Advances in Clinical Medicine
Vol.
12
No.
07
(
2022
), Article ID:
53621
,
9
pages
10.12677/ACM.2022.127931
C-反应蛋白、白介素-6在醒后卒中的研判价值
潘树超1,张婷婷1,翟迎凯1,游振宁1,汪元浚2*
1青海大学第一附属医院,青海 西宁
2青海大学第一附属医院老年科,青海 西宁
收稿日期:2022年6月13日;录用日期:2022年7月6日;发布日期:2022年7月14日

摘要
目的:探讨醒后卒中(WUS)患者血清C-反应蛋白(CRP)、白介素-6(IL-6)水平与病情严重程度的相关性,分析其诊断WUS的价值。方法:选择2020年1月至2021年6月我院老年病科和神经内科收治的153例WUS患者(WUS组)和72例体检者(对照组),根据美国(NHISS)评分将WUS组分为轻度亚组(43例)、中度亚组(69例)和重度亚组(41例),根据脑梗死大小分为大梗死亚组(35例)、小梗死亚组(80例)、腔隙性梗死亚组(38例)。检测血清CRP、IL-6水平,分析WUS发病因素以及CRP、IL-6诊断WUS的价值。结果:WUS组血清CRP、IL-6水平均高于对照组(P < 0.05)。重度亚组、中度亚组患者血清CRP、IL-6水平高于轻度亚组(P < 0.05),大梗死亚组、小梗死亚组患者血清CRP、IL-6水平高于腔隙性梗死亚组(P < 0.05)。阻塞性睡眠呼吸暂停综合征,高水平CRP、高水平IL-6是WUS的危险因素(P < 0.05)。CRP、IL-6诊断WUS的曲线下面积为0.743、0.683,联合CRP、IL-6诊断为0.895,高于单独CRP、IL-6 (z = 4.288、5.022,P < 0.05)。结论:WUS患者血清CRP、IL-6水平均增高,且与梗死面积增大、神经缺损加重有关,可作为WUS诊断的辅助指标。
关键词
醒后卒中,C-反应蛋白,白介素-6,神经缺损程度,梗死体积

The Diagnostic Value of C-Reactive Protein and Interleukin-6 in Post-Wake Stroke
Shuchao Pan1, Tingting Zhang1, Yingkai Zhai1, Zhenning You1, Yuanjun Wang2*
1The First Affiliated Hospital of Qinghai University, Xining Qinghai
2Department of Gerontology, The First Affiliated Hospital of Qinghai University, Xining Qinghai
Received: Jun. 13th, 2022; accepted: Jul. 6th, 2022; published: Jul. 14th, 2022

ABSTRACT
Objective: To investigate the correlation between the levels of C-reactive protein (CRP) and interleukin-6 (IL-6) and the severity of disease in patients with wake-up stroke (WUS), and to analyze the value of the diagnosis of WUS. Methods: 153 WUS patients (WUS group) admitted to the department of Neurology of our hospital and 72 physical examination subjects (control group) from January 2020 to June 2021 were selected. The WUS group was divided into mild subgroup (43 cases), moderate subgroup (69 cases) and severe subgroup (41 cases) according to the national institutes of health stroke scale (NHISS) score. According to the size of cerebral infarction, they were divided into major infarction subgroup (35 cases), minor infarction subgroup (80 cases) and lacunar infarction subgroup (38 cases). Serum CRP and IL-6 levels were detected to analyze the pathogenesis of WUS and the value of CRP and IL-6 in the diagnosis of WUS. Results: The levels of serum CRP and IL-6 in WUS group were higher than those in control group (P < 0.05). The serum levels of CRP and IL-6 in severe and moderate subgroups were higher than those in mild subgroup (P < 0.05), and the levels of CRP and IL-6 in major and minor subgroups were higher than those in lacunar subgroup (P < 0.05). Obstructive sleep apnea syndrome, high levels of CRP and IL-6 were risk factors for WUS (P < 0.05). The area under the curve of CRP and IL-6 in the diagnosis of WUS were 0.743 and 0.683, and the combined diagnosis of CRP and IL-6 was 0.895, which was higher than that of CRP and IL-6 alone (z = 4.288 and 5.022, P < 0.05). Conclusion: The serum CRP and IL-6 levels of WUS patients are increased, which are related to the increase of infarct size and the aggravation of nerve defect, and can be used as auxiliary indicators for the diagnosis of WUS.
