Advances in Clinical Medicine
Vol.
14
No.
03
(
2024
), Article ID:
82569
,
9
pages
10.12677/ACM.2024.143718
miR-423-3p通过下调CARNS1促进乳腺癌细胞恶性生物学行为
李金洋,陈东旭,傅腾超,吴琍*
青岛大学附属医院乳腺病诊疗中心,山东 青岛
收稿日期:2024年2月14日;录用日期:2024年3月9日;发布日期:2024年3月14日

摘要
目的:探讨miR-423-3p对乳腺癌细胞恶性生物学行为的影响及作用机制。方法:体外培养人乳腺癌细胞MDA-MB-231、MDA-MB-468、MCF-7、BT-549及正常乳腺上皮细胞MCF-10A,采用实时荧光定量PCR (RT-qPCR)法和Western Blot方法,分别检测miR-423-3p及CARNS1 mRNA和蛋白相对表达量。选取MCF-7细胞,将细胞分为转染mimics NC组(A组)、转染miR-423-3p mimics组(B组)、转染inhibitors NC组(C组)、转染inhibitors组(D组)。在239T细胞中分别转染并分为CARNS1-3'-UTR-wt+miR-423-3p mimics (E组)、CARNS1-3'-UTR-wt-mimics NC (F组)、CARNS1-3'-UTR-mut+miR-423-3p mimics (G组)及CARNS1-3'-UTR-mut+mimics NC (H组)。采用RT-qPCR和Western Blot法,检测A~D组细胞miR-423-3p及CARNS1 mRNA和蛋白表达情况,采用CCK8法、细胞划痕实验、Transwell法和流式细胞术检测细胞增殖、迁移、侵袭和凋亡情况。在线数据库预测miR-423-3p与CARNS1基因3'非翻译区(3'-UTR)的互补结合位点,双荧光素酶报告基因实验验证预测结果。结果:RT-qPCR结果显示,MCF-7、MDA-MB-231、MDA-MB-468及BT-549细胞中miR-423-3p的相对表达量均明显高于正常乳腺上皮细胞MCF-10A (P < 0.05),RT-qPCR和Western Blot检测结果显示,CARNS1 mRNA以及蛋白的相对表达量均明显低于正常乳腺上皮细胞MCF-10A (P < 0.05)。CCK8实验结果显示,在细胞培养的第24、48、72小时,B组细胞增殖活力明显高于A组,D组明显低于C组。细胞划痕实验显示,B组细胞愈合率明显高于A组(P < 0.05)。D组细胞愈合率明显低于C组(P < 0.05)。Transwell实验结果显示,过表达miR-423-3p后可促进乳腺癌细胞侵袭,而抑制miR-423-3p后会抑制乳腺癌细胞的侵袭,差异有统计学意义(P < 0.05)。流式细胞实验结果显示,B组较A组凋亡能力明显降低(P < 0.05),D组较C组凋亡能力明显增强(P < 0.05)。starBase数据库预测miR-423-3p与CARNS1基因3'非翻译区(3'-UTR)存在互补结合位点,双荧光素酶报告基因实验结果显示,E组较F组细胞相对荧光素酶显著下调(P < 0.05),G组与F组细胞间相对荧光素酶活性差异无统计学意义(P > 0.05)。RT-qPCR检测结果显示,B组细胞的miR-423-3p相对表达量明显高于A组(P < 0.05),而CARNS1 mRNA相对表达量明显低于A组(P < 0.05),D组细胞的miR-423-3p相对表达量明显低于C组(P < 0.05),而CARNS1 mRNA相对表达量明显高于C组(P < 0.05)。结论:miR-423-3p可通过靶向调控CARNS1促进乳腺癌细胞的恶性生物学行为。
关键词
乳腺癌,微小RNA,miR-423-3p,CARNS1

miR-423-3p Promotes Malignant Biological Behavior in Breast Cancer Cells by Downregulating CARNS1
Jinyang Li, Dongxu Chen, Tengchao Fu, Li Wu*
Diagnosis and Treatment Centre of Breast Diseases, The Affiliated Hospital of Qingdao University, Qingdao Shandong
Received: Feb. 14th, 2024; accepted: Mar. 9th, 2024; published: Mar. 14th, 2024

ABSTRACT
Objective: To investigate the effect and mechanism of miR-423-3p on the malignant biological behavior of breast cancer cells. Methods: Human breast cancer cells MDA-MB-231, MDA-MB-468, MCF-7, BT-549 and normal breast epithelial cells MCF-10A were cultured in vitro, and the relative mRNA and protein expressions of miR-423-3p and CARNS1 were detected by real-time quantitative PCR (RT-qPCR) and Western Blot, respectively. MCF-7 cells were divided into mimics NC transfection group (group A), miR-423-3p mimics group (group B), transfection inhibitors NC group (group C), and transfection inhibitors group (group D). 