Advances in Clinical Medicine
Vol.
10
No.
11
(
2020
), Article ID:
38945
,
7
pages
10.12677/ACM.2020.1011426
81例Ph染色体阳性急性淋巴细胞 白血病患者的遗传学特征 及疗效分析
孔琦,赵春亭*,崔渤莉,王彩曌,闫思琪,费海荣,李田兰,刘珊珊
青岛大学附属医院血液内科,山东,青岛
收稿日期:2020年11月2日;录用日期:2020年11月23日;发布日期:2020年11月30日

摘要
目的:研究Ph染色体阳性急性淋巴细胞白血病(Ph+ALL)患者的遗传学特征,分析影响患者疾病缓解和复发以及患者生存状况的因素。方法:收集2002年1月至2020年5月期间于青大附院初诊的Ph+ALL患者共81例,收集患者的一般资料、遗传学相关检查、治疗方案等,分析不同遗传学特点和不同治疗方案患者的病情转归及生存状况。结果:81例患者中有46例(56.79%)患者为P210基因阳性,有34例(41.98%)患者为P190基因阳性,1例(1.23%)患者同时存在P190和P210基因阳性。在随访期内发生ABL激酶突变的患者有15例(18.25%),P210融合基因组的突变率为10.87%,P190融合基因组的突变率为29.41%,二者有显著性统计学差异(P = 0.036)。有42例患者在第一次诱导缓解化疗中加用酪氨酸激酶抑制剂(TKIs),该组患者的缓解率为69.05%,有39例患者在第一次诱导缓解治疗中单用传统化疗方案,缓解率为43.59%,二者有显著性统计学差异(P = 0.021)。随访期内获得完全缓解的患者有69例,其中伊马替尼治疗组的复发率为61.70%,达沙替尼治疗组的复发率为31.82%,二者有显著性统计学差异(P = 0.021)。根据患者的治疗方案进行分组,比较各组患者的生存曲线,其中伊马替尼治疗组和达沙替尼治疗组(P = 0.003)、随访期内行造血干细胞移植(HSCT)组和未移植组(P = 0.010)的生存曲线不同。结论:在Ph+ALL患者中,P210比P190更多见。BCR/ABL融合基因为P190可能为ABL激酶突变的危险因素。第一次诱导缓解化疗即加用TKIs的患者比单用传统化疗方案治疗的患者有更高的缓解率。伊马替尼治疗组的复发率高于达沙替尼治疗组的复发率。选用达沙替尼治疗、完全缓解后行HSCT治疗可以改善患者的生存状况。
关键词
急性淋巴细胞白血病,Ph染色体,BCR/ABL,P190,P210,ABL激酶突变,伊马替尼,达沙替尼,造血干细胞移植

Genetic Characteristics and Curative Effect Analysis of 81 Patients with Ph Chromosome Positive Acute Lymphoblastic Leukemia
Qi Kong, Chunting Zhao*, Boli Cui, Caizhao Wang, Siqi Yan, Hairong Fei, Tianlan Li, Shanshan Liu
Department of Hematology, Affiliated Hospital of Qingdao University, Qingdao Shandong

Received: Nov. 2nd, 2020; accepted: Nov. 23rd, 2020; published: Nov. 30th, 2020

ABSTRACT
Objective: To study the genetic characteristics of patients with Phchromosome positive acute lymphoblastic leukemia (Ph+ALL) and to analyze factors that influence remission and recurrence of the disease and survival of the patients. Methods: There are 81 patients who were initial diagnosed with Ph+ALL in their first visiting the Affiliated Hospital of Qingda University from January 2002 to May 2020. We collected their general information, genetic tests and treatment plans and analyzed the prognosis and survival status of patients with different genetic characteristics and treatment plans. Results: Among the 81 patients, 46 (56.79%) cases have P210 gene, 34 (41.98%) cases have P190 gene, and 1 (1.23%) case has both P190 and P210 gene. During the follow-up period, there were 15 patients (18.25%) generating ABL kinase mutation, the mutation rate of P210- cases is 10.87%, and P190-case is 29.41%, showing a statistically significant difference (P = 0.036). There were 42 patients being treated with tyrosine kinase inhibitors (TKIs) in the first induction chemotherapy, with a remission rate of 69.05%, and 39 patients being treated with conventional chemotherapy alone, with a remission rate of 43.59%, showing a statistically significant difference (P = 0.021). There were 69 patients who achieved complete remission during the follow-up period, among which the recurrence rate of the imatinib-treatment group is 61.70% and that of the Dasatinib-treatment group is 31.82%, showing statistically significant difference (P = 0.021). Patients were grouped according to their treatment regimen and the survival curves of patients in each group were compared. The survival curves of the imatinib-treatment group and dasatinib-treatment group (P = 0.003), the hematopoietic stem cell transplantation (HSCT) group and the non-trans- plantation group are different (P = 0.010). Conclusion: In Ph+ALL, P210 is more common than P190. P190 may be a risk factor for ABL kinase mutation. Patients treated with TKIs for the first induction therapy had higher remission rates than those treated with conventional chemotherapy alone. The recurrence rate of the imatinib group was higher than that of the dasatinib group. The survival of patients treated with dasatinib and treated with HSCT after complete remission can be improved.
