Pharmacy Information
Vol.06 No.02(2017), Article ID:20577,9
pages
10.12677/PI.2017.62009
Analysis and Countermeasure of the Physical Stability of Nanosuspension
Cui Wang, Fanglian Yi, Libiao Luan*
School of Pharmacy, China Pharmaceutical University, Nanjing Jiangsu

Received: May 1st, 2017; accepted: May 19th, 2017; published: May 22nd, 2017

ABSTRACT
Nanosuspension is an important form of pharmaceutical science; it can solve most problems of drug solubility, improve the bioavailability of drugs, but its physical stability problem has been the bottleneck of application and industrialization. In this paper, the representative research was summarized, analyzed, sorted and summarized to provide and analyze countermeasures for the preparation of physical stability of the nanosuspension.
Keywords:Nanosuspension, Stability, Stabilizer
影响纳米混悬剂的物理稳定性的因素 及应对策略分析
王翠,易方莲,栾立标*
中国药科大学药学院,江苏 南京

收稿日期:2017年5月1日;录用日期:2017年5月19日;发布日期:2017年5月22日

摘 要
纳米混悬剂是药物制剂学中的一种重要剂型,其能够解决多数难溶性药物的溶解度问题,提高药物的生物利用度,但是其物理稳定性问题一直是其应用及产业化的瓶颈。本文总结近年来国内外具有代表性研究,进行分析、整理、归纳,为制备物理稳定的纳米混悬剂提供应对对策并进行分析。
关键词 :纳米混悬剂,稳定性,稳定剂

Copyright © 2017 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
纳米混悬剂(nanosuspensions)是亚微米级粒径大小的药物颗粒依靠表面活性剂的电荷效应或立体效应稳定地混悬在溶液中而形成的一种胶体分散体系。其中药物的平均粒径小于1 μm,一般在200~500 nm之间 [1] 。纳米混悬剂可解决大多数难溶性药物的溶解度问题,提高难溶性药物的生物利用度,产生生物黏附性、提高药物化学稳定性等 [2] ;而其较低的主体变异性,食物消除效应,剂量均衡性,是纳米粒的潜在效应 [3] 。纳米混悬剂的上述优点吸引国内外的药学工作者把更多的精力投入到纳米混悬剂的研究中。然而,在纳米混悬液中,药物容易发生沉降、聚集、降解、变性,这导致了纳米混悬液不稳定。正是因为纳米混悬液的不稳定,使得纳米混悬液很难进行工业化生产,从而最终应用到临床。因此,解决纳米混悬剂的不稳定性是一个尤为迫切的问题,值得科研工作者进行深入研究。本文从纳米混悬剂的物理稳定性进行介绍,并对其理论机制及其应对策略进行综述。
2. 常见物理稳定性问题
纳米混悬剂的性质对不同给药方式的影响尤为重要,例如:对于眼用给药途径来说,粒子大小和形状相互作用共同决定纳米混悬剂的刺激性,有尖锐角和边的粒子相比于等距粒子与钝角的粒子刺激强,其形态学研究对于眼部给药系统来说,是必要考察项目 [4] ;对于肺部给药系统来说,团聚与聚集会影响药物的沉积量和位点,从而影响药效 [5] ;在静脉给药系统中,团聚与聚集可能引起毛细血管栓塞或阻塞血液的流通 [6] ,因此其物理稳定性研究对于肺部给药及静脉给药尤为重要;对于经皮给药系统来说,其粒径大小对于溶出度及透皮吸收速率影响显著,应给予相应关注。
