Advances in Analytical Chemistry
Vol.08 No.02(2018), Article ID:25194,11
pages
10.12677/AAC.2018.82010
Hydrotalcite Application in Electrochemical Sensing and Testing
Liya Cheng1*#, Chaolin Wang2*, Meiqin Wang3*, Yaping Ding2#
1Institute of Geological Experiment of Anhui Province, Hefei Anhui
2Department of Chemistry, Shanghai University, Shanghai
3Heze Vocational College for Home Economics, Shanxian County Shandong

Received: Apr. 29th, 2018; accepted: May 14th, 2018; published: May 30th, 2018

ABSTRACT
Layered double hydroxides (LDHs) are a family of anionic clays consisting of positively charged brucite-like layers among which are located anions and water molecules. The preparation methods of LDHs are diverse, and the LDH film usually uses layer-by-layer self-assembly method, dropping method and electrochemical deposition method and other methods to modify the electrode. Hydrotalcite has the obvious advantages in the sensing and detection and in the field of electrochemical analytical chemistry. Therefore, in recent years, domestic and international analysis and sensor technology workers in the extensive attention, and a large number of literatures have been reported. According to the principle of sensing and detection, the preparation method of modified electrode, this paper summarizes and evaluates the application of bioanalysis, and discusses the future direction of development.
Keywords:Layered Double Hydroxides, Modification Electrode, Electrochemical Sensing, Electrochemical Test
纳米水滑石在电化学传感与检测中的应用
程丽娅1*#,王朝琳2*,王美芹3*,丁亚萍2#
1安徽省地质实验研究所,安徽 合肥
2上海大学化学系,上海
3菏泽家政职业学院,山东 单县

收稿日期:2018年4月29日;录用日期:2018年5月14日;发布日期:2018年5月30日

摘 要
水滑石是由带正电荷的金属氢氧化物层和带负电荷的层间阴离子构成的层状双羟基金属复合氧化物(LDH),其制备方法多样,而LDH薄膜通常使用层层自组装法、滴涂法以及电化学沉积法等方法修饰电极。水滑石这种层状结构的材料在传感和检测中体现出明显的优势,在电分析化学领域中有着重要的应用,发挥巨大作用。因此近年来受到国内外分析与传感科技工作者的广泛关注,并且,有大量文献报道了该方面的研究工作。本文按照传感与检测的原理、修饰电极的制备方法,对生物分析检测的应用进行总结与评价,并讨论了未来的发展方向。
关键词 :LDHs,修饰电极,电化学传感,电化学检测

Copyright © 2018 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
水滑石类化合物是一种阴离子型无机层状黏土材料,由于它的主体成份一般是由两种金属的氢氧化物构成,又称为层状双羟基复合金属氢氧化物(Layered Double Hydroxide,简写为LDH) [1]。LDH的主片层是带正电荷的双金属氢氧化物,是LDH纳米片状材料的前体,LDH可通过一定的方法剥离得到正电荷型的LDH纳米片状材料 [2]。这大大拓展了LDH材料的应用范围,可利用LDH纳米片状材料作为基元构筑新型的二维纳米复合材料 [3]。近年来,水滑石的应用愈发广泛,在国内外,水滑石材料在医药、生物、催化、吸附、电化学、光化学、水处理和环境保护等许多领域展现出广阔的应用前景。另一方面,基于剥离的LDH纳米片的主客体相互作用,可构筑具有新型结构特点的纳米复合材料,研究表明该类材料可应用于发光、超疏水、防腐、传感器等许多领域 [4] - [16]。而在电化学传感领域,水滑石纳米材料的应用也十分广泛,在环境监测、食品安全和生物医学领域都有重要的应用价值 [17] - [22]。
2. 水滑石纳米片的简介
LDH作为LDH纳米片状材料的前体,典型的LDH是镁铝碳酸根型,其结构类似于水镁石Mg(OH)2,水镁石正八面体结构中心为Mg2+,六个顶点为OH,相邻的正八面体通过羟基共用边相互连接形成片层,片层间对顶相叠排列在一起,片层间通过氢键缔合。位于层上的Mg2+可在一定的范围内被半径相似的Al3+同晶取代,使得Mg2+、Al3+、OH-离子层带正电荷,这些正电荷被位于层间的 中和, 与层板之间通过静电引力以及氢键结合起来,使LDH结构整体保持电中性。LDH是由层间阴离子及带正电荷层板通过非共价键堆积而组装成的化合物,片层中的金属离子可在一定的范围内被半径相似的其它金
属离子同晶取代,故LDH的化学组成可以理想的表示为: ,其中Mp+