Keywords:Wake-Up Stroke, C-Reactive Protein, Interleukin-6, Degree of Nerve Defect, Volume of Infarction

Copyright © 2022 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
醒后卒中(wake-up stroke, WUS)是睡眠觉醒后由本人或他人发现的缺血性脑卒中,占所有缺血性脑卒中的1/4 [1],由于WUS发病时间不明确,大多数患者丧失静脉溶栓和机械取栓等再灌注治疗机会,导致其预后较差 [2]。WUS发病机制尚不清楚,现有研究普遍认为WUS与睡眠紊乱有关,阻塞性睡眠呼吸暂停患者罹患缺血性脑卒中的风险明显增加 [3]。而睡眠紊乱,尤其是阻塞性睡眠呼吸暂停可诱发全身炎症反应,导致血管内皮损伤以及功能障碍,促使动脉粥样硬化形成 [4],进而发生缺血性脑卒中。因此推测炎性因子可能与WUS存在一定关系,C-反应蛋白(C-reactive protein, CRP)是一种急性炎症蛋白,在严重感染或主要组织损伤的急性反应期间其浓度迅速增加,CRP被认为是血管疾病的主要介质,可与低密度脂蛋白结合于动脉粥样硬化斑块中,参与缺血性脑卒中发病过程 [5]。白介素-6 (Interleukin-6, IL-6)是神经系统中主要细胞因子,在神经损伤后浓度明显增高,与缺血性脑卒中发病和临床结局有关 [6]。CRP、IL-6是否可以作为WUS的生物学标志物尚不清楚,鉴于此,本研究拟探讨血清CRP、IL-6水平与WUS发病以及病情的关系,以期为临床诊治提供参考。
2. 资料与方法
2.1. 临床资料
选择2020年1月至2022年6月我院神经内科收治的153例WUS患者(WUS组),纳入标准:① 根据病史、症状体征、神经系统检查以及颅脑影像检查初次诊断为缺血性脑卒中,符合《中国急性缺血性脑卒中诊治指南2018》诊断流程和标准 [7];② 醒后发病;③ 年龄18周岁上,家属均知情同意本研究。排除标准:① 日间或非入睡期间发生的缺血性脑卒中;② 脑出血,脑外伤;③ 颅脑肿瘤,脑血管畸形;④ 近6个月内颅内手术或介入治疗史;⑤ 合并其它部位恶性肿瘤、感染、免疫性疾病患者。根据入院24 h评估的美国国立卫生研究院卒中量表(national institutes of health stroke scale, NIHSS)评分将患者分为轻度亚组(NIHSS评分 < 6分,43例),中度亚组(6分 ≤ NIHSS评分 < 13分,69例),重度亚组(NIHSS评分 ≥ 14分,41例) [8],根据入院CT/MRI扫描结果结合Adama分型标准分为大梗死亚组(脑梗死区直径 > 310 mm,35例)、小梗死亚组(直径115~310 mm,80例)、腔隙性梗死亚组(直径 < 115 mm,38例) [9]。另选择体检中心接诊的72例体检志愿者为对照组,对照组和实验组在一般临床资料上无差异,且均排除心脑血管疾病,感染或自身免疫性疾病。本研究已经获得我院伦理委员会批准。
2.2. 实验室检测
WUS组入院当日(健康者体检当日)采集静脉血完善实验室检查。血标本处理:取干燥试管标本中血液凝固后上清液离心(上海迈皋科学仪器有限公司M16RS高速离心机,参数:3000 r/min,离心半径10 cm,离心时间5 min)后取血清−20℃保存备检。抗凝试管混匀后上机检测。Aristo特定蛋白分析仪(深圳国赛生物技术有限公司)检测血清CRP水平,试剂盒购自上海酶联生物科技有效公司。Kayto RT-6000酶标仪(深圳雷杜公司)运用双抗体夹心免疫层析法测定血清IL-6水平,试剂盒购自北京博奥生物技术公司。BS-280全自动生化分析仪(深圳迈瑞公司)检测空腹血糖(fasting plasma glucose, FPG)、甘油三酯(triglycerides, TG)、总胆固醇(total cholesterol, TC)水平。西门子ADVIA 2120全自动血细胞分析仪检测白细胞(white blood cells, WBC)、血小板(platelet, PLT)计数。