2.1. 形态学改变
在制备纳米混悬剂的过程中,会改变活性药物的本身形态,其原因可能是所选用的稳定剂和药物之间的相互作用有关。
Janne R等人 [7] 研究发现,以卵磷脂为稳定剂制备布地奈德纳米混悬剂的透射电镜扫描(TME)图像表明,其纳米粒的形态近似椭圆形,用泊洛沙姆F68作为稳定剂制备的布地奈德的透射电镜扫描(TME)图像表明,其纳米粒的形态近似长方形(如图1)。
Yancai W等人 [8] 使用不同节枝率的嵌段式聚合物mPEGC作为稳定剂,制备一种新型抗肿瘤药物DM的纳米混悬剂。此混悬剂在原子力显微镜(AFM)下的纳米粒形态为棒状柱体。
2.2. 晶型改变
纳米混悬剂的制备过程中,药物的状态可能会由晶型状态转变为无定型态。无定型态的药物其具有增加溶解度和奥斯瓦尔成熟的特征,由此会导致絮凝和聚集,因此应关注纳米混悬剂储存过程中药物晶型的变化 [9] 。

Figure 1. TEM images of the freshly prepared crystalline nanosuspensions of budesonide stabilized with (a) LEC (asolectin from soybean) and (b) F68 (Pluronic F68) [7]
图1. 卵磷脂为稳定剂的布地奈德纳米混悬剂(a);泊洛沙姆F68作为稳定剂的布地奈德纳米混悬剂(b) [7]
Zakir F等人 [6] 采用乳化超声法,以不同表面活性剂作为稳定剂,制备两性霉素B纳米混悬剂,结果表明所制备的两性霉素B的纳米粒的晶型并没有改变。HasegawaY等人 [10] 采用湿磨法,以泊洛沙姆407 (P407)为稳定剂,制备吡罗昔康(PXC)纳米混悬剂,其结晶度的检测结果表明,吡罗昔康(PXC)纳米混悬剂和冻干纳米制剂中吡罗昔康(PXC)是以无定型状态和晶体状态存在,分析可能是泊洛沙姆407 (P407)的聚环氧乙烷链(PEO)分布在纳米粒的外层,与药物表面发生强烈的吸附。Pireddu R等人 [11] 采用湿磨法,以泊洛沙姆188 (Poloxamer 188)为稳定剂,制备两种不同晶型的双氯芬酸纳米混悬剂。应用差示扫描(DSC)曲线分析原料药及其纳米混悬剂表明,Poloxamer 188尖锐的吸热峰在50℃,双氯芬酸晶型1 (HD1)尖锐的吸热峰在176.9℃,双氯芬酸晶型2 (HD2)尖锐的吸热峰在176.93℃,在相应的位置,制备的两种不同晶型的双氯芬酸纳米混悬剂是没有尖锐的吸热峰,说明晶型已经变为无定型状态(如图2)。
2.3. 聚集
纳米混悬剂是一种热力学不稳定体系,粒径很小,比表面积大,产生很高的表面能,纳米粒子间具有相互聚集的趋势以降低其整个体系表面能,因而容易发生相互聚集的现象 [12] 。聚集可引起药物微粒的快速沉降、晶体生长等现象,从而造成药物剂量分布不均匀、静脉注射时堵塞毛细血管等问题。纳米混悬剂聚集的机理是由于奥斯瓦尔成熟,小的粒子比大的粒子拥有更高的饱和溶解度,由此制造了大粒子
和小粒子浓度差。大粒子周围存在饱和溶液,使其不断析出,在小粒子周围存在不饱和溶液,使其不断溶解,当使用不合适药物的稳定剂制备纳米混悬剂,会导致小粒子溶解,较大的粒子增大,发生聚集现象 [13] 。
纳米粒的电位是评价纳米混悬液分散性和聚集稳定性的重要参数指标。有研究 [4] 表明,绝对值小于30 mV的胶体体系是稳定的物理体系。当使用空间位阻稳定剂时,电位可以作为表面吸附率的指标。因此,通过药物表面覆盖率降低绝对电位值,是一种物理稳定的状态表现。
2.4. 沉降
因分散介质与微粒的密度不同,微粒在重力的作用下发生的定向运动而使分散体系发生相分离的过程称为沉降 [14] 。可以通过如下三种方式减缓混悬剂沉降速度:1) 减小纳米粒的粒径;2) 降低固体微粒与分散介质间的密度差;3) 增大分散介质的黏度 [12] 。第一种方法可以通过制备出细小粒径的微粒使其布朗运动作用超过重力作用而实现混悬液稳定。后两种方法主要通过向混悬剂中加入适当的稳定剂加以实现。
