu, J., Zhang, R.L., Chen, B.H., et al. (2022) Injectable Reactive Oxygen Species-Responsive Hydrogel Dressing with Sustained Nitric Oxide Release for Bacterial Ablation and Wound Healing. Advanced Functional Materials, 32, Article ID: 2202857. https://doi.org/10.1002/adfm.202202857
- 47. Li, F., Zang, M.S., Hou, J.X., et al. (2021) Cascade Catalytic Nanoplatform Constructed by Laterally-Functionalized Pil-larArenes for Antibacterial Chemodynamic Therapy. Journal of Materials Chemistry B, 9, 5069-5075. https://doi.org/10.1039/D1TB00868D
- 48. Ramani, K., Cormack, T., Cartwright, A.N.R., et al. (2022) Regulation of Peripheral Inflammation by a Non-Viable, Non-Colonizing Strain of Commensal Bacteria. Frontiers in Immunology, 13, Article 768076. https://doi.org/10.3389/fimmu.2022.768076
- 49. Rohner, N.A., Learn, G.D., Wiggins, M.J., Woofter, R.T. and von Recum, H.A. (2021) Characterization of Inflammatory and Fibrotic Encapsulation Responses of Implanted Materials with Bacterial Infection. ACS Biomaterials Science & Engineering, 7, 4474-4482. https://doi.org/10.1021/acsbiomaterials.1c00505
- 50. Tokarz-Deptuła, B., Palma, J., Baraniecki, Ł., et al. (2021) What Function Do Platelets Play in Inflammation and Bacterial and Viral Infections? Frontiers in Immunology, 12, Arti-cle 770436. https://doi.org/10.3389/fimmu.2021.770436
- 51. Yan, X., Yang, J., Wu, J., et al. (2021) Antibacterial Carbon Dots/Iron Oxychloride Nanoplatform for Chemodynamic and Photothermal Therapy. Colloid and Interface Sci-ence Communications, 45, Article ID: 100552. https://doi.org/10.1016/j.colcom.2021.100552
- 52. Xiao, Y., Xu, M., Lv, N., et al. (2021) Dual Stimu-li-Responsive Metal-Organic Framework-Based Nanosystem for Synergistic Photothermal/Pharmacological Antibacterial Therapy. Acta Biomaterialia, 122, 291-305. https://doi.org/10.1016/j.actbio.2020.12.045
- 53. Silva-Freitas, E.L., Pontes, T.R.F., Araujo-Neto, R.P., et al. (2017) Design of Magnetic Polymeric Particles as a Stimulus-Responsive System for Gastric Antimicrobial Therapy. AAPS Pharmscitech, 18, 2026-2036. https://doi.org/10.1208/s12249-016-0673-1
- 54. Naseri, E. and Ahmadi, A. (2022) A Review on Wound Dress-ings: Antimicrobial Agents, Biomaterials, Fabrication Techniques, and Stimuli-Responsive Drug Release. European Polymer Journal, 173, Article ID: 111293. https://doi.org/10.1016/j.eurpolymj.2022.111293
- 55. Liu, Y., Mao, S., Zhu, L., Chen, S. and Wu, C. (2021) Based on Tannic Acid and Thermoresponsive Microgels Design a Simple and High-Efficiency Multifunctional Antibacterial Coating. European Polymer Journal, 153, Article ID: 110498. https://doi.org/10.1016/j.eurpolymj.2021.110498
- 56. Duan, Y., He, K., Zhang, G. and Hu, J. (2021) Photore-sponsive Micelles Enabling Codelivery of Nitric Oxide and Formaldehyde for Combinatorial Antibacterial Applications. Biomacromolecules, 22, 2160-2170. https://doi.org/10.1021/acs.biomac.1c00251
- 57. Zhang, H., Xie, M.Y., Chen, H.H., et al. (2021) Gas-Mediated Cancer Therapy. Environmental Chemistry Letters, 19, 149-166. https://doi.org/10.1007/s10311-020-01062-1
- 58. Gong, W., Xia, C. and He, Q. (2022) Therapeutic Gas Delivery Strategies. WIREs Nanomedicine and Nanobiotechnology, 14, e1744. https://doi.org/10.1002/wnan.1744
- 59. Zhou, Y., Yang, T., Liang, K. and Chandrawati, R. (2021) Metal-Organic Frameworks for Therapeutic Gas Delivery. Advanced Drug Delivery Reviews, 171, 199-214. https://doi.org/10.1016/j.addr.2021.02.005
- 60. Tang, Y.N., Qin, Z., Yin, S.Y. and Sun, H. (2021) Transition Metal Oxide and Chalcogenide-Based Nanomaterials for Antibacterial Activities: An Overview. Nanoscale, 13, 6373-6388. https://doi.org/10.1039/D1NR00664A
- 61. Gao, F., Li, X., Zhang, T., et al. (2020) Iron Nanoparticles Augmented Chemodynamic Effect by Alternative Magnetic Field for Wound Disinfection and Healing. Journal of Controlled Release, 324, 598-609. https://doi.org/10.1016/j.jconrel.2020.06.003
NOTES
*通讯作者。