Keywords:Acute Lymphoblastic Leukemia, Ph Chromosome, BCR/ABL, P190, P210, ABL Kinase Mutation, Imatinib, Dasatinib, Hematopoietic Stem Cell Transplantation

Copyright © 2020 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
急性淋巴细胞白血病(ALL)是一种异质性的血液系统疾病,以骨髓、外周血和其他器官中未成熟的淋巴细胞增殖为特征。Ph+ALL患者占ALL患者的20%~30% [1],Ph染色体阳性往提示预后不良,近年来TKIs的使用使该类患者的预后得到一定改善,但治疗过程中某些患者出现ABL激酶突变使患者面临更换药物的选择及预后的改变。目前,国内外对Ph+ALL患者的研究均有报道,但由于存在地域差异,不同种族间Ph+ALL患者的临床特点不同。本文收集了2002年1月至2020年5月期间于青大附院初诊的81例Ph+ALL患者的临床资料,重点回顾性分析了患者的遗传学特征及不同治疗方案患者的疗效及生存状况。
2. 病例资料
收集2002年1月至2020年5月期间于青大附院初诊的ALL患者共256例,所有患者经MICM诊断标准 [2] 确诊,其中Ph染色体阳性的患者有81例(31.64%),阴性的患者175例(68.36%)。本论文重点回顾性分析该81例Ph+ALL患者的遗传学特征及疗效。对81例Ph+ALL患者进行随访,随访时间为12~2117天。本研究中81例Ph+ALL患者的年龄区间为13~76岁,男性患者有41例(50.62%),女性患者有40例(49.38%)。所观察病例一经确诊,在排除化疗禁忌后采用以VDP (长春地辛 + 蒽环类药物 + 地塞米松)为基础的诱导缓解化疗方案。其中有42例(51.85%)在第一次诱导缓解化疗中加用TKIs,余39例(48.15%)未加用TKIs,这39例患者中有35例在后续治疗中加用TKIs。所有加用TKIs治疗的患者中有54例(70.13%)选用伊马替尼,有23例(29.87%)选用达沙替尼治疗。在随访期内共有19例(23.46%)患者接受HSCT治疗。本论文所有信息的采集已经过患者同意,且严格保密,符合医学伦理要求。
3. 分析方法及工具
应用IBM SPSS Statistics 24.0软件对所收集数据进行统计学分析。率的比较采用卡方检验,生存分析采用Log-Rank检验,以P < 0.05为差异有统计学意义。
4. 结果
4.1. 细胞遗传学特点
81例患者初诊时行骨髓染色体、基因检测,除Ph染色体阳性外,伴有其他染色体核型异常的有19例(23.46%),所见其余染色体异常有-7,+8,-9,-16,-17,-18,-20,+X,-Y,idem,der (9),der(12),der(14),der(16),der(22),del (20),del(3),add(20),t(1; 2),t(2; 16),t(2; 5),t(2:22),t(8;9)。采用荧光定量PCR基因检测技术,有46例(56.79%)患者为P210基因阳性,34例(41.98%)患者为P190基因阳性,1例(1.23%)患者同时存在P190和P210基因阳性。随访期内所观察的81例患者中有15 (18.52%)例患者出现ABL激酶突变,根据患者的临床特征分组,分析不同临床特征的患者ABL激酶突变率的差异(表1),发现P190融合基因组的患者ABL激酶突变率为29.41%,P210融合基因组的突变率为10.87%,二者有显著性统计学差异(P = 0.036)。