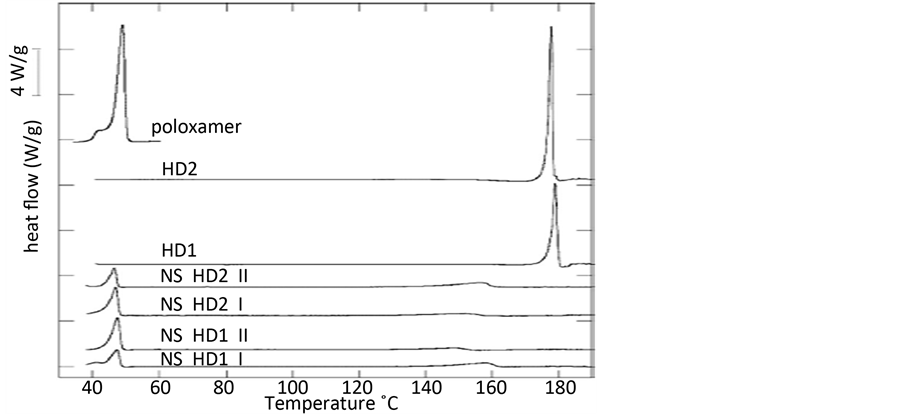
Figure 2. DSC thermograms of poloxamer, bulk HD2 and HD1 and nanosuspensions (NS HD2 II, NS HD2 I, NS HD1 II, NS HD1 I) [11]
图2. 差示扫描法(DSC)曲线分析:辅料(poloxamer188);原料药(HD2, HD1);纳米混悬剂I (NS HD2 II, NS HD2 I, NS HD1 II, NS HD1) [11]
3. 纳米混悬剂物理稳定性的应对策略分析
3.1. 新型纳米混悬剂的稳定剂的探究
在纳米混悬剂制备和储存过程中为避免颗粒聚集和沉降,稳定剂是必不可少的。稳定剂分为传统稳定剂和新型稳定剂。传统稳定剂包括离子型稳定剂和非离子型稳定剂。离子型稳定剂的静电斥力稳定机理可用经典的Derjaguin-Landau-Verwey-Overbeek (DLVO)理论解释,是指胶体在溶剂中既有静电斥力又存在相互吸引。其静电斥力源于双电层的重叠(EDL),从而防止胶体凝聚。双电层的作用分别是:1) 第一层吸引在粒子表面,具有中和体系电子的作用,2) 另一层是离子的扩散层,提供静电斥力 [12] 。常用的离子型表面活性剂包括十二烷基硫酸钠(SDS)、月桂硫酸钠(SLS) [15] ,卵磷脂(lecithin) [7] 。
非离子稳定剂的空间位阻稳定机理可以根据吉布斯自由能表示:ΔG = ΔH − TΔS。+ΔG表示稳定悬浮,而−ΔG表示粒子聚集。如果介质是一种维持稳定剂效应的溶剂,粒子碰撞时吸附在粒子上的稳定层
不能互相渗透,减少稳定剂的稳定层相互作用,产生+ΔG。另一方面,如果分散介质是一种破坏稳定剂效应的溶剂,吸附在粒子上的稳定层可能产生渗透动力并诱发粒子聚集(如图3) [12] 常用非离子表面活性剂包括泊洛沙姆® (Pluronic®) [16] 、吐温-80 [6] 、吐温-60 [15] 、聚乙烯醇(PVA) [17] 、聚乙二醇(PEG)、聚乙稀吡咯烷酮(PVP) [18] 、羟丙甲基纤维素(HPMC) [19] 。有研究表明在任何稳定剂存在的情况下,都可以制备药物的纳米混悬剂,但是PVA作为物理稳定剂时,仅起到维持理想黏度,减少纳米微粒沉降的作用 [5] 。当前研究 [20] 表明,开发和寻找新型稳定剂是解决纳米混悬剂物理稳定性最佳的方法。(及对于纳米混悬剂技术是很重要的一方面)。例如开发两亲性表面活性剂作为稳定剂,可以有效提高纳米混悬剂的稳定性,其作用机制主要是通过减小粒子的表面能,形成能量屏障,阻止粒子聚集与结晶生长 [21] 。
Weisan P等人 [22] 采用沉淀–超声法,制备卡维地洛纳米混悬剂,并检测其溶解度和生物利用度,实验证明维生素e琥珀酸酯(VES)是一种有效的联合稳定剂,可以进一步研究维生素e琥珀酸酯(VES)这种新型稳定剂是否可以用于制备其他药物的纳米混悬剂。Tang Xing等人 [3] 通过研究一种用于制备固体分散体的嵌段式聚合物soluplus®,探究其是否可以作为稳定剂用于制备非诺贝特(FBT)纳米混悬剂产品。研究结果表明:soluplus®可以有效提高纳米混悬剂的稳定性,其原因可能是Soluplus®内包药物FBT,使
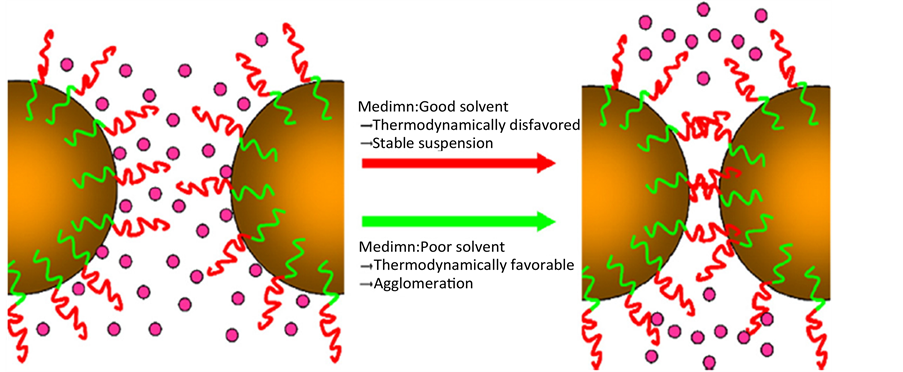
Figure 3. Steric stabilization mechanisms [12]
图3. 空间位阻稳定机理 [12]