239T cells were transfected and divided into CARNS1-3'-UTR-wt miR-423-3p mimics (group E), CARNS1-3'-UTR-wt-mimics NC (group F), CARNS1-3'-UTR-mut miR-423-3p mimics (group G) and CARNS1-3'-UTR-mut mimics NC (group H). The mRNA and protein expressions of miR-423-3p and CARNS1 in group A~D cells were detected by RT-qPCR and Western Blot, and the proliferation, migration, invasion and apoptosis of cells were detected by CCK8 method, cell scratch assay, Transwell method and flow cytometry. The complementary binding sites of miR-423-3p and the 3 untranslated region (3'-UTR) of CARNS1 gene were predicted by an online database, and the prediction results were verified by double luciferase reporter gene assays. Results: RT-qPCR results showed that the relative expression levels of miR-423-3p in MCF-7, MDA-MB-231, MDA-MB-468 and BT-549 cells were significantly higher than those in normal mammary epithelial cells MCF-10A (P < 0.05), and the relative expression levels of mRNA and protein in CARNS1 were significantly lower than those in normal mammary epithelial cells (P < 0.05). The results of CCK8 experiments showed that at the 24th, 48th and 72nd hours of cell culture, the cell proliferation activity of group B was significantly higher than that of group A, and that of group D was significantly lower than that of group C. The cell scratch test showed that the cell healing rate of group B was significantly higher than that of group A (P < 0.05). The cell healing rate of group D was significantly lower than that of group C (P < 0.05). The results of Transwell assay showed that overexpression of miR-423-3p could promote the invasion of breast cancer cells, while inhibition of miR-423-3p would inhibit the invasion of breast cancer cells, and the difference was statistically significant (P < 0.05). The results of flow cytometry showed that the apoptosis ability of group B was significantly lower than that of group A (P < 0.05), and the apoptosis ability of group D was significantly higher than that of group C (P < 0.05). The starBase database predicted that miR-423-3p had complementary binding sites in the 3' untranslated region (3'-UTR) of CARNS1 gene, and the results of double luciferase reporter gene assay showed that the relative luciferase of group E was significantly down-regulated compared with group F (P < 0.05), and there was no significant difference in relative luciferase activity between group G and group F (P > 0.05). The results of RT-qPCR showed that the relative expression of miR-423-3p in group B was significantly higher than that in group A (P < 0.05), while the relative expression of mRNA in CARNS1 was significantly lower than that in group A (P < 0.05), the relative expression of miR-423-3p in group D was significantly lower than that in group C (P < 0.05), and the relative expression of mRNA in CARNS1 was significantly higher than that in group C (P < 0.05). Conclusion: miR-423-3p can promote the malignant biological behavior of breast cancer cells by targeting CARNS1.