2.3. 临床资料收集
收集受试者年龄、性别、体质量指数、吸烟史、饮酒史、基础疾病、是否合并阻塞性睡眠呼吸暂停综合征、入院收缩压和舒张压、实验室指标;以及基础疾病情况,包括:高血压、2-糖尿病、高脂血症、高同型半胱氨酸血症、慢阻肺、冠心病、颈动脉狭窄、心房颤动等。
2.4. 统计学分析
SPSS 25.00进行数据分析,Shapiro-Wilk检验计量资料符合正态分布以( )表示,采用独立样本t检验。以率(%)表示计数资料采用c2检验。多因素Logistic回归分析WUS发病危险因素,受试者工作特征曲线(Receiver operator characteristics curve, ROC)分析CRP、IL-6诊断WUS的价值,检验水准α = 0.05。
3. 结果
3.1. 基线资料以及实验室指标比较
WUS组高血压比例、糖尿病比例、高脂血症比例、阻塞性睡眠呼吸暂停综合征比例、收缩压、舒张压、WBC、TG、TC、FPG、CRP、IL-6水平均高于对照组(P < 0.05),其它比较差异无统计学意义(P > 0.05),见表1。
Table 1. Comparison of baseline data and laboratory indicators [ /cases (%)]
表1. 基线资料以及实验室指标比较[( )/例(%)]
3.2. 不同病情血清CRP、IL-6水平比较
不同神经缺损程度、不同梗死大小WUS患者血清CRP、IL-6水平差异显著(P < 0.05),重度亚组、中度亚组患者血清CRP、IL-6水平高于轻度亚组(P < 0.05),中度亚组患者血清CRP、IL-6水平高于轻度亚组(P < 0.05)。大梗死亚组、小梗死亚组患者血清CRP、IL-6水平高于腔隙性梗死亚组(P < 0.05),小梗死亚组患者血清CRP、IL-6水平高于腔隙性梗死亚组(P < 0.05),见表2。
Table 2. Serum CRP and IL-6 levels in different patients ( )
表2. 不同病情血清CRP、IL-6水平差异( )
注:与轻度亚组/腔隙性梗死亚组比较,aP < 0.05,与中度亚组/小梗死亚组比较,bP < 0.05。
3.3. 影响WUS发病的危险因素分析
以是否发生WUS为因变量(赋值:0 = 否,1 = 是),以高血压、糖尿病、高脂血症、阻塞性睡眠呼吸暂停综合征、收缩压、舒张压、WBC、TG、TC、FPG、CRP、IL-6为自变量(赋值见表3),ENTER法筛选变量,最终阻塞性睡眠呼吸暂停综合征,高水平CRP、高水平IL-6是WUS的危险因素(P < 0.05),见表4。
Table 3. Assignment
表3. 赋值
Table 4. The Logistic regression equations affecting the onset of WUS
表4. 影响WUS发病的Logistic回归方程
3.4. CRP、IL-6诊断WUS的价值分析
CRP、IL-6诊断WUS的曲线下面积为0.743、0.683,联合CRP、IL-6诊断WUS的曲线下面积为0.895,高于单独CRP、IL-6 (z = 4.288、5.022,P = 0.000、0.000),见表5和图1。
Table 5. Efficacy of CRP and IL-6 in the diagnosis of WUS
表5. CRP、IL-6诊断WUS的效能
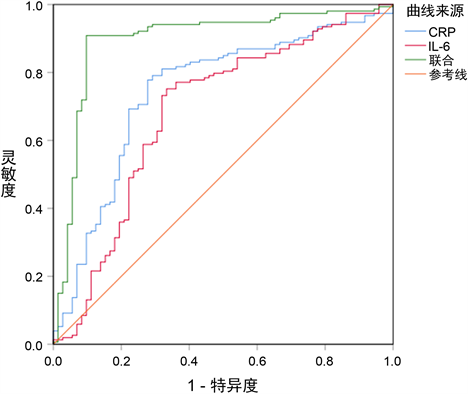
Figure 1. ROC diagram of CRP and IL-6 diagnostic of WUS
图1. CRP、IL-6诊断WUS的ROC图
4. 讨论