Table 1. The difference of ABL kinase mutation rate among patients with different clinical characteristics
表1. 不同临床特征患者ABL激酶突变率的差异
4.2. 缓解情况
所有患者经过诱导化疗1疗程后复查骨髓,有46例(56.79%)患者达CR,10例(12.35%)患者达PR,25例(30.86%)患者NR。第一次诱导化疗时加用TKIs的患者有42例,达CR的有29例(69.05%),未加用TKIs的患者有39例,达CR的有17例(43.59%),二者有统计学差异(P = 0.021),随访期内获得CR的共有69例(85.19%),有12 (14.81%)例患者在随访期内持续未缓解。
4.3. 复发及生存情况
在69例CR的患者中有36例复发,伊马替尼治疗组的复发率为61.7%,达沙替尼治疗组的复发率为31.82%,二者有统计学差异(P = 0.021),且伊马替尼治疗组和达沙替尼治疗组患者的生存曲线不同(P = 0.031) (图1),行HSCT治疗组患者的生存曲线和未行HSCT治疗组患者的生存曲线不同(P = 0.01) (图2)。
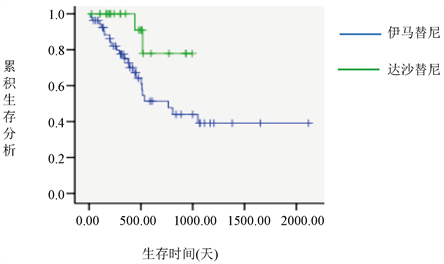
Figure 1. Survival curves of the imatinib and dasatinib groups
图1. 伊马替尼治疗组和达沙替尼治疗组的生存曲线
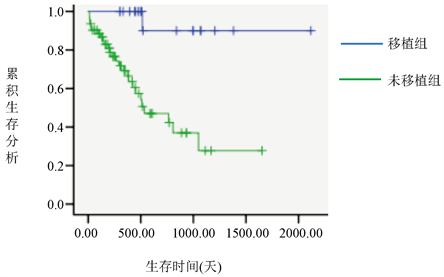
Figure 2. Survival curves for the transplanted and non-transplanted groups
图2. 移植组和未移植组的生存曲线
5. 讨论
在ALL中BCR/ABL常见的亚型有P190和P210,本课题所收集患者中P210 (56.79%)比P190 (41.98%)多见,这与国内一研究结果相似,其所收集的49例患者中融合基因P190占38.5%,P210占61.5% [3],此特点不同于慢性粒细胞白血病患者,其融合基因P190比P210更常见 [4]。1例患者同时存在P190和P210,该患者在达沙替尼联合诱导化疗1疗程后复查骨髓达CR,后续达沙替尼及化疗巩固维持治疗,随访1年余仍完全缓解状态。
BCR/ABL融合基因已经成为ALL患者治疗的重要靶点,目前一线的标准治疗方案是TKIs联合化疗,TKIs能够阻断BCR/ABL融合基因编码肿瘤蛋白的ATP结合位点,以阻止下游增殖信号通路的激活而发挥作用 [5]。在国外GRAAPH-2003研究中,45例初诊的Ph+ ALL患者,在标准诱导化疗的基础上添加伊马替尼治疗,4年OS及DFS非别为52%和43% [6]。而在另一项LALA-94研究中154例新诊断的Ph+ ALL患者,接受标准的化疗方案进行诱导治疗,4年OS及DFS均为20% [7]。UKALLXII/ECOG2993研究266例Ph+ALL患者的4年OS,比较无伊马替尼组、早期使用伊马替尼组、晚期使用伊马替尼组的结果显示早期使用伊马替尼联合化疗的诱导治疗4年OS更高 [8]。国外一长达13年的随访研究,28例Ph+ALL患者在给予HYPER-CVAD化疗方案联合伊马替尼治疗后,继续给予伊马替尼为基础的巩固维持治疗,结果提示治疗3个月达到深度分子学缓解(CMR/MMR)的患者5年DFS更高 [9]。还有研究显示移植前后接受伊马替尼治疗可以显著提高OS,且移植后立即启动伊马替尼维持治疗的患者5年DFS和OS更高 [10] [11] [12]。