Keywords:Breast Cancer, microRNA, miR-423-3p, CARNS1

Copyright © 2024 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
乳腺癌(Breast Cancer, BC)是女性最常见的恶性肿瘤之一,其发病率和死亡率均位于女性癌症首位 [1] 。微小RNA (microRNA, miRNA)是一类长度为19~22个核苷酸的非编码RNA [2] ,可通过与靶基因mRNA的3'-UTR碱基互补配对,调控mRNA的翻译和降解,影响细胞的增殖、凋亡等生物学过程 [3] 。近年来相关研究表明,miRNA可以通过调控癌细胞中的各种代谢途径来调节基因表达,进而促进或抑制癌症进展 [4] 。miR-423-3p可通过靶向CYBRD1激活FAK信号通路促进肺腺癌上皮间质转化,进而促进肿瘤生长 [5] 。miR-423-3p可通过调控SUFU影响结肠癌细胞对5-Fu的敏感性 [6] ,此外,还有研究结果显示miR-423-3p在胶质瘤组织中显著上调,且miR-423-3p表达增加与胶质瘤患者的不良预后相关,并可通过下调PANX2促进胶质瘤的生长、进展 [7] 。然而,关于miR-423-3p在乳腺癌中的作用及机制研究尚未见报道,本文主要探究miR-423-3p对乳腺癌细胞增殖、迁移、侵袭和凋亡的影响,以及miR-423-3p与CARNS1基因的靶向调控关系,为乳腺癌的诊断和治疗提供新的靶点。
2. 材料和方法
2.1. 细胞与试剂
乳腺癌细胞MDA-MB-231、MDA-MB-468、MCF-7、BT-549及正常乳腺上皮细胞MCF-10A来自青岛大学医学部分子生物学与生物化学实验室。DMEM培养基、PBS、胰蛋白酶消化液、青/链霉素混合液购于上海源培生物科技有限公司,胎牛血清购于美国Gibco公司,CCK8试剂盒、多聚甲醛、结晶紫染液购于上海碧云天生物技术有限公司,逆转录试剂盒、Trizol购于南京诺唯赞生物科技公司,mimics及inhibitors转染试剂购于上海吉玛基因,CARNS1、GADPH抗体、二抗购于Proteintech公司,双荧光素酶报告基因检测盒购于北京全式金生物技术有限公司。
2.2. 细胞培养及分组
将乳腺癌细胞MDA-MB-231、MDA-MB-468、MCF-7、BT-549及正常乳腺上皮细胞MCF-10A置于含有10%胎牛血清和1%青/链霉素混合液的DMEM培养基中,在37℃、5% CO2的细胞培养箱中培养,于细胞融合度达80%~90%时传代,取对数生长期的细胞,用于后续实验。
在6孔板中培养MCF-7细胞并孵育过夜,当细胞融合度达60%~80%时使用转染试剂进行转染,分为mimics NC组(A组)、转染miR-423-3p mimics组(B组)、转染inhbitors NC组(C组)、转染inhibitors组(D组)。
2.3. RT-qPCR检测miR-423-3p和CARNS1的表达
在MDA-MB-231、MDA-MB-468、MCF-7、BT-549、MCF-10A细胞悬液中加入Trizol试剂提取RNA,逆转录为cDNA并扩增。上机条件:① 在95℃下预变性3 min;② 95℃,15 s;55℃,20 s;72℃,15 s,循环40次。miR-423-3p的内参为U6,CARNS1的内参为GAPDH。采用2−ΔΔCt计算miR-423-3p和CARNS1的相对表达量。各引物序列见表1。
2.4. Western Blot检测CARNS1的表达
收集上述细胞于EP管中,加入裂解液提取蛋白,使用BCA试剂盒测定蛋白浓度,通过SDS-PAGE分离蛋白质并转移至PVDF膜,使用5%脱脂牛奶封闭1 h,加入内参GAPDH一抗(1:1000)、目的基因CARNS1一抗(1:5000),在4℃冰箱孵育过夜。使用TBST漂洗3次,每次10 min。然后加入二抗室温孵育1 h,再次用TBST洗膜3次,每次10 min。使用ECL化学发光,成像系统显影、拍照并测量灰度值。CARNS1蛋白相对表达量 = CARNS1蛋白条带灰度值/内参GAPDH蛋白条带灰度值。
2.5. CCK8实验
将转染后的细胞悬液接种至96孔板中(10 μL/孔),分别加入10 μL的CCK8溶液,置于细胞培养箱内孵育1 h后,使用酶标仪测定各孔在波长450 nm处的吸光值(A)。
Table 1. PCR primers
表1. PCR引物
2.6. 细胞划痕实验
将转染后的各组细胞接种于6孔板,待细胞生长至铺满整孔时,用200 μL移液管枪头沿直尺垂直划痕,后加入无血清培养基,在0和48 h使用显微镜拍摄记录。