为一价或二价(多为二价)的金属阳离子,M3+为三价的金属阳离子,x为M3+/(Mp+ + M3+)的摩尔比,可在0.1至0.5之间变化,超出此范围则会引入杂相,An−为插入层间的阴离子,可以是有机、无机、同多或杂多、配合客体阴离子,m为结晶水的数目。可位于主体层板上金属阳离子,二价如Mg2+、Ni2+、Zn2+、Mn2+、Cu2+、Co2+、Pd2+、Fe2+等,三价如A13+、Cr3+、Co3+、Fe3+等均可以形成LDH。其结构类似于水镁石Mg(OH)2,由MgO6八面体共用棱边而形成单元主体层板 [23] - [34]。LDH的结构示意图见图1。
3. 水滑石纳米片的制备
LDH纳米片状材料是通过剥离LDH前体得到的,LDH的层板内存在强的共价键作用,而层间则是弱的相互作用力,因此层与层容易被其它分子撑开或剥离,而层板结构不受影响。阳离子型层状材料相对来说易于剥离,而LDH由于片层电荷密度很高,因此要实现其片层的剥离比较困难。LDH纳米片的剥离研究,经过了数年的研究与进展,逐渐实现了使用更简单环保的方法对LDH进行剥离 [35] [36] [37] [38]。
3.1. 水滑石纳米片的制备
日本的Sasaki课题组通过研究发现, 插层的大片层LDH在甲酰胺中可以实现层板剥离 [39]。采用这种方法制备的层板剥离LDH呈规整的六方形结构,粒径可以达到1 µm,厚度小于1 nm。除了 ,他们还发现 和十二烷基磺酸根等插层的LDH也能够在甲酰胺中剥离 [40]。他们还提出了LDH在甲酰胺中溶胀剥离的机理如图2所示。随后,Sasaki课题组对LDH和LDH的剥离进行了一系列系统的工作 [41] [42] [43] [44] [45]。2012年,Wang等 [46] 对LDH的剥离和应用最新进展做了综述。2014年,我国的段雪院士对LDH的剥离及在催化中应用的最新进展做了系统综述 [47]。最近,O’Hare [48] 课题组又提出了一种新的剥层方法——反相微乳液一步合成法。采用这种方法可以直接制备出层板剥离LDH粉体材料。归纳总结以上文献报道LDH的剥离过程主要分为两个步骤:首先是以合适的阴离子插入层间,削弱层板之间的相互作用力,然后在合适的溶剂中回流直至形成剥离的LDH胶体溶液。由于LDH层板二维纳米尺度,LDH剥离后理想的状态为澄清透明的。

Figure 1. Structure of LDHs
图1. LDH的结构示意图

Figure 2. Peeling process of hydrotalcite
图2. 水滑石剥离过程示意图
3.2. 水滑石纳米片状复合材料的制备
水滑石纳米复合材料(nanocomposites)是指由经过筛选的,有一定数量比的两种或两种以上不同物理或化学性质的物质以微观或宏观的形式复合而成的多相固体材料,其中至少有一种材料在一维方向是处于纳米级。纳米复合材料的结构由简单到复杂,种类越来越多,合成方法也越来越多样,其中应用最广泛的复合合成法主要有溶胶-凝胶法、共沉淀法、离子交换法、微乳液法、气相沉积法、水热法、自组装法、溶剂蒸发法和机械球磨法等 [49] [50] [51] [52] [53]。研究表明,由于纳米分散相具有较大的表面积以及强的界面相互作用,纳米复合材料不仅能克服两种材料自身的缺点,使原有性能得到发挥,而且由于协同效应及其它作用使得该纳米复合材料还可能具有原材料所不具备的特殊性能和功能,从而在机械结构材料、光电子功能材料、磁性功能材料、化学和生物材料、医学功能材料和热学功能材料等领域具有广阔的应用前景。
4. 电化学传感器简介
传感技术包括信息交换、信息处理及接口技术三部分,其中信息交换为传感技术的核心内容,即所谓的传感器。电化学传感器与其他的传感器一样,一般由感应器、换能器、检测器三个部分组成。
4.1. 电化学传感器的原理
电化学传感器同光化学传感器和热化学传感器一样,是化学传感器的一个子类,它能够利用被检测对象的化学反应所产生的电位、电流、电阻、电容等信号变化,对其进行灵敏、快速而准确的信号转换和检测,而且能够实现直接、自动化、小型化和智能化的检测。在工作过程中,电化学传感器中的感受器与待测物质进行识别并与之发生物理,生物或者化学反应并产生信号,产生的反应信号经特定的换能器将这种感知信号转换成可识别的、与目标物质浓度成比例的电信号,从而达到定性或定量的分析检测目标物质。其工作原理如图3所示。
4.2. 电化学传感器的制备
对于电化学传感器,工作电极其核心部件之一,它的发展对各种电化学传感器的发展起到了关键作用。因此如何使电极能够提高电子转移的速度且可以有选择地进行所期望的反应,从而实现对特定物质的检测,一直是电化学工作者的目标 [54]。在早期的研究中,金、铂、碳和甘汞是常用的电极材料,这些材料不是价格高昂,就是有毒有害,而且简单的裸电极/电解液界面行为无法充分地反映研究界面复杂的反应特征,并且这种简单的电极界面经常不能实现生物、化学分子与电极间有效的电子传递 [55] ,此外对所有溶液中的电活性物质都有响应。所以,人们迫切需要一种简单的,经济的,特异性的电极进行对生物、化学分子的电化学分析。因此,在电极表面上进行一定功能化的化学修饰引起了人们的关注。
化学修饰电极的制备是电化学传感器的关键,它研究修饰电极表面的微结构和其界面反应,促进了电化学及电化学传感器的发展和应用。电极的修饰方法的设计、操作步骤等对电化学传感器的灵敏度、
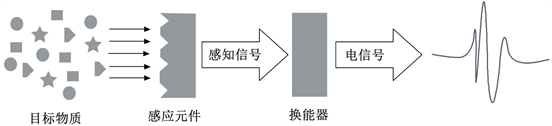
Figure 3. Structure and basic principle of electrochemical sensor
图3. 电化学传感器的结构及其基本原理
抗干扰性、重现性和稳定性都有直接的影响。化学修饰电极是用化学或物理的方法,将特定功能的分子、离子、聚合物等固定在工作电极表面,实现功能化。按修饰的方法一般可分为共价键合、化学吸附、聚合物覆盖三种方式,在电极表面固定具有独特化学性质的修饰成分,制造某种特殊的微结构,赋予电极特定的化学和电化学性质以实现特异性检测。目前常作修饰电极的材料主要有金属及其氧化物、粘土类、电活性薄膜、离子交换膜、导电聚合膜、分子筛类等。修饰方法没有严格的界限,研究中常常综合不同方法对电极进行化学修饰 [56]。
5. 水滑石纳米片在电化学传感与检测中的应用