FBT不直接暴露在水中,形成凝胶作用而缓慢释放,弱化了奥斯瓦尔效应。本实验证明:soluplus®可用于制备稳定性良好的纳米混悬剂,并能提高溶解度和生物利用度。羟丙基-β-环糊精(HP-β-CD)是一种高水溶性的表面活性剂,成本低廉,毒性小,可用于增加水难溶性物质的溶解度。Yan Xie等人 [23] 的实验证明羟丙基-β-环糊精(HP-β-CD)可制备出粒径大小为380 nm和电位为−28 mV的杨酶酮纳米混悬剂,其具有良好的稳定性。羟丙基-βb-环糊精(HP-β-CD)可以减少介质和药物之间的表面张力,开发其作为纳米混悬剂的稳定剂的新用途,增加制剂的多样性是很有价值的。由于因常见的稳定剂较弱的稳定作用和长期治疗的毒副作用的限制,Wua Wei等人 [24] 以吲哚美辛药物为模型,探究食物蛋白质(大豆蛋白、血清分离蛋白、乳球蛋白)作为纳米混悬剂的新型稳定剂的可能性。由于热变性和随后吸附在药物粒子表面的原因,包埋的疏水分子暴露有助于稳定药物纳米混悬剂。分析蛋白质稳定纳米混悬剂的机理包括静电斥力和空间位阻。但是,值得注意的是当pH值高于或低于等电点,两亲性食物的蛋白质可以作为稳定剂。而pH值在等电点时,蛋白质的电能为0,其纳米稳定作用受限制,此时导致粒子聚集。
当前研究 [20] 表明,开发和寻找新型稳定剂是解决纳米混悬剂物理稳定性最常用的方法,但稳定剂的选择是一个艰辛且很复杂的过程,需要药学工作者潜下心来深入研究。
3.2. 候选药物的本身性质对于制备纳米混悬剂的影响
Maya G等人 [19] 通过研究六种活性药物(naproxen, A, B, C, D and E)的物理性质和四种稳定剂(SLS, DOSS, TPGS and Pluronic F127)之间的效应,分析药物的性质,纵观药物和稳定剂之间相互作用以给制备稳定纳米混悬剂产品处方提供关键参数。研究表明,制备有效的纳米混悬剂产品,药物本身的油水分配系数(logP)、热函与制备稳定的纳米混悬剂产品处方的可行性直接相关。结果如图4所示:对于球磨法制备的候选活性药物,不论对于空间位阻稳定剂还是静电斥力稳定剂,可以看出logP和成功制备药物纳米混悬剂直接相关,若其有较高的热函、油水分配系数,既可以应用静电斥力也可以应用空间位阻稳定剂制备纳米混悬剂;当其具有较低的热函、油水分配系数,则不能成为制备稳定的纳米混悬剂产品的候选药物。
另外,静电斥力稳定剂是粒子表面静电团决定的,其由药物的官能团和离解度决定。此时球磨介质的PH和药物的pKa对于制备过程中的稳定有重大影响。
3.3. 非离子型聚合物稳定剂间氢键作用调控粒径大小
Yanping B等人 [25] 研究,采用沉淀法,制备无定形的白藜芦醇纳米混悬剂,研究应用PVA/PVP这一对高聚物制备粒径可控的固体纳米粒。研究分析,选择吐温和PVA作为纳米混悬剂的稳定剂时,此时非离子聚合物PVP是以一种非奥斯瓦尔成熟的机理控制纳米混悬剂的粒径大小。分析原因可能是PVA聚合度及流动性高于PVP,这样PVA具有更强大的结合白藜芦醇的趋势。因此,当PVP加入含有吐温和PVA的纳米混悬剂中,其与PVA相互作用嫁接成氢键桥,而非是与PVA竞争药物离子表面,其作用机理可以归结为氢键诱导的聚集和结块(图5、图6)。
3.4. 一种钠盐对稳定剂的影响
有研究 [26] [27] 表明:在霍夫梅斯尔的一列阴离子对聚合物的溶解度和对固体表面的吸附有重要影响。而聚合物的吸附作为自由能的补偿,可以减少熵损失 [28] 。有研究 [29] 指出固体表面聚合物的吸附,通过如空间位阻效应似的较弱的相互作用,是纳米混悬剂稳定的关键。Hassa A等人 [17] 的研究指出:以PVP作为磺胺甲嘧啶纳米混悬剂的稳定剂,利用钠盐(碘化钠、氯化钠、硫酸钠)的阴离子可以增加聚合物PVP对于药物粒子的吸附,以增加纳米粒的稳定性,减小粒径。机理可能是按霍夫梅斯尔的离子理论,其阴离子硫酸根离子可以破坏水中的氢键结构,降低药物离子的水合作用,增加PVP和粒子表面的吸附,硫酸离子在相对高的浓度,使得更多的PVP吸附于离子表面,因此增强纳米粒的稳定效应 [30] 。
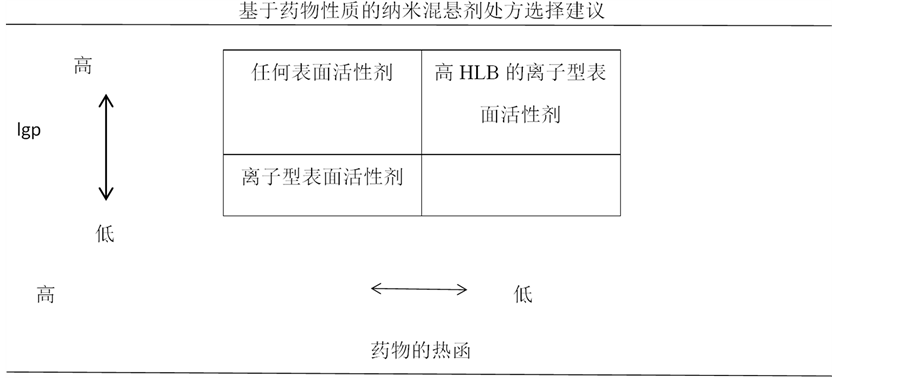
Figure 4. Proposed generic formulation of nanosuspension based on drug properties [19]
图4. 基于药物性质制备纳米混悬剂处方的建议 [19]
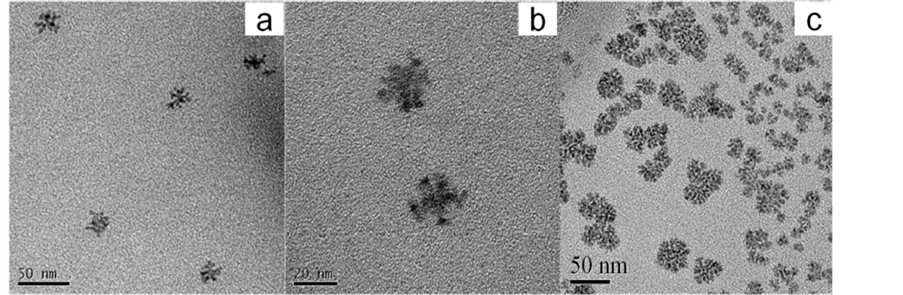
Figure 5. TEM micrographs of Resveratrol nanosuspension containing Tween and PVA (a), Resveratrol nanosus- pension containing Tween and PVP (b), Resveratrol nanosuspension containing Tween, PVP and PVA (c) [25]