2.7. Transwell检测实验
取对数生长期的细胞,用PBS和无血清培养基先后洗涤,使用无血清培养基培养,调整浓度至2 × 105/mL。将Matrigel胶加入Transwell小室的上室,加入150 μL细胞悬液,在下室加入800 μL含10%血清的培养基,置于培养箱培养24 h。使用4%多聚甲醛溶液固定,结晶紫染色。在倒置显微镜下计算中间和四周5个视野的细胞数,取平均值。
2.8. 流式细胞术
取转染后的细胞使用PBS洗涤3次,置于六个孔板中,每孔含有加入5 µL FITC抗体和5 µL PI抗体,室温下避光染色30 min,再次PBS洗涤细胞,使用流式细胞仪和BD Cell Quest Pro软件分析细胞凋亡的比例。
2.9. 双荧光素酶报告基因测定
starBase数据预测miR-423-3p与CARNS1基因的结合位点。构建CARNS1野生型(wt)和突变型(mut)重组质粒,将239T细胞接种于6孔板中培养,分别转染CARNS1-3'-UTR-wt+miR-423-3p mimics (E组)、CARNS1-3'-UTR-wt-mimics NC (F组)、CARNS1-3'-UTR-mut+miR-423-3p mimics (G组)及CARNS1-3'-UTR-mut+mimics NC (H组),24 h后,使用双荧光素酶报告基因试剂盒于酶标仪中测定荧光素酶活性。
2.10. RT-qPCR检测转染后细胞中miR-423-3p和CARNS1 mRNA和蛋白的表达
在转染后细胞中分别加入Trizol试剂提取RNA,逆转录为cDNA并扩增。miR-423-3p以U6为内参照,CARNS1以GAPDH为内参照,使用RT-qPCR检测转染后各组细胞miR-423-3p和CARNS1的表达,上机条件同前。各引物序列见表1。收集转染后的MCF-7细胞,加入裂解液提取蛋白,使用BCA试剂盒测定蛋白浓度,使用Western Blot检测各组转染后细胞中CARNS1蛋白的表达量。
2.11. 统计学分析
使用SPSS 26.0软件进行分析,所有实验均独立重复3次,结果取平均值。正态分布的计量资料以均数 ± 标准差( ± s)表示,两组比较采用t检验,多组间比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
3. 结果
3.1. miR-423-3p和CARNS1 mRNA及蛋白在乳腺癌细胞的表达情况
RT-qPCR实验结果表明,miR-423-3p在乳腺癌细胞MDA-MB-231、MDA-MB-468、MCF-7、BT-549中的表达较正常乳腺上皮细胞MCF-10A显著上调,表达量分别为4.012 ± 0.438,1.652 ± 0.111,5.983 ± 0.431,2.808 ± 0.267,1.000 ± 0.056,差异有统计学意义(P < 0.05),在MCF-7中表达最高。而CARNS1在乳腺癌中显著下调,上述各细胞系中CARS1 mRNA的表达量分别为0.449 ± 0.053,0.601 ± 0.056,0.174 ± 0.010,0.330 ± 0.020,1.000 ± 0.062 (P < 0.05),在MCF-7中表达最低。Western Blot结果显示,上述各细胞系中CARNS1蛋白相对表达量分别为0.660 ± 0.026,0.809 ± 0.022,0.278 ± 0.011,0.508 ± 0.028,1.000 ± 1.019,差异有统计学意义(P < 0.05)。
3.2. miR-423-3p对乳腺癌细胞增殖能力的影响
CCK-8实验结果显示,miR-423-3p mimics组(B组)较mimics NC组(A组)增殖活力明显升高,转染inhibitors组(D组)较转染inhbitors NC组(C组)组增殖活力明显降低,差异有统计学意义(P < 0.05)。见表2。
3.3. miR-423-3p对乳腺癌细胞迁移能力的影响
细胞划痕实验结果表明,mimics NC组(A组)、转染miR-423-3p mimics组(B组)、转染inhbitors NC组(C组)、转染inhibitors组(D组)细胞愈合率分别为39.540 ± 5.897,72.910 ± 3.292,39.750 ± 2.780,20.260 ± 1.789,其中miR-423-3p mimics组(B组)较mimics NC组(A组)愈合率明显升(P < 0.05),转染inhibitors组(D组)较转染inhbitors NC组(C组)组愈合率明显降低(P < 0.05)。见图1。