水滑石(LDH)作为一种重要的无机层状材料 [57] [58] ,近年来在其他领域 [59] - [64] 吸引了越来越多的关注。而在电化学领域,含有过渡金属的LDH由于具有优异的电荷传输,氧化还原催化活性,片层的正电荷、阴离子交换、大的表面积和生物相容性等 [65] 特性已成为潜在的有吸引力的表面改性电极的材料。然而,进一步应用受到了限制主要是因为一般方法合成的LDH是粉末状的,附着能力差,导致很难修饰于电极基质上。此外,许多电活性分子或大分子不能插入层间的类,只能吸附在材料表面导致负荷率低,而且聚合严重,容易解吸。因此通过不同的修饰方法将LDH修饰到电极上,从而使电化学传感器的电化学性能达到更优异的效果。
5.1. 水滑石纳米片层层自主装法修饰电极在电分析中的应用
静电层层自组装法(Layer-by-Layer assembly, LBL)的基本原理是:以静电作用为驱动力,将基底分别浸入带有相反电荷溶液中,在基底表面,带相反电荷的物质交替沉积构造具有一定厚度的多层膜体系。由于LBL自组装薄膜结构简单、制备相对容易等特点而引起了研究人员极大的兴趣。
LDH通过剥离,获得带正电的二维各向异性的单一纳米片状材料。这些纳米片可以作为优秀的基元构筑有机–无机多层超薄膜UTFs [66] [67]。这启发了利用带正电的LDH纳米片和带负电荷的电活性物质交替修饰电极制备电化学传感器。这种UTFs具有两个优点:首先,由于是分子水平的组装,电活性物质均匀分散提高了物质和修饰电极之间的电子转移速率;其次,由于均匀和有取向的组装,提高了LDH 纳米片在电极表面上的附着力,从而促进了长期的稳定使用。
已经有报道了一些利用静电层层(LBL)自组装法方法制备了UTFs修饰的电化学传感器。用静电LBL自组装法制备了基于NiAl/LDH纳米片和铬黑T (EBT)的UTFs修饰在玻碳电极(GCE)上的电化学传感器。其制备方法为将预处理的电极交替在带负电的EBT溶液(0.5mM)和NiAl/LDH纳米片悬浮液中浸泡,以上一系列的组装操作被重复了n次(1次组装为分别在EBT溶液和NiAl-LDH纳米片悬浮液中浸泡1次),获得(EBT/LDH)nUTFs修饰电极,记为(EBT/LDH)nUTFs/GCE。之后对水杨酸进行检测,具有良好的电化学信号,修饰电极和裸电极对比显示出其电流响应大数倍,峰电流明显说明修饰电极有很强的催化能力。检测SA的线性范围为浓度从0.01到100 μM。最低检出限约为0.003 μM (S/N = 3),灵敏度是860 μAmM−1cm−2 [68]。
萘酚绿B (NGB)又称酸性绿1,有独特的化学、物理和电子属性,是一种无机纳米粒子,可作为电活性物质修饰在电极表面,具有较高的稳定性以及有着良好的重复性。因此可以使用LBL自组装法可以将 NGB和LDHs修饰在氧化铟锡(ITO)电极上,从而检测抗坏血酸(AA),随着AA浓度的增加,响应电流会变大,最后得出的结论为AA的检测线性响应范围为1.2~55.2 µM,最低检出限是0.51 µM [69]。
通过LBL的方法还可制作羧甲基-β-环糊精(CMCD)Mg-Al水滑石(LDH/CMCD)的电极,其制备方法为将预处理的电极CMCD溶液(0.5 mM)和LDH纳米片悬浮液中浸泡修饰到玻碳电极上,从而可以制备传感器进行应用 [70]。
5.2. 电沉积法修饰电极在电分析中的应用
电化学沉积法(EFD)是通过外电场作用时LDHs带点胶体粒子沉积到电极表面上的一种成膜方法。这种方法不需要复杂的设备,而且操作简单,沉积率高,具有与基底结合力强等优点,被人们广泛关注。薄膜的均匀程度以及厚度取决于胶体溶液的浓度、分散度、电压、通电时间等因素。
将制备的钴锰水滑石(CoMn-LDH)的悬浮液在ITO电极上进行修饰,并使溶剂在室温下蒸发,形成均匀且超薄的LDH膜。将改性的ITO电极在含有HAuCl4的磷酸盐缓冲溶液中浸渍从而吸附AuCl4离子,之后使用电沉积的方法将金纳米颗粒(AuNP)修饰在电极上来制造用于过氧化氢(H2O2)的电化学传感器。最后得出结论其线性范围为0.1 μM至1.27 mM,最低检出限为0.06 μM [71]。
通过共沉淀法将合成的镍铝水滑石(NiAl-LDH)悬浮液修饰在电极上,再将将壳聚糖和乙酸的混合物滴在电极表面上直到干燥,最后通过电沉积发沉积金纳米粒子(AuNP),从而制备出一种新型灵敏的非那吡啶电化学传感器。该传感器表现出良好的电化学性能,其检测限为0.009 μM [72]。
采用成核/晶化隔离法合成了镁铝水滑石(MgAl-LDH)纳米颗粒,利用电沉积法将其修饰到氧化铟锡导电玻璃电极表面,在制备了铂纳米颗粒/水滑石复合修饰电极,使该电极对过氧化氢具有较好的电催化性能。基于镁铝水滑石良好的生物相容性,将葡萄糖氧化酶进一步修饰到该电极表面,实现了对葡萄糖高灵敏的电化学检测,检出限达1.0 μmol/L [73]。
还可以通过电沉积的方法沉积铜纳米线来制备电极。先将制备的LDH修饰在玻碳电极上之后,再将电极在CuCl2溶液中浸渍进行电沉积,从而制作了测定多柔比星的电化学传感器,最终结果表明线性浓度范围为10.0~2110.0 nM,检测限为0.02 nM [74]。
5.3. 滴涂法修饰电极在电分析中的应用
滴涂法是化学修饰电极常用的方法,其制作过程就是将制备好的材料进行分散,然后使用移液枪将材料滴到电极上,在红外灯下烤干,之后在进行一系列的电化学行为。这种方法制作简单方便,用时短,低成本,可以进行广泛应用。比如通过一步合成法合成NiFe-LDH,通过滴涂法修饰在电极上从而制作了无酶葡萄糖传感器,其结果显示出Ni基纳米材料对非酶葡萄糖传感器显示出显着的电化学催化活性,其最低检出限为0.59 μM [75]。
使用滴涂法修饰电极,还可以制作基于巯基乙酸插层的Mg-Al LDH (Mg-Al-TGA LDH)高灵敏度和选择性电化学传感器。制备的电极可以用于痕量检测金属汞。最终结果表明其检测范围为2.0~800 nM,最低检出限为0.8 nM [76]。
通过共沉淀法制备NiAl-LDH,将Fe3O4和LDH混合进行分散,再通过滴涂法修饰在玻碳电极上,从而制备了一种具有磁性的新型电化学传感器。并使用差分脉冲伏安法(DPV)作为敏感电化学方法研究了修饰电极测定痕量曲马多(TRA)的性能。最后结果表明其线性范围为1.0~200.0 μM,最低检出限为0.3 μM [77]。
将制备好的CoFe-LDHs悬浮液在去离子水中分散,之后与碳量子点(C-Dots)进行混合搅拌,然后再加入辣根过氧化物酶(HRP)搅拌,最终通过滴涂法修饰在玻碳电极上制备一种HRP电化学生物传感器。结果表明由C-Dots/LDH固定的HRP保留了酶的活性,表现出良好的电催化还原活性和对H2O2的优异电化学催化性能。其线性范围是0.1~23.1 μM,检测限为0.04 μM [78]。