另外,坚持足量用药是长期疗效的保证,有人研究87例成人Ph+ ALL患者治疗过程中使用伊马替尼的剂量强度(实际给予伊马替尼剂量与计划给予伊马替尼剂量的比值,IDI)对生存率的影响,结果显示IDI ≥ 90%组比IDI < 90%组的患者RFS及OS更高 [13]。综合上述研究表明早期、足量、全程使用伊马替尼治疗可以有效改善患者的临床结局。本研究中81例患者按照指南推荐于常规治疗阶段选用VDP为基础的方案诱导缓解化疗,其中42例患者在第一次诱导缓解化疗中加TKIs,该组患者的缓解率为69.05%,39例患者在第一次诱导缓解治疗中单用传统化疗方案,缓解率为43.59%,这同样说明早期加用TKIs可改善疾病的缓解状况。
第一代和第二代TKIs在靶向作用于酪氨酸激酶位点的顺序上有所不同,国外也有研究表明二代TKIs为基础的方案治疗Ph+ ALL,OS及RFS均优于一代TKIs [14]。本例研究中随访期内获得完全缓解的患者有69例,伊马替尼治疗组的复发率为61.70%,达沙替尼治疗组的复发率为31.82%,并且伊马替尼治疗组和达沙替尼治疗组患者的生存曲线不同,提示选用达沙替尼治疗可改善患者的生存状况。
尽管TKIs的广泛使用在Ph+ALL患者中产生了良好的应答率,使患者的生存时间显著延长,但TKIs的耐药仍是目前面临的一大挑战,目前发现与耐药相关的基因突变位点有100余种,有研究显示存在联合突变比单独T315I突变耐药率高20倍 [15],突变之外的耐药因素亦待进一步研究。现阶段早期识别ABL激酶突变是预测疾病复发和耐药的有效手段,对指导预后和治疗方案的选择有重要意义。本研究中,有15例(21.74%)患者出现ABL激酶突变,P190融合基因组的ABL激酶突变率为29.41%,P210融合基因组的突变率为10.87% (P = 0.036)。提示P190阳性可能为激酶突变的危险因素。目前对有ABL激酶突变的复发性Ph+ALL患者有效治疗中,造血干细胞移植治疗可改善患者的预后,本例研究中,随访期内移植组和未移植组的患者的生存曲线亦不同(P = 0.01),行造血干细胞移植治疗可改善患者的生存。国外也有研究表明,即使在TKIs时代,HSCT仍是Ph+ALL患者的首选治疗 [8]。
文章引用
孔 琦,赵春亭,崔渤莉,王彩曌,闫思琪,费海荣,李田兰,刘珊珊. 81例Ph染色体阳性急性淋巴细胞白血病患者的遗传学特征及疗效分析
Genetic Characteristics and Curative Effect Analysis of 81 Patients with Ph Chromosome Positive Acute Lymphoblastic Leukemia[J]. 临床医学进展, 2020, 10(11): 2805-2811. https://doi.org/10.12677/ACM.2020.1011426
参考文献
- 1. Forghieri, F., Luppi, M. and Potenza, L. (2015) Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Hematology, 20, 618-619. https://doi.org/10.1179/1024533215Z.000000000402
- 2. 中华医学会血液学分会, 中国抗癌协会血液肿瘤专业委员会. 中国成人急性淋巴细胞白血病诊断与治疗专家共识[J]. 中华血液学杂志, 2012, 33(9): 789-792.
- 3. 刘娟, 张闰, 葛峥, 林忠琨, 柳萍, 仇海荣, 李建勇, 等. 217例成人急性淋巴细胞白血病遗传学特征及其临床预后意义的研究[J]. 中华医学遗传学杂志, 2013, 30(2): 1003-9406.