Table 2. Comparison of MCF-7 cell proliferation activity
表2. MCF-7细胞增殖活力比较
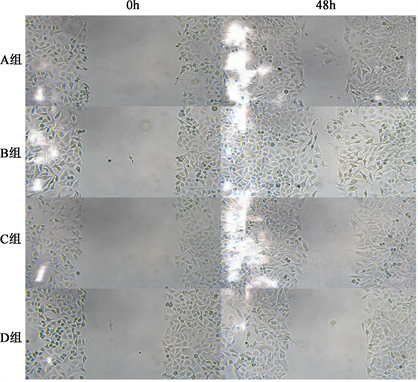
Figure 1. Comparison of the migration ability of MCF-7 cells in groups A~D after transfection
图1. 转染后A~D组MCF-7细胞迁移能力比较
3.4. miR-423-3p对乳腺癌细胞侵袭能力的影响
Transwell实验结果显示,过表达miR-423-3p后可促进乳腺癌细胞侵袭,而敲除miR-423-3p后会抑制乳腺癌细胞的侵袭,差异有统计学意义(P < 0.05)。见图2。
3.5. miR-423-3p对乳腺癌细胞凋亡的影响
流式细胞术结果显示,过表达miR-423-3p后可抑制乳腺癌细胞凋亡,而敲除miR-423-3p后会促进乳腺癌细胞的凋亡,差异有统计学意义(P < 0.05)。
3.6. miR-423-3p与CARNS1在乳腺癌中的靶向关系
通过starbase在线数据库预测miR-423-3p与CARNS1具有结合位点(图3)。双荧光素酶活性报告实验结果显示E~H组间细胞相对荧光素酶活性值差异有统计学意义(p < 0.05),其中E组较F组细胞相对荧光素酶显著下调(p < 0.05),G组与F组细胞间相对荧光素酶活性差异无统计学意义(p > 0.05)。见图4。
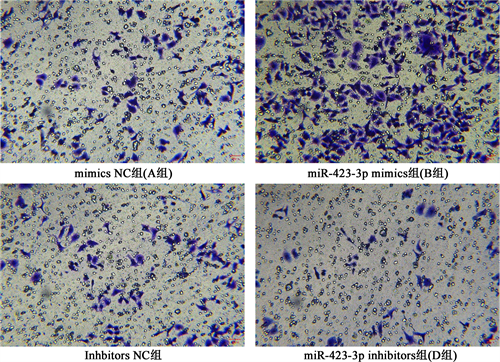
Figure 2. Comparison of the invasion ability of MCF-7 cells in groups A~D after transfection
图2. 转染后A~D组MCF-7细胞侵袭能力比较
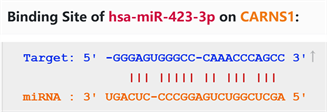
Figure 3. Predicted binding sites of miR-423-3p and CARNS1
图3. miR-423-3p与CARNS1的预测结合位点
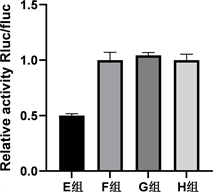
Figure 4. Dual luciferase activity reporter assay to detect the targeting relationship between miR-423-3p and CARNS1
图4. 双荧光素酶活性报告实验检测miR-423-3p与CARNS1的靶向关系
3.7. miR-423-3p抑制CARNS1在乳腺癌中的表达
RT-qPCR实验结果显示,A~D各组细胞miR-423-3p相对表达量分别为1.000 ± 0.014,3.168 ± 0.289,1.013 ± 0.064,0.457 ± 0.043,差异有统计学意义(P < 0.05)。其中,miR-423-3p mimics组(B组)较mimics NC组(A组)组显著升高,转染inhibitors组(D组)较转染inhbitors NC组(C组)组显著降低。上述各细胞系中CARS1 mRNA的表达量分别为1.000 ± 0.091,0.526 ± 0.019,1.008 ± 0.043,3.161 ± 0.142,差异有统计学意义(P < 0.05)。其中,miR-423-3p mimics组(B组)较mimics NC组(A组)组显著降低,转染inhibitors组(D组)较转染inhbitors NC组(C组)组显著升高。
4. 讨论
随着近年来医疗技术的飞速发展,包括手术、放疗、化疗、内分泌、靶向和免疫治疗等乳腺癌的诊治能力大幅提高,然而仍有部分患者病情进展迅速,预后较差 [8] 。因此,探索新的治疗方式对乳腺癌患者尤为重要。miRNA通过与mRNA的3'-UTR区结合发挥作用,促进其降解并抑制miRNA翻译,在细胞生长、分化、增殖、凋亡等阶段发挥重要作用 [9] [10] [11] [12] ,与乳腺癌的发生、发展密切相关 [13] 。本文的研究对象miR-423-3p在肺癌 [5] [14] 、肝癌 [15] 、结肠癌 [6] 、前列腺癌 [16] 、胶质瘤 [7] 等肿瘤进展可作为促癌基因,而在某些肿瘤中亦可作为抑癌基因 [17] 。本研究结果显示,miR-423-3p在乳腺癌细胞中高表达,通过CCK-8实验、细胞划痕实验、Transwell和流式细胞术等细胞功能实验证明,miR-423-3p促进乳腺癌细胞的恶性生物学行为,具有致癌基因的作用。