在水介质中,将1-天冬酰胺作为预嵌入剂制备了超薄剥离的NiAl层状双氢氧化物(ELDH)纳米片。将制备好的NiAl-LDH悬浮液滴涂在干净的玻碳电极表面上,然后再修饰一层壳聚糖,从而制作了改性得玻璃电极来检测的双酚A (BPA)。所得传感器表现出良好的再现性,选择性和可接受的稳定性,通过差示脉冲伏安法(DPV)用于检测BPA的线性范围为0.02~1.51 μM宽,检测限为6.8 nM [79]。
使用滴涂法将一步合成法合成的花状分层的H-NiAl/LDHs修饰到电极上,使用时间电流曲线法分别检测对苯二酚和儿茶酚,其最终结果表明电化学信号响应良好,对苯二酚的检测范围是0.01~140 μM,检出限为0.003 μM,儿茶酚的检测范围是0.01~400 μM,检出限为0.003 μM [80]。
通过共沉淀法制备NiMgFe/LDH,并将其滴涂到经葡萄糖氧化酶(GOD)改性的玻碳电极上,从而制备了一种葡萄糖酶生物传感器,该传感器对葡萄糖的检测范围是1~20 mM,检出限0.12 mM [81]。
5.4. 其他修饰电极方法在电分析中的应用
除却滴涂,层层自组装,电沉积法之外,还有一系列其他修饰电极的方法。例如通过共沉淀法在碱性介质中成功制备了CoNiAl-LDH,之后将GQDs/CoNiAl-LDH纳米复合材料按比例与石墨和石蜡混合,直到混合均匀的到糊状物。然后将混合均匀的糊状物牢固地包装在玻璃管的空腔,从而制备了GQDs/ CoNiAl-LDH碳糊电极来构建一种无酶葡萄糖电化学传感器。最终结果表明其电化学性能良好,线性范围为0.01~14.0 mM,检测限为6 μM [82]。
同样适用制作碳糊电极的方法,可以制作一种MgAl-LDH/GO/MWCNT新型碳糊电极来检测罂粟碱(PAP)。该电化学传感器低成本、易于制备,在优化条件下,PAP的线性范围是0.10~100 μM,检测限为0.04 μM [83]。
NiAl-LDH薄膜可以用原位生长技术修饰到碳布电极上来制作一种有着良好的灵敏性和较快相应的无酶葡萄糖电化学生物传感器。由于碳布电极可以将其裁剪成不同的形状和大小,从而满足不同的检测需求并且保持着良好的电化学活性。此外,碳布电极其成本较低且制备方法简单,有利于其更加广泛的应用。结果最终表明,随着葡萄糖浓度的增加,其响应越大,其检测的线性范围为1~329 µM,最低检测限为0.22 µM [84]。
6. 结论与展望
在过去的几十年里,人们对水滑石材料的研究付出了巨大的努力,为其性质、合成和应用建立起了一个丰富的数据库。水滑石纳米片状材料和其复合材料不仅够将纳米片的电子、化学和物理方面的优良特性得到充分发挥和得到新的特性,而且也能够极大地丰富、发展纳米片状材料的制备和应用,这些预测已经取得了丰硕的研究成果,尤其是在电化学传感器领域。但在合成材料方法,修饰制备电极过程中还有许多的问题需要进一步的研究和完善。将制备纳米片状材料的技术和分析方法相结合,进一步提高电化学传感器的各种性能,将是电分析化学未来进一步发展的方向。
基金项目
国家自然科学基金资助课题(21671132)。
文章引用
程丽娅,王朝琳,王美芹,丁亚萍. 纳米水滑石在电化学传感与检测中的应用
Hydrotalcite Application in Electrochemical Sensing and Testing[J]. 分析化学进展, 2018, 08(02): 74-84. https://doi.org/10.12677/AAC.2018.82010
参考文献
- 1. Rives, V. (2001) Layered Double Hydroxides: Present and Future. Nova Science Publishers, New York.
- 2. Lee, S.Y. and Kim, S.J. (2002) Expansion of Smectite by Hexadecyltrimethylammonium. Clays and Clay Minerals, 50, 435-445.
https://doi.org/10.1346/000986002320514163 - 3. Kong, X.G., Rao, X.Y., Han, J.B., Wei, M. and Duan, X. (2010) Layer-by-Layer Assembly of Bi-Protein/Layered Double Hydroxide Ultrathin Film and Its Electrocatalytic Behavior for Catechol. Biosensors and Bioelectronics, 26, 549-554.
https://doi.org/10.1016/j.bios.2010.07.045 - 4. Feng, L., Li, S., Li, Y., Li, H., Zhang, L., Zhai, J., Song, Y., Liu, B., Jiang, L. and Zhu, D. (2002) Super-Hydrophobic Surfaces: From Natural to Artificial. Advanced Materials, 50, 435-455.
https://doi.org/10.1002/adma.200290020 - 5. Guo, Z., Zhou, F., Hao, J. and Liu, W. (2005) Stable Biomimetic Super-Hydrophobic Engineering Materials. Journal of the American Chemical Society, 127, 15670-1567.
https://doi.org/10.1021/ja0547836 - 6. Chen, H.Y., Zhang, F.Z., Fu, S.S. and Duan, X. (2006) In Situ Mi-crostrueture Control of Oriented Layered Double Hydroxide Monolayer Films with Curved Hexagonal Crystals as Super Hydrophobic Materials. Advanced Materials, 18, 3089-3093.