- 4. Komorowski, L., Fidyt, K., Patkowska, E. and Firczuk, M. (2020) Philadelphia Chromosome-Positive Leukemia in the Lymphoid Lineage—Similarities and Differences with the Myeloid Lineage and Specific Vulnerabilities. International Journal of Molecular Sciences, 2020, 5776. https://doi.org/10.3390/ijms21165776
- 5. Dalle, I.A., Kantarjian, H.M., Short, N.J., Konopleva, M., Jain, N., et al. (2019) Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia at First Relapse in the Era of Tyrosine Kinase Inhibitors. American Journal of Hematology, 94, 1388-1395. https://doi.org/10.1002/ajh.25648
- 6. Tanguy-Schmidt, A., Rousselot, P., et al. (2013) Long-Term Follow-Up of the Imatinib GRAAPH-2003 Study in Newly Diagnosed Patients with de Novo Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A GRAALL Study. Biology of Blood and Marrow Transplantation, 19, 150-155. https://doi.org/10.1016/j.bbmt.2012.08.021
- 7. Lickliter, J.D., TayoIor, K., Szer, J., et al. (2015) An Imatinib-Only Window Followed by Imatinib and Chemotherapy for Philadelphia Chromosome-Positive Acute Leukemia: Long-Term Results of the CMLALL1 Trial. Leukemia & Lymphoma, 56, 630-638. https://doi.org/10.3109/10428194.2014.925547
- 8. Fielding, A.K., Rowe, J.M., Buck, G., Foroni, L., Gerrard, G., et al. (2014) UKALLXII/ECOG2993: Addition of Imatinib to a Standard Treatment Regimen Enhances Long-Term Outcomes in Philadelphia Positive Acute Lymphoblastic Leukemia. Blood, 123, 843-850. https://doi.org/10.1182/blood-2013-09-529008
- 9. Daver, N., Thomas, D., Ravandi, F., et al. (2015) Final Report of a Phase II Study of Imatinib Mesylate with Hyper-CVAD for the Front-Line Treatment of Adult Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Haematologica, 100, 653-661. https://doi.org/10.3324/haematol.2014.118588
- 10. Mizuta, S., Matsuo, K., Nishiwaki, S., Imai, K., et al. (2014) Pretransplant Administration of Imatinib for Allo-HSCT in Patients with BCR-ABL-Positive Acute Lymphoblastic Leukemia. Blood, 123, 2325-2332. https://doi.org/10.1182/blood-2013-11-538728
- 11. Nishiwaki, S., Miyamura, K., Kato, C., Terakura, S., et al. (2010) Impact of Post-Transplant Imatinib Administration on Philadelphia Chromosome-Positive Acute Lymphoblastic Leukaemia. Anticancer Research, 30, 2415-2418.
- 12. Chen, H., Liu, K.Y., Xu, L.P., Liu, D.H., Chen, Y.H., et al. (2012) Administration of Imatinib after Allogeneic Hematopoietic Stem Cell Transplantation May Improve Disease-Free Survival for Patients with Philadelphia Chromosome-Positive Acute Lymphobla Stic Leukemia. Journal of Hematology & Oncology, 5, Article No. 29. https://doi.org/10.1186/1756-8722-5-29
- 13. Lim, S.-N., Joo, Y.-D., Lee, K.-H., Kim, D.-Y., Lee, J.-H., et al. (2015) Long-Term Follow-Up of Imatinib plus Combination Chemotherapy in Patients with Newly Diagnosed Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. American Journal of Hematology, 90, 1013-1020. https://doi.org/10.1002/ajh.24137
- 14. Akahoshi, Y., Nishiwaki, S., Mizuta, S., Ohashi, K., et al. (2019) Tyrosine Kinase Inhibitor Prophylaxis after Transplant for Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Cancer Science, 110, No. 10. https://doi.org/10.1111/cas.14167
- 15. Pavlovsky, C., Chan, O., Talati, C. and Pinilla-Ibarz, J. (2019) Ponatinib in the Treatment of Chronic Myeloid Leukemia and Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia. Future Oncology, 15, 257-269. https://doi.org/10.2217/fon-2018-0371
NOTES
*通讯作者。