CARNS1 (肌肽合酶1)是一种蛋白质编码基因,主要参与肌肽生物合成和组氨酸降解 [18] 。肌肽最初是在骨骼肌中发现的,是一种天然存在的含有二肽的组氨酸,它可以作为细胞内pH缓冲剂和抗氧化剂 [19] 。熊峰等发现,CARNS1在冠心病中可能是一个保护性因素 [20] 。此外,还有研究显示注射肌肽可抑制大鼠脾脏的交感神经活动,减少大鼠结肠癌细胞的增殖 [21] 。本研究结果提示,CARNS1在乳腺癌细胞中表达降低。双荧光素酶基因报告实验验证了miR-423-3p与CARNS1的靶向关系,并通过RT-qPCR和Western Blot实验进一步验证,转染miR-423-3p mimics组miR-423-3p表达明显升高,而CARNS1 mRNA及蛋白表达水平显著降低;转染miR-423-3p inhibitors组miR-423-3p表达被显著抑制,CARNS1 mRNA及蛋白表达水平明显升高。由此可证明,CARNS1受到miR-423-3p的负向调控。
综上所述,本研究证明miR-423-3p是一个起促癌作用的miRNA,而CARNS1是一种抑癌基因。在乳腺癌细胞中,miR-423-3p通过抑制CARNS1的表达,促进乳腺癌细胞的增殖、迁移、侵袭并抑制其凋亡。由于miRNA参与调控多个靶基因及信号通路,miR-423-3p调控CARNS1促癌的具体机制及相关通路还需进一步研究。
文章引用
李金洋,陈东旭,傅腾超,吴 琍. miR-423-3p通过下调CARNS1促进乳腺癌细胞恶性生物学行为
miR-423-3p Promotes Malignant Biological Behavior in Breast Cancer Cells by Downregulating CARNS1[J]. 临床医学进展, 2024, 14(03): 415-423. https://doi.org/10.12677/ACM.2024.143718
参考文献
- 1. Sung, H., Ferlay, J., Siegel, R.L., et al. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians, 71, 209-249. https://doi.org/10.3322/caac.21660
- 2. Murugesan, M. and Premkumar, K. (2021) Integrative miRNA-mRNA Functional Analysis Identifies miR-182 as a Potential Prognostic Biomarker in Breast Cancer. Molecular Omics, 17, 533-543. https://doi.org/10.1039/D0MO00160K
- 3. An, M., Zang, X., Wang, J., et al. (2021) Comprehensive Analysis of Differentially Expressed Long Noncoding RNAs, miRNAs and mRNAs in Breast Cancer Brain Metastasis. Epigenomics, 13, 1113-1128. https://doi.org/10.2217/epi-2021-0152
- 4. He, B., Zhao, Z., Cai, Q., et al. (2020) miRNA-Based Biomarkers, Therapies, and Resistance in Cancer. International Journal of Biological Sciences, 16, 2628-2647. https://doi.org/10.7150/ijbs.47203
- 5. Ma, J., Huang, W., Zhu, C., et al. (2021) MiR-423-3p Activates FAK Sig-naling Pathway to Drive EMT Process and Tumor Growth in Lung Adenocarcinoma through Targeting CYBRD1. Journal of Clinical Laboratory Analysis, 35, E24044. https://doi.org/10.1002/jcla.24044
- 6. 赵楠, 张涛, 魏东, 等. MiR-423-3p通过靶向SUFU调节结肠癌细胞对5-Fu的敏感性[J]. 中国组织化学与细胞化学杂志, 2021, 30(2): 152-158.