https://doi.org/10.1002/adma.200600615 - 7. Zhang, F.Z., Zhao, L.L., Chen, H.Y., Xu, S.L., Evans, D.G. and Duan, X. (2008) Corrosion Resistance of Super Hydrophobic Layered Double Hydroxide Films on Aluminum. An-gewandte Chemie International Edition, 47, 2466-2469.
https://doi.org/10.1002/anie.200704694 - 8. Yan, D., Lu, J., Wei, M., Han, J., Ma, J., Li, F., Evans, D.G. and Duan, X. (2009) Ordered Poly(p-phenylene)/Layered Double Hydroxide Ultrathin Films with Blue Luminescence by Layer-by-Layer Assembly. Angewandte Chemie International Edition, 48, 3073-3076.
https://doi.org/10.1002/anie.200900178 - 9. Yan, D., Lu, J., Chen, L., Qin, S., Ma, J., Wei, M., Evans, D.G. and Duan, X. (2010) A Strategy to the Ordered Assembly of Functional Small Cation with Layered Double Hydroxides for Luminescent Ultra-Thin Films. Chemical Communications, 46, 5912-5914.
https://doi.org/10.1039/c0cc00522c - 10. Yan, D., Lu, J., Ma, J., Wei, M., Qin, S., Chen, L., Evans, D.G. and Duan, X. (2010) Thin Film of Coumarin-3-Carboxylate and Surfactant Co-Intercalated Layered Double Hydroxide with Polarized Photoluminescence: A Joint Experimental and Molecular Dynamies Study. Journal of Materials Chemistry, 20, 5016-5024.
https://doi.org/10.1039/b924821h - 11. Yan, D., Lu, J., Ma, J., Wei, M., Wang, X., Evans, D.G. and Duan, X. (2010) Anionic Poly(p-Phenylenevinylene)/ Layered Double Hydroxide Ordered Ultrathln Films with Multiple Quantum Well Structure: A Combined Experimental and Theoretical Study. Langlnuir, 26, 7007-7014.
https://doi.org/10.1021/la904228b - 12. Yan, D., Lu, J., Wei, M., Qin, S., Chen, L., Zhang, S., Evans, D.G. and Duan, X. (2011) Heterogeneous Transparent Ultrathin Films with Tunable-Color Luminescence Based on the Assembly of Photoactive Organic Molecules and Layered Double Hydroxides. Advanced Functional Materials, 21, 2497-2505.
https://doi.org/10.1002/adfm.201002446 - 13. Zhang, L., Zhang, X., Shen, L., et al. (2012) High-Current Capacitive Behavior of Graphene/CoAI-Layered Double Hydroxide Composites as Electrode Material for Supercapacitors. Journal of Power Sources, 199, 395-401.
https://doi.org/10.1016/j.jpowsour.2011.10.056 - 14. Avila, P, Montes, M. and Miro, E.E. (2005) Monolithic Reactors for Environmental Applications: A Review on Preparation Technologies. Chemical Engineering Journal, 109, 11-36.
https://doi.org/10.1016/j.cej.2005.02.025 - 15. Tomasic, V. and Jovc, F. (2006) State-of-the-Art in the Monolithic Catalysts/Reactors. Applied Catalysis A, 311, 112-121.
https://doi.org/10.1016/j.apcata.2006.06.013 - 16. Lu, Z., Zhang, F., Lei, X., Yang, L., Xu, S. and Duan, X. (2008) In Situ Growth of Layered Double Hydroxide Films on Anodic Aluminum Oxide/Aluminum and Its Catalytic Feature in Aldol Condensation of Acetone. Chemical Engineering Science, 63, 4055-4062.
https://doi.org/10.1016/j.ces.2008.05.007 - 17. Shan, D., Cosnier, S. and Mousty, C. (2003) Layered Double Hydroxides: An Attractive Material for Electrochemical Biosensor Design. Analytical Chemistry, 75, 3872-3879.
https://doi.org/10.1021/ac030030v - 18. Han, E., Shan, D., Xue, H. and Cosnier, S. (2007) Hybrid Material Based on Chitosan and Layered Double Hydroxides: Characterization and Application to the Design of Amperometric Phenol Biosensor. Biomacromolecules, 8, 971-975.
https://doi.org/10.1021/bm060897d - 19. Shan, D., Yao, W. and Xue, H. (2007) Electrochemical Study of Ferro-cenemethanol-Modified Layered Double Hydroxides Composite Matrix: Application to Glucose Amperometric Bio-sensor. Biosensors and Bioelectronics, 23, 432-437.
https://doi.org/10.1016/j.bios.2007.06.007 - 20. Kong, X., Rao, X., Han, J., Wei, M. and Duan, X. (2010) Layer-by-Layer Assembly of Bi-Protein/Layered Double Hydroxide Ultrathin Film and Its Electrocatalytic Behavior for Catechol. Biosensors and Bioelectronics, 26, 549-554.
https://doi.org/10.1016/j.bios.2010.07.045 - 21. Cosnier, S., Mousty, C., Gondran, C. and Lepellec, A. (2006) Entrapment of Enzyme within Organic and Inorganic Materials for Biosensor Applications: Comparative Study. Mate-rials Science and Engineering: C, 26, 442-447.
https://doi.org/10.1016/j.msec.2005.10.077 - 22. Zhao, J., Kong, X., Shi, W., Shao, M., Han, J., Wei, M., Evans, D.G. and Duan, X. (2011) Self-Assembly of Layered Double Hydroxide Nanosheets/Au Nanoparticles Films for En-zyme-Free Electrocatalysis of Glucose. Journal of Materials Chemistry, 21, 13926-13933.
https://doi.org/10.1039/c1jm12060c - 23. Constantino, V.R.L. and Pinnavaia, T.J. (1994) Structure-Reactivity Relationships for Basic Catalysts Derived from a Mg2+/A13+/ Layered Double Hydroxide. Catalysis Letters, 23, 361-368.
https://doi.org/10.1007/BF00811370 - 24. Cavani, F., Trifiro, F. and Vaccari, A. (1991) Hy-drotalcite-Type Anionic Clays: Preparation, Properties and Application. Catalysis Today, 11, 173-301.
https://doi.org/10.1016/0920-5861(91)80068-K - 25. Reichle, W.T. (1986) Anionic Clay Minerals. Chemtech, 6, 58-63.