图5. 透射电镜扫描图:白藜芦醇/吐温-80/PVA纳米混悬剂(a);白藜芦醇/吐温-80/PVA纳米混悬剂(b);白藜芦醇/吐温-80/PVA/PVP纳米混悬剂(c) [25]
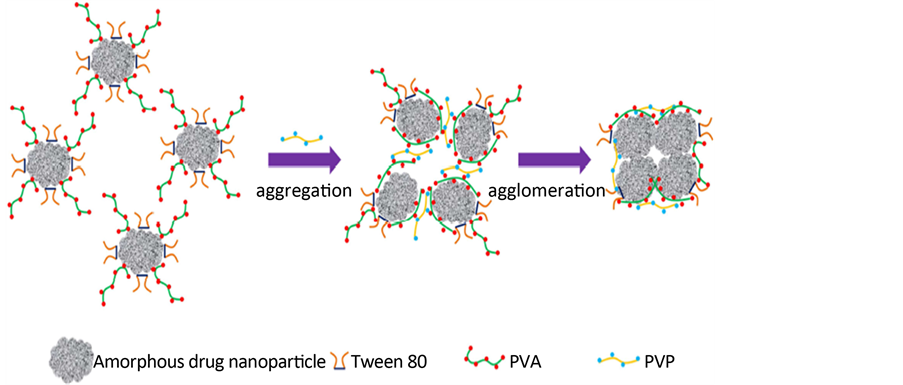
Figure 6. A plausible scheme of nanoparticles aggregation induced by PVA-PVP Interaction [25]
图6. PVA-PVP相互作用诱导的纳米粒聚集的机理模拟 [25]
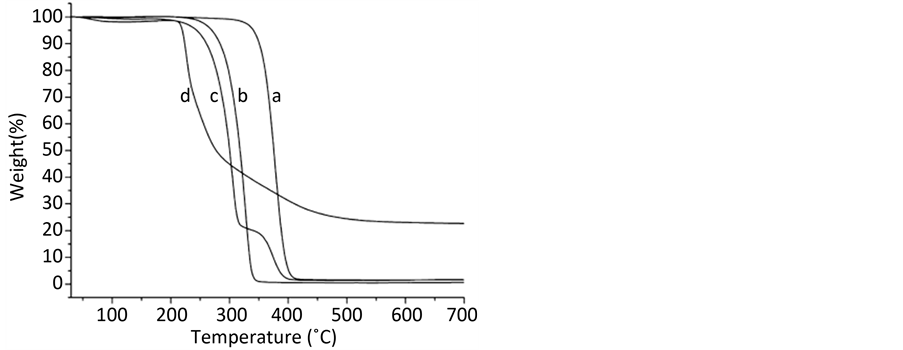
Figure 7. TGA results of (a) poloxamer 188, (b) mannitol, (c) PTXNS and (d) raw PTX [16]
图7. 热重量分析图P188 (a),甘露醇(b),PTXNS (c)、PTX (d) [16]
3.5. 表征方式对稳定剂稳定纳米混悬剂的研究
研究纳米混悬剂的不同表征方式,推动纳米混悬剂的质量评价,从而建立适宜的开发手段。在分子水平上研究纳米混悬剂,可以帮助我们了解各种纳米粒的结构,为新的纳米混悬剂处方设计提供关键信息。Moribe K等人 [10] 采用湿磨法,以泊洛沙姆407 (P407)为稳定剂,制备吡罗昔康(PXC)纳米混悬剂,并通过先进的光谱技术评估药物与稳定剂的分子状态。应用核磁共振仪(NMR)检测溶液,探究纳米粒的层结构的构成。结合核磁共振(NMR)谱与拉曼(Raman)光谱研究混悬态的纳米粒,揭示PXC和P407的分子状态,阐明纳米混悬剂的结构。Yong L等人 [16] 结合高速分散和高压均质法,采用乳化法以泊洛沙姆188 (P188)和甘露醇制备紫杉醇纳米混悬液(PTXNS),研究PTX和PTXNS热性能,以热重分析法(TGA)为检测手段,分析紫杉醇纳米混悬剂的性质及紫杉醇纳米混悬剂中紫杉醇的含量。紫杉醇原料药在217℃~276℃温度区间内,快速失重,紫杉醇纳米混悬剂中紫杉醇的快速失重是220到320℃(如图7),这一结果说明,含有泊洛沙姆188和甘露醇的PTXNS展现了泊洛沙姆188和甘露醇的性质。也说明了PTXNS中的PTX因具有粒径较小,因此有更大的表面能,这使得PTX更容易失重挥发并且提前降解。PTXNS在323到407℃快速降解,是因为大多数的PTX在PTXNS中开始降解。结合TGA的结果进一步说明PTXNS中泊洛沙姆188的含量非常少,PTX在PTXNS中的含量仅有3.42%。
4. 结论与展望
纳米混悬剂能够解决多数难溶性药物的溶解度问题,提高药物的生物利用度,因此纳米混悬制剂技术将是研究的一个热点,但是其物理稳定性问题一直是其应用及产业化的瓶颈。因此亟需开展该剂型物理稳定性的基础研究,改进制备方法,开发新的稳定剂。本文总结影响纳米混悬剂物理稳定性的问题,在此基础上提出相应的应对策略,为制备稳定性好、生物利用度高的纳米混悬剂的研究提供参考。
文章引用
王 翠,易方莲,栾立标. 影响纳米混悬剂的物理稳定性的因素及应对策略分析
Analysis and Countermeasure of the Physical Stability of Nanosuspension[J]. 药物资讯, 2017, 06(02): 46-54. http://dx.doi.org/10.12677/PI.2017.62009
参考文献 (References)
- 1. Keck, C.M. and Müller, R.H. (2006) Drug Nanocrystals of Poorly Soluble Drugs Produced by High Pressure Homogenisation. European Journal of Pharmaceutics & Biopharmaceutics, 62, 3-16.