- 7. Xu, J., He, J., Huang, H., et al. (2018) MicroRNA-423-3p Promotes Glioma Growth by Targeting PANX2. Oncology Letters, 16, 179-188. https://doi.org/10.3892/ol.2018.8636
- 8. Loibl, S., Poortmans, P., Mor-row, M., et al. (2021) Breast Cancer. The Lancet, 397, 1750-1769. https://doi.org/10.1016/S0140-6736(20)32381-3
- 9. Fridrichova, I. and Zmetakova, I. (2019) MicroRNAs Con-tribute to Breast Cancer Invasiveness. Cells, 8, Article No. 1361. https://doi.org/10.3390/cells8111361
- 10. Petri, B.J. and Klinge, C.M. (2020) Regulation of Breast Cancer Metastasis Signaling by miRNAs. Cancer and Metastasis Re-views, 39, 837-886. https://doi.org/10.1007/s10555-020-09905-7
- 11. Song, M., Liu, J., Zheng, X., et al. (2021) MiR-148a-3p Targets CEMIP to Suppress the Genesis of Gastric Cancer Cells.Biochemical and Biophysical Research Communications, 575, 42-49. https://doi.org/10.1016/j.bbrc.2021.08.039
- 12. Wang, W., Dong, J., Wang, M., et al. (2018) MiR-148a-3p Suppresses Epithelial Ovarian Cancer Progression Primarily by Targeting C-Met. Oncology Letters, 15, 6131-6136. https://doi.org/10.3892/ol.2018.8110
- 13. Iacona, J.R. and Lutz, C.S. (2019) MiR-146a-5p: Ex-pression, Regulation, and Functions in Cancer. Wiley Interdisciplinary Reviews: RNA, 10, E1533. https://doi.org/10.1002/wrna.1533
- 14. Liu, Y., Huang, R., Xie, D., et al. (2021) ZNF674-AS1 Antagonizes MiR-423-3p to Induce G0/G1 Cell Cycle Arrest in Non-Small Cell Lung Cancer Cells. Cell & Molecular Bio Letters, 26, Article No. 6. https://doi.org/10.1186/s11658-021-00247-y
- 15. Liu, Z. and Cui, Y. (2021) Bim’s Effect on the Expression of MiR-423-3p in Promoting Primary Hepatic Cancer (PHC) and Role of MiR-423-3p in PHC Proliferation and Invasion. Biochemical Genetics, 59, 1247-1259. https://doi.org/10.1007/s10528-021-10052-z
- 16. Guo, T., Wang, Y., Jia, J., et al. (2020) The Identification of Plasma Exosomal MiR-423-3p as a Potential Predictive Biomarker for Prostate Cancer Castration-Resistance Develop-ment by Plasma Exosomal MiRNA Sequencing. Frontiers in Cell and Developmental Biology, 8, Article ID: 602493. https://doi.org/10.3389/fcell.2020.602493
- 17. Yan, X., Zhang, S., Jia, J., et al. (2022) Exosomal MiR-423-3p In-hibits Macrophage M2 Polarization to Suppress the Malignant Progression of Cervical Cancer. Pathology—Research and Practice, 235, Article ID: 153882. https://doi.org/10.1016/j.prp.2022.153882
- 18. Drozak, J., Veiga-Da-Cunha, M., Vertommen, D., et al. (2010) Molecular Identification of Carnosine Synthase as ATP-Grasp Domain-Containing Protein 1 (ATPGD1). Journal of Bi-ological Chemistry, 285, 9346-9356. https://doi.org/10.1074/jbc.M109.095505
- 19. Artioli, G.G., Sale, C. and Jones, R.L. (2019) Carnosine in Health and Disease. European Journal of Sport Science, 19, 30-39. https://doi.org/10.1080/17461391.2018.1444096
- 20. Xiong, F., Mao, R., Zhao, R., et al. (2022) Plasma Exosomal S1PR5 and CARNS1 as Potential Non-Invasive Screening Biomarkers of Coronary Heart Disease. Frontiers in Cardi-ovascular Medicine, 9, Article ID: 845673. https://doi.org/10.3389/fcvm.2022.845673
- 21. Horii, Y., Shen, J., Fujisaki, Y., et al. (2012) Effects of L-Carnosine on Splenic Sympathetic Nerve Activity and Tumor Proliferation. Neuroscience Letters, 510, 1-5. https://doi.org/10.1016/j.neulet.2011.12.058
NOTES
*通讯作者Email: wuliqd@163.com