- 26. Velu, S., Ramaswamy, V. and Sivasanker, S. (1997) New Hydrotalcite-Like Anionic Containing Zr4+ in the Layers. Chemical Communications, 21, 2107-2108.
https://doi.org/10.1039/a704752e - 27. Velu, S., Suzuki, K., Osaki, T., Ohashi, F. and Tornura, S. (1999) Synthesis of New Sn Incorporated Layered Double Hydroxides and Their Evolution to Mixed Oxides. Materials Research Bulletin, 34, 1707-1717.
https://doi.org/10.1016/S0025-5408(99)00168-3 - 28. Zerig, H.C., Xu, Z.P. and Qian, M. (1998) Synthesis of Non-at-Containing Hydrotalcite-Tike Compound. Chemistry of Materials, 10, 2277-2283.
https://doi.org/10.1021/cm9802503 - 29. Fernandez, J.M., Barriga, C., Ulibarri, M.A., Labajos, F.M. and Rives, V. (1994) Preparation and Thermal Stability of Manganese-Containing Hydrotalcite. Journal of Materials Chemistry, 4, 1117-1121.
https://doi.org/10.1039/JM9940401117 - 30. 刘俊杰, 李峰, Evans, D.C., 段雪. 由Mg-Fe(IIrFe(III)LDHs层状前体制备MgFe20尖晶石的研究[J]. 化学学报, 2003, 61: 51-57.
- 31. 冯拥军, 李殿卿, 李春喜, 王子镐, Evans, D.G., 段雪. Cu-Ni-Mg-Al-CO3四元LDHs的合成及结构分析[J]. 化学学报, 2003, 61: 78-83.
- 32. Brindley, G.W. and Kikkawa, S. (1980) Thermal Behavior of Hydrotalcite and of Anion-Exchanged Forms of Hydrotalcite. Clays and Clay Minerals, 28, 87-91.
https://doi.org/10.1346/CCMN.1980.0280202 - 33. Zhao, Y., Li, F., Zhang, R., Evans, D. and Duan, X. (2002) Preparation of Layered Double-Hydroxide Nanomaterials with a Uniform Crystallite Size Using a New Method Involving Separate Nucleation and Aging Steps. Chemistry of Materials, 14, 4286-4291.
https://doi.org/10.1021/cm020370h - 34. Miyata, S. (1983) Anion-Exchange Properties of Hydrotalcite-Like Compounds. Clays and Clay Minerals, 31, 305-312.
https://doi.org/10.1346/CCMN.1983.0310409 - 35. Adachi, P., Forano, C. and Besse, J. (1983) Delamination of Layered Double Hydroxides by Use of Surfactants. Chemical Communications, 1, 91-92.
https://doi.org/10.1039/a908251d - 36. Hibino, T. and Jones, W. (2001) New Approach to the Delamination of Layered Double Hydroxides. Journal of Materials Chemistry, 11, 1321-1323.
https://doi.org/10.1039/b101135i - 37. Jobbagy, M. and Regazzoni, A.E. (2004) Delamination and Restaeldng of Hybrid Layered Double Hydroxides Assessed by in Situ XRD. Journal of Colloid and Interface Science, 275, 345-348.
https://doi.org/10.1016/j.jcis.2004.01.082 - 38. Hibino, T. and Kobayashi, M. (2005) Delamination of Layered Double Hydroxides in Water. Journal of Materials Chemistry, 15, 653-658.
https://doi.org/10.1039/b416913a - 39. Liu, Z.P., Ma, R.Z., Osada, M., Lyi, N., Ebina, Y., Takada, K. and Sasaki, T. (2006) Sythesis, Anion Exchange, and Delaminating of Co-AI Layered Double Hydroxide: Assembly of the Exfoli-ated Nanosheet/Polyamine Composite Films and Magneto-Optical Studies. Journal of the American Chemical Society, 128, 4872-4880.
https://doi.org/10.1021/ja0584471 - 40. Ma, R.Z., Ebina, Y., Lyi, N. and Sasaki, T. (2005) Positively Charged Nanosheets Derived via Total Delamination of Layered Donble Hydroxides. Chemistry of Materials, 17, 4386-4391.
https://doi.org/10.1021/cm0510460 - 41. Ma, R., Liu, Z., Li, L., Lyi, N. and Sasaki, T. (2006) Exfoliating Layered Double Hydroxides in Formamide: A Method to Obtain Positively Charged Nanosheets. Journal of Materials Chemistry, 16, 3809-3813.
https://doi.org/10.1039/b605422f - 42. Ma, R., Liu, Z., Takada, K., Lyi, N., Bando, Y. and Sasaki, T. (2007) Synthesis and Exfoliation of Co-Fe Layered Double Hydroxides: An Innovative Topochemical Approach. Journal of the American Chemical Society, 129, 5257-5263.
https://doi.org/10.1021/ja0693035 - 43. Ma, R., Liang, J., Takada, K. and Sasaki, T. (2001) Topochemical Synthesis of Co-Fe Layered Double Hydroxides at Varied Fe/Co Ratios: Unique Intercalation of Triiodide and Its Profound Effect. Journal of the American Chemical Society, 133, 613-620.
https://doi.org/10.1021/ja1087216 - 44. Ma, R., Takada, K., Fukuda, K., Lyi, N., Bando, Y. and Sasaki, T. (2008) Topochemical Synthesis of Monometallic Co2+-Co3+ Layered Double Hydroxide and Its Exfoliation into Positively Charged Co(OH)2 Nanosheets. Angewandte Chemie International Edition, 47, 86-89.
https://doi.org/10.1002/anie.200703941 - 45. Liang, J., Ma, R., lyi, N., Ebina, Y., Takada, K. and Sasaki, T. (2010) Topochemical Synthesis, Anion Exchange, and Exfoliation of Co-Ni Layered Double Hydroxides: A Route to Positively Charged Co-Ni Hydroxide Nanosheets with Tunable Composition. Chemistry of Materials, 22, 371-378.
https://doi.org/10.1021/cm902787u - 46. Wang, Q. and O’Hare, D. (2012) Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chemical Reviews, 112, 4124-4155.
https://doi.org/10.1021/cr200434v - 47. Fan, G., Li, F., Evans, D.G. and Duan, X. (2014) Catalytic Applications of Layered Double Hydroxides: Recent Advances and Perspectives. Chemical Society Reviews, 43, 7040-7066.
https://doi.org/10.1039/C4CS00160E - 48. Hu, G., Wang, N. and O’Hare, D. (2006) One-Step Synthesis and AFM Imagining of Hydrophobic LDH Monolayers. Chemical Communications, 287-289.