- 2. Möschwitzer, J., Achleitner, G., Pomper, H., et al. (2004) Development of an Intravenously Injectable Chemically Stable Aqueous Omeprazole Formulation Using Nanosuspension Technology. European Journal of Pharmaceutics & Biopharmaceutics, 58, 615-619.
- 3. Yang, H., Teng, F., Wang, P., et al. (2014) Investigation of a Nanosuspension Stabilized by Soluplus® to Improve Bioavailability. International Journal of Pharmaceutics, 477, 88-95.
- 4. Ahuja, M., Dhake, A.S., Sharma, S.K., et al. (2011) Diclofenac-Loaded Eudragit S100 Nanosuspension for Ophthalmic Delivery. Journal of Microencapsulation, 28, 37-45. https://doi.org/10.3109/02652048.2010.523794
- 5. Jacobs, C. and Müller, R.H. (2002) Production and Characterization of a Budesonide Nanosuspension for Pulmonary Administration. Pharmaceutical Research, 19, 189-194. https://doi.org/10.1023/A:1014276917363
- 6. Zakir, F., Sharma, H., Kaur, K., et al. (2011) Nanocrystallization of Poorly Water Soluble Drugs for Parenteral Administration. Journal of Biomedical Nanotechnology, 7, 127-129. https://doi.org/10.1166/jbn.2011.1234
- 7. Raula, J., Rahikkala, A., Halkola, T., et al. (2013) Coated Particle Assemblies for the Concomitant Pulmonary Administration of Budesonide and Salbutamol Sulphate. International Journal of Pharmaceutics, 441, 248-254.
- 8. Du, J., Zhou, Y., Wang, L., et al. (2016) Effect of PEGylated Chitosan as Multifunctional Stabilizer for Deacetyl Mycoepoxydience Nanosuspension Design and Stability Evaluation. Carbohydrate Polymers, 153, 471-481.
- 9. Sharma, P., Zujovic, Z.D., Bowmaker, G.A., et al. (2011) Evaluation of a Crystalline Nanosuspension: Polymorphism, Process Induced Transformation and in Vivo Studies. International Journal of Pharmaceutics, 408, 138-151.
- 10. Hasegawa, Y., Higashi, K., Yamamoto, K., et al. (2015) Direct Evaluation of Molecular States of Piroxicam/Polox- amer Nanosuspension by Suspended-State NMR and Raman Spectroscopies. Molecular Pharmaceutics, 12, 1564- 1572. https://doi.org/10.1021/mp500872g
- 11. Pireddu, R., Sinico, C., Ennas, G., et al. (2015) Novel Nanosized Formulations of Two Diclofenac Acid Polymorphs to Improve Topical Bioavailability. European Journal of Pharmaceutical Sciences, 77, 208-215.
- 12. Wu, L., Zhang, J. and Watanabe, W. (2011) Physical and Chemical Stability of Drug Nanoparticles. Advanced Drug Delivery Reviews, 63, 456-469.
- 13. Ali, H.S., York, P. and Blagden, N. (2009) Preparation of Hydrocortisone Nanosuspension through a Bottom-Up Nanoprecipitation Technique Using Microfluidic Reactors. International Journal of Pharmaceutics, 375, 107-113.
- 14. 谢向阳, 陈晨, 廖祥茹, 韩亮. 纳米混悬剂的物理稳定性研究进展[J]. 国际药学研究杂志, 2011, 1(5): 369-374.
- 15. Taneja, S., Shilpi, S. and Khatri, K. (2016) Formulation and Optimization of Efavirenz Nanosuspensions Using the Precipitation-Ultrasonication Technique for Solubility Enhancement. Artificial Cells, Nanomedicine, and Biotechnology, 44, 978-984.
- 16. Yong, L., Zhao, X., Zu, Y., et al. (2015) Preparation and Characterization of Paclitaxel Nanosuspension Using Novel Emulsification Method by Combining High Speed Homogenizer and High Pressure Homogenization. International Journal of Pharmaceutics, 490, 324-333.
- 17. Lou, H., Liu, M., Qu, W., et al. (2013) The Influence of Sodium Salts (Iodide, Chloride And Sulfate) on the Formation Efficiency of Sulfamerazine Nanocrystals. Pharmaceutical Development & Technology, 19, 548-555. https://doi.org/10.3109/10837450.2013.805777
- 18. Mishra, B., Sahoo, J. and Dixit, P.K. (2016) Enhanced Bioavailability of Cinnarizine Nanosuspensions by Particle Size Engineering: Optimization and Physicochemical Investigations. Materials Science & Engineering C: Materials for Biological Applications, 63, 62-69.
- 19. George, M. and Ghosh, I. (2012) Identifying the Correlation Between Drug/Stabilizer Properties and Critical Quality Attributes (CQAs) of Nanosuspension Formulation Prepared by wet Media Milling Technology. European Journal of Pharmaceutical Sciences, 48, 142-152.