- 49. Duan, X., Wang, J. and Lieber, C.M. (2000) Synthesis and Optical Properties of Gallium Arsenide Nanowires. Applied Physics Letters, 76, 1116-1119.
https://doi.org/10.1063/1.125956 - 50. Sanz, N., Baldeck, P. and Ibanez, A. (2000) Organic Nano-crystals Embedded in Sol-Gel Glasses for Optical Applications. Synthetic Metals, 115, 229-234.
https://doi.org/10.1016/S0379-6779(00)00340-4 - 51. 宋崇林, 王军, 沈美庆, 泰永宁. 干燥条件对纳米晶体LaCoO3的结构与合成机理的影响[J]. 应用化学, 1998(15): 65-67.
- 52. Gissibl, B., Wilhelm, D., Würschum, R., Herrig, H., Müller, F., Kelsch, M., Reimann, K., Phillipp, F., Beck, H.P., Hempelmann, R. and Schaefer, H.E. (1997) Electron Microscopy of Nanocrystalline BaTiO3. Nanostructured Materials, 8, 619-622.
https://doi.org/10.1016/S0965-9773(97)00139-6 - 53. 张昭, 彭少方, 刘栋昌. 无机精细化工工艺学[M]. 北京: 化学工业出版社, 2002: 295-301.
- 54. 刘有芹, 颜芸, 沈含熙. 化学修饰电极的研究及其分析应用[J]. 化学研究与应用, 2006(18): 337-343.
- 55. 刘霄. 聚合物修饰电极在生化检测中的应用研究[D]: [硕士学位论文]. 上海: 上海大学.
- 56. 王玉龙. 基于钙钛矿型复合氧化物的电化学传感器研究[D]: [硕士学位论文]. 上海: 上海大学.
- 57. Fogg, A.M., Green, V.M., Harvey, H.G. and O’Hare, D. (1999) New Separation Science Using Shape-Selective Ion Exchange Intercalation Chemistry. Advanced Materials, 11, 1466-1469.
3.0.CO;2-1>https://doi.org/10.1002/(SICI)1521-4095(199912)11:17<1466::AID-ADMA1466>3.0.CO;2-1 - 58. Dong, Y., Sheng, Q.L., Zheng, J.B. and Tang, H.S. (2014) A Nonenzymatic Reduced Glutathione Sensor Based on Ni-Al LDHs/MWCNTs Composites. Analytical Methods, 6, 8598-8603.
https://doi.org/10.1039/C4AY01702A - 59. Dong, X.Y., Wang, L., Wang, D., Li, C. and Jin, J. (2012) Lay-er-by-Layer Engineered Co-Al Hydroxide Nanosheets/Graphene Multilayer Films as Flexible Electrode for Superca-pacitor. Langmuir, 28, 293-298.
https://doi.org/10.1021/la2038685 - 60. Li, M.G., Ji, H.Q., Wang, Y.L., Liu, L. and Gao, F. (2012) MgFe-Layered Double Hydroxide Modified Electrodes for Direct Electron Transfer of Heme Proteins. Biosensors and Bioelectronics, 38, 239-244.
https://doi.org/10.1016/j.bios.2012.05.035 - 61. Basile, F., Benito, P., Fornasari, G., Rosetti, V., Scavetta, E., Tonelli, D. and Vaccari, A. (2009) Electrochemical Synthesis of Novel Structured Catalysts for H-2 Production. Applied Catalysis B, 91, 563-572.
https://doi.org/10.1016/j.apcatb.2009.06.028 - 62. Cui, L., Meng, X.M., Xu, M.R., Shang, K., Ai, S.Y. and Liu, Y.P. (2011) Electro-Oxidation Nitrite Based on Copper Calcined Layered Double Hydroxide and Gold Nanoparticles Modified Glassy Carbon Electrode. Electrochimica Acta, 56, 9769-9774.
https://doi.org/10.1016/j.electacta.2011.08.026 - 63. Zhang, X.Q., Zeng, M.G., Li, S.P. and Li, X.D. (2014) Methotrexate Intercalated Layered Double Hydroxides with Different Particle Sizes: Structural Study and Controlled Release Properties. Colloids and Surfaces B: Biointerfaces, 117, 98-106.
https://doi.org/10.1016/j.colsurfb.2014.02.018 - 64. Zhao, Q., Tian, S.L., Yan, L.X., Zhang, Q.L. and Ning, P. (2015) Novel HCN Sorbents Based on Layered Double Hydroxides: Sorption Mechanism and Performance. Journal of Hazardous Materials, 285, 250-258.
https://doi.org/10.1016/j.jhazmat.2014.11.045 - 65. Li, D.W., Li, Y.T., Song, W. and Long, Y.T. (2010) Simultaneous Determination of Dihydroxybenzene Isomers Using Disposable Screen-Printed Electrode Modified by Multiwalled Carbon Nanotubes and Gold Nanoparticles. Analytical Methods, 2, 837-843.
https://doi.org/10.1039/c0ay00076k - 66. Li, L., Ma, R.Z., Ebina, Y., Fukuda, K., Takada, K. and Sasaki, T. (2007) Layer-by-Layer Assembly and Spontaneous Flocculation of Oppositely Charged Oxide and Hydroxide Nanosheets into Inorganic Sandwich Layered Materials. Journal of the American Chemical Society, 129, 8000-8007.
https://doi.org/10.1021/ja0719172 - 67. Yan, D.P., Lu, J., Wei, M., Han, J.B., Ma, J., Lin, F., Evans, D.G. and Duan, X. (2009) Ordered Poly(p-phenylene)/Layered Double Hydroxide Ultrathin Films with Blue Luminescence by Layer-by-Layer Assembly. Angewandte Chemie International Edition, 48, 3073-3076.