- 20. Ezhilarasi, P.N., Karthik, P., Chhanwal, N., et al. (2013) Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food and Bioprocess Technology, 6, 628-647. https://doi.org/10.1007/s11947-012-0944-0
- 21. Meriskoliversidge, E. and Liversidge, G.G. (2011) Nanosizing for Oral and Parenteral Drug Delivery: A Perspective on Formulating Poorly-Water Soluble Compounds Using Wet Media Milling Technology. Advanced Drug Delivery Reviews, 63, 427-440.
- 22. Liu, D., Xu, H., Tian, B., et al. (2012) Fabrication of Carvedilol Nanosuspensions through the Anti-Solvent Precipitation-Ultrasonication Method for the Improvement of Dissolution Rate and Oral Bioavailability. AAPS PharmSciTech, 13, 295-304. https://doi.org/10.1208/s12249-011-9750-7
- 23. Hong, C., Dang, Y., Lin, G., et al. (2014) Effects of Stabilizing Agents on the Development of Myricetin Nanosuspension and Its Characterization: An in Vitro and in Vivo Evaluation. International Journal of Pharmaceutics, 477, 251- 260.
- 24. He, W., Lu, Y., Qi, J., et al. (2013) Food Proteins as Novel Nanosuspension Stabilizers for Poorly Water-Soluble Drugs. International Journal of Pharmaceutics, 441, 269-278.
- 25. Bi, Y., Liu, J., Wang, J., et al. (2015) Particle Size Control and the Interactions between Drug and Stabilizers in an Amorphous Nanosuspension System. Journal of Drug Delivery Science & Technology, 29, 167-172.
- 26. Zhang, Y., Furyk, S., Bergbreiter, D.E., et al. (2005) Specific Ion Effects on the Water Solubility of Macromolecules: PNIPAM and the Hofmeister Series. Journal of the American Chemical Society, 127, 14505-14510. https://doi.org/10.1021/ja0546424
- 27. Van, E.B., Froyen, L., Van, H.J., et al. (2008) Drying of Crystalline Drug Nanosuspensions—The Importance of Surface Hydrophobicity on Dissolution Behavior upon Redispersion. European Journal of Pharmaceutical Sciences, 35, 127-135.
- 28. Zhang, Y. and Cremer, P.S. (2006) Interactions between Macromolecules and Ions: The Hofmeister Series. Current Opinion in Chemical Biology, 10, 658-663.
- 29. Lee, J., Lee, S.J., Choi, J.Y., et al. (2005) Amphiphilic Amino Acid Copolymers as Stabilizers for the Preparation of Nanocrystal Dispersion. European Journal of Pharmaceutical Sciences, 24, 441-449.
- 30. Ploehn, H.J. and Russel, W.B. (1990) Interactions between Colloidal Particles and Soluble Polymers. Advances in Chemical Engineering, 15, 137-228.
- 31. Keck, C.M. and Müller, R.H. (2006) Drug Nanocrystals of Poorly Soluble Drugs Produced by High Pressure Homogenisation. European Journal of Pharmaceutics & Biopharmaceutics, 62, 3-16.
- 32. Möschwitzer, J., Achleitner, G., Pomper, H., et al. (2004) Development of an Intravenously Injectable Chemically Stable Aqueous Omeprazole Formulation Using Nanosuspension Technology. European Journal of Pharmaceutics & Biopharmaceutics, 58, 615-619.
- 33. Yang, H., Teng, F., Wang, P., et al. (2014) Investigation of a Nanosuspension Stabilized by Soluplus® to Improve Bioavailability. International Journal of Pharmaceutics, 477, 88-95.
- 34. Ahuja, M., Dhake, A.S., Sharma, S.K., et al. (2011) Diclofenac-Loaded Eudragit S100 Nanosuspension for Ophthalmic Delivery. Journal of Microencapsulation, 28, 37-45. https://doi.org/10.3109/02652048.2010.523794
- 35. Jacobs, C. and Müller, R.H. (2002) Production and Characterization of a Budesonide Nanosuspension for Pulmonary Administration. Pharmaceutical Research, 19, 189-194. https://doi.org/10.1023/A:1014276917363
- 36. Zakir, F., Sharma, H., Kaur, K., et al. (2011) Nanocrystallization of Poorly Water Soluble Drugs for Parenteral Administration. Journal of Biomedical Nanotechnology, 7, 127-129. https://doi.org/10.1166/jbn.2011.1234
- 37. Raula, J., Rahikkala, A., Halkola, T., et al. (2013) Coated Particle Assemblies for the Concomitant Pulmonary Administration of Budesonide and Salbutamol Sulphate. International Journal of Pharmaceutics, 441, 248-254.
- 38. Du, J., Zhou, Y., Wang, L., et al. (2016) Effect of PEGylated Chitosan as Multifunctional Stabilizer for Deacetyl Mycoepoxydience Nanosuspension Design and Stability Evaluation. Carbohydrate Polymers, 153, 471-481.
- 39. Sharma, P., Zujovic, Z.D., Bowmaker, G.A., et al. (2011) Evaluation of a Crystalline Nanosuspension: Polymorphism, Process Induced Transformation and in Vivo Studies. International Journal of Pharmaceutics, 408, 138-151.
- 40. Hasegawa, Y., Higashi, K., Yamamoto, K., et al. (2015) Direct Evaluation of Molecular States of Piroxicam/Polox- amer Nanosuspension by Suspended-State NMR and Raman Spectroscopies. Molecular Pharmaceutics, 12, 1564- 1572. https://doi.org/10.1021/mp500872g
- 41. Pireddu, R., Sinico, C., Ennas, G., et al. (2015) Novel Nanosized Formulations of Two Diclofenac Acid Polymorphs to Improve Topical Bioavailability. European Journal of Pharmaceutical Sciences, 77, 208-215.
- 42. Wu, L., Zhang, J. and Watanabe, W. (2011) Physical and Chemical Stability of Drug Nanoparticles. Advanced Drug Delivery Reviews, 63, 456-469.
- 43. Ali, H.S., York, P. and Blagden, N. (2009) Preparation of Hydrocortisone Nanosuspension through a Bottom-Up Nanoprecipitation Technique Using Microfluidic Reactors. International Journal of Pharmaceutics, 375, 107-113.
- 44. 谢向阳, 陈晨, 廖祥茹, 韩亮. 纳米混悬剂的物理稳定性研究进展[J]. 国际药学研究杂志, 2011, 1(5): 369-374.
- 45. Taneja, S., Shilpi, S. and Khatri, K. (2016) Formulation and Optimization of Efavirenz Nanosuspensions Using the Precipitation-Ultrasonication Technique for Solubility Enhancement. Artificial Cells, Nanomedicine, and Biotechnology, 44, 978-984.
- 46. Yong, L., Zhao, X., Zu, Y., et al. (2015) Preparation and Characterization of Paclitaxel Nanosuspension Using Novel Emulsification Method by Combining High Speed Homogenizer and High Pressure Homogenization. International Journal of Pharmaceutics, 490, 324-333.
- 47. Lou, H., Liu, M., Qu, W., et al. (2013) The Influence of Sodium Salts (Iodide, Chloride And Sulfate) on the Formation Efficiency of Sulfamerazine Nanocrystals. Pharmaceutical Development & Technology, 19, 548-555. https://doi.org/10.3109/10837450.2013.805777
- 48. Mishra, B., Sahoo, J. and Dixit, P.K. (2016) Enhanced Bioavailability of Cinnarizine Nanosuspensions by Particle Size Engineering: Optimization and Physicochemical Investigations. Materials Science & Engineering C: Materials for Biological Applications, 63, 62-69.
- 49. George, M. and Ghosh, I. (2012) Identifying the Correlation Between Drug/Stabilizer Properties and Critical Quality Attributes (CQAs) of Nanosuspension Formulation Prepared by wet Media Milling Technology. European Journal of Pharmaceutical Sciences, 48, 142-152.
- 50. Ezhilarasi, P.N., Karthik, P., Chhanwal, N., et al. (2013) Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food and Bioprocess Technology, 6, 628-647. https://doi.org/10.1007/s11947-012-0944-0
- 51. Meriskoliversidge, E. and Liversidge, G.G. (2011) Nanosizing for Oral and Parenteral Drug Delivery: A Perspective on Formulating Poorly-Water Soluble Compounds Using Wet Media Milling Technology. Advanced Drug Delivery Reviews, 63, 427-440.
- 52. Liu, D., Xu, H., Tian, B., et al. (2012) Fabrication of Carvedilol Nanosuspensions through the Anti-Solvent Precipitation-Ultrasonication Method for the Improvement of Dissolution Rate and Oral Bioavailability. AAPS PharmSciTech, 13, 295-304. https://doi.org/10.1208/s12249-011-9750-7
- 53. Hong, C., Dang, Y., Lin, G., et al. (2014) Effects of Stabilizing Agents on the Development of Myricetin Nanosuspension and Its Characterization: An in Vitro and in Vivo Evaluation. International Journal of Pharmaceutics, 477, 251- 260.
- 54. He, W., Lu, Y., Qi, J., et al. (2013) Food Proteins as Novel Nanosuspension Stabilizers for Poorly Water-Soluble Drugs. International Journal of Pharmaceutics, 441, 269-278.
- 55. Bi, Y., Liu, J., Wang, J., et al. (2015) Particle Size Control and the Interactions between Drug and Stabilizers in an Amorphous Nanosuspension System. Journal of Drug Delivery Science & Technology, 29, 167-172.
- 56. Zhang, Y., Furyk, S., Bergbreiter, D.E., et al. (2005) Specific Ion Effects on the Water Solubility of Macromolecules: PNIPAM and the Hofmeister Series. Journal of the American Chemical Society, 127, 14505-14510. https://doi.org/10.1021/ja0546424
- 57. Van, E.B., Froyen, L., Van, H.J., et al. (2008) Drying of Crystalline Drug Nanosuspensions—The Importance of Surface Hydrophobicity on Dissolution Behavior upon Redispersion. European Journal of Pharmaceutical Sciences, 35, 127-135.
- 58. Zhang, Y. and Cremer, P.S. (2006) Interactions between Macromolecules and Ions: The Hofmeister Series. Current Opinion in Chemical Biology, 10, 658-663.
- 59. Lee, J., Lee, S.J., Choi, J.Y., et al. (2005) Amphiphilic Amino Acid Copolymers as Stabilizers for the Preparation of Nanocrystal Dispersion. European Journal of Pharmaceutical Sciences, 24, 441-449.
- 60. Ploehn, H.J. and Russel, W.B. (1990) Interactions between Colloidal Particles and Soluble Polymers. Advances in Chemical Engineering, 15, 137-228.