https://doi.org/10.1002/anie.200900178 - 68. Wang, C.L., Shen, M.J., Ding, Y.P., Zhao, D.S., et al. (2017) Facile Preparation of Multilayer Ultrathin Films Based on Eriochrome Black T/NiAl-Layered Double Hydroxide Nanosheet, Characterization and Application in Amperometric Detection of Salicylic Acid. Journal of Electroanalytical Chemistry, 785, 131-137.
https://doi.org/10.1016/j.jelechem.2016.12.008 - 69. Kong, X.G., Shi, W.Y., Zhao, J.W., Wei, M. and Duan, X. (2011) Layer-by-Layer Assembly of Electroactive Dye/Inorganic Matrix Film and Its Application as Sensor for Ascorbic Acid. Talanta, 85, 493-498.
https://doi.org/10.1016/j.talanta.2011.04.020 - 70. Gong, J.M., Han, X.M. and Zhu, X.L. (2014) Layer-by-Layer Assembled Multilayer Films of Exfoliated Layered Double Hydroxide and Carboxymethyl-β-Cyclodextrin for Selective Capacitive Sensing of Acephatemet. Biosensors and Bioelectronics, 61, 379-385.
https://doi.org/10.1016/j.bios.2014.05.044 - 71. Xu, L., Lian, M.L. and Chen, X. (2017) Amperometric Sensing of Hydrogen Peroxide via an ITO Electrode Modified with Gold Nanoparticles Electrodeposited on a CoMn-Layered Double Hydroxide. Microchimica Acta, 184, 3989-3996.
https://doi.org/10.1007/s00604-017-2428-4 - 72. Taei, M., Hasanpour, F., Dinian, M. and Dehghani, E. (2017) Au Nanoparticles Decorated Reduced Graphene Oxide/Layered Double Hydroxide Modified Glassy Carbon as a Sensitive Sensor for Electrocatalytic Determination of Phenazopyridine. Measurement, 99, 90-97.
https://doi.org/10.1016/j.measurement.2016.12.012 - 73. Xu, L., Lin, Y.Q. and Chen, X. (2016) Electrodeposition of Platinum Nanoparticles on MgAl-Layered Double Hydroxide Modified Indium Tin Oxide Electrode for Electrochemical Glucose Biosensor. Chemical Journal of Chinese Universities, 37, 442-447.
- 74. Taei, M., Hasanpour, F. and Dehghani, E. (2015) Electrodepositing of Copper Nanowires on Layered Double Hydroxide Film Modified Glassy Carbonelectrode for the Determination of Doxorubicin. Journal of the Taiwan Institute of Chemical Engineers, 54, 183-190.
- 75. Lu, Y., Jiang, B., Fang, L. and Fan, S.Y. (2017) Highly Sensitive Nonenzymatic Glucose Sensor Based on 3D Ultrathin NiFe Layered Double Hydroxide Nanosheets. Electroanalysis, 29, 1755-1761.
https://doi.org/10.1002/elan.201700025 - 76. Asadpour-Zeynali, K. and Amini, R. (2017) A Novel Voltammetric Sensor for Mercury(II) Based on Mercaptocarboxylic Acid Intercalated Layered Double Hydroxide Nanoparticles Modified Electrode. Sensors and Actuators B: Chemical, 246, 961-968.
- 77. Madrakianr, T., Alizadeh, S., Bahram, M. and Afkhami, A. (2016) A Novel Electrochemical Sensor Based on Magneto LDH/Fe3O4 Nanoparticles @ Glassy Carbon Electrode for Voltammetric Determination of Tramadol in Real Samples. Ionics, 23, 1005-1015.
https://doi.org/10.1007/s11581-016-1871-2 - 78. Wang, Y.L., Wang, Z.C., Rui, Y.P. and Li, M. (2015) Horseradish Peroxidase Immobilization on Carbon Nanodots/CoFe Layered Double Hydroxides: Direct Electrochemistry and Hydrogen Peroxide Sensing. Biosensors and Bioelectronics, 64, 57-62.
https://doi.org/10.1016/j.bios.2014.08.054 - 79. Zhan, T.R., Song, Y. and Tan, Z.W. (2017) Electrochemical Bisphenol A Sensor Based on Exfoliated Ni2Al-Layered Double Hydroxide Nanosheets Modified Electrode. Sensors and Actuators B: Chemical, 238, 962-971.
- 80. Shen, M.J., Zhang, Z. and Ding, Y.P. (2016) Synthesizing NiAl-Layered Double Hydroxide Microspheres with Hierarchical Structure and Electrochemical Detection of Hydroquinone and Catechol. Microchemical Journal, 124, 209-214.
https://doi.org/10.1016/j.microc.2015.08.011 - 81. Xu, Y.H., Liu, X.J., Ding, Y.P. and Luo, L.Q. (2011) Preparation and Electrochemical Investigation of a Nano-Structured Material Ni2+/MgFe Layered Double Hydroxide as a Glucose Biosensor. Applied Clay Science, 52, 322-327.
https://doi.org/10.1016/j.clay.2011.03.011 - 82. Samuei, S., Fakkar, J. and Rezvani, Z. (2017) Synthesis and Characterization of Graphene Quantum Dots/CoNiAl-Layered Double-Hydroxide Nanocomposite: Application as a Glucose Sensor. Analytical Biochemistry, 521, 31-39.
https://doi.org/10.1016/j.ab.2017.01.005 - 83. Rezaei, B., Heidarbeigy, M. and Ensafi, A.A. (2015) Electrochemical Determination of Papaverine on Mg-Al Layered Double Hydroxide/Graphene Oxide and CNT Modified Carbon Paste Electrode. IEEE Sensors Journal, 16, 3496-3503.
https://doi.org/10.1109/JSEN.2016.2533429 - 84. Xu, J., Wang, Q., Wang, X., Xiang, Q., Liang, B., Chen, D., et al. (2013) Flexible Asymmetric Supercapacitors Based upon Co9S8 Nanorod Co3O4@RuO2 Nanosheet Arrays on Carbon Cloth. ACS Nano, 7, 5453-5462.
https://doi.org/10.1021/nn401450s
NOTES
*等同贡献。
#通讯作者。