脑卒中是全球第二大死亡原因,也是导致长期残疾的第一大原因 [10],睡眠呼吸暂停是脑卒中的独立危险因素,增加约两倍的脑卒中发病风险 [11]。与日间脑卒中相比,WUS患者伴重度睡眠呼吸暂停的比例明显增高 [12],患有睡眠呼吸暂停且合并低氧血症的患者更容易在睡眠觉醒后发生脑卒中 [13]。炎症反应是缺血性脑卒中发病的主要病理基础,炎症介质合成和释放可促使血小板活化,趋化因子激活,细胞间黏附分子表达,并粘附迁移于血管内皮表面,导致管壁血栓和动脉粥样硬化斑块形成,造成血管内堵塞 [14]。炎症反应与睡眠呼吸暂停也存在密切关系,夜间反复呼吸暂停可引起慢性缺氧和高碳酸血症,引起氧化应激反应和炎症细胞因子激活,引发血管内皮细胞损伤和功能障碍 [15],诱发脑血管疾病。由此可见,炎症反应可能参与WUS发病机制,与其发生和病情存在密切关系。
CRP是一种五聚体蛋白,由五个相同的非共价结合亚基组成,以Ca2+依赖性方式与PCh结合激活经典的补体途径,参与免疫和炎症反应过程 [16]。CRP是人类先天宿主防御机制的主要组成部分,在严重感染或炎症状态下其浓度可能增加1000倍以上 [16]。动脉粥样硬化始于血管壁的脂质浸润、内皮功能障碍和慢性低度炎症,CRP也参与其中,在白细胞介素(IL)-6、IL-1β和肿瘤坏死因子(TNF)促炎作用下CRP在肝脏和脂肪组织中大量合成,通过激活补体系统诱导细胞凋亡、血管细胞活化、白细胞募集、脂质积累、血小板聚集,最终形成动脉粥样硬化斑块以及血栓 [17]。CRP被认为是缺血性脑卒中的生物标志物,CRP水平升高与是缺血性脑卒中较差的临床结果相关 [18]。睡眠呼吸暂停患者唾液中CRP水平也明显升高 [19],CRP参与阻塞性睡眠呼吸暂停综合征介导的炎症反应、脂质代谢异常和内皮功能障碍过程 [20]。本研究发现CRP水平在WUS患者血清中明显升高,是与WUS发病的危险因素,且与WUS神经缺损严重程度,脑梗死面积均有关,表明CRP同样参与WUS发病过程。推测可能的机制为,CRP诱发的慢性炎症反应导致持续的动脉粥样硬化进程,同时受夜间呼吸暂停影响,血氧含量降低,血液碳酸含量增加,加剧炎症反应和血管内皮功能损伤,导致脑血管收缩和舒张功能,并兴奋交感神经,引起血压和心率增加 [21],最终在多种因素作用下促使WUS的发生。
IL-6是一种用于维持体内平衡的原型细胞因子,在自分泌、旁分泌和内分泌信号传导中发挥重要作用,参与肝脏急性期反应的调节、B细胞刺激、调节T细胞和效应T细胞间平衡调节、代谢以及神经功能调节等多种生理过程 [22]。正常情况下血液中IL-6水平非常低,但在感染、炎症或组织损伤状态下迅速产生,并通过刺激急性期反应,激活免疫反应促进宿主防御,IL-6过度持续合成可引起急性全身炎症反应综合征和自身免疫疾病 [23]。已知CRP是动脉粥样硬化血栓形成的下游生物标志物,而IL-6则是动脉粥样硬化的上游标志物,IL-6作为“二级信使”细胞因子,可促使肝脏产生CRP,还可直接参与动脉粥样硬化斑块形成和不稳定过程 [24]。缺血性脑卒中血清IL-6水平升高,且与脑梗死体积以及神经功能损伤程度呈正相关 [25],IL-6在阻塞性睡眠呼吸暂停综合征患者中同样升高,参与低度慢性炎症反应和睡眠呼吸障碍过程 [26]。本研究发现IL-6与WUS也存在密切关系,IL-6水平升高是WUS的危险因素,并可引起脑梗死体积增加和神经缺损加重。分析可能的机制为:缺血性脑卒中后,野生型tPA与脂蛋白相关受体或NMDA受体结合加剧血管收缩,上调IL-6水平,进一步加剧脑血管损伤和脑组织病理损害 [27]。
ROC分析结果显示CRP、IL-6诊断WUS均具有较高的价值,而联合两项指标后诊断效能明显提高,说明同时检测血清CRP、IL-6水平,综合参考两项指标可为WUS诊断提供更可靠的信息。
本研究局限性:本研究未能根据WUS患者前循环、后循环情况进行细化分析与对比,造成结果偏倚,今后可增加样本量对不同类型脑梗死患者的影响因素进行分析。
综上,WUS患者血清CRP、IL-6水平均明显升高,CRP、IL-6水平升高是WUS的危险因素,且与神经缺损程度加重以及脑梗死体积增加有关,联合CRP、IL-6可更有效地诊断WUS。
文章引用
潘树超,张婷婷,翟迎凯,游振宁,汪元浚. C-反应蛋白、白介素-6在醒后卒中的研判价值
The Diagnostic Value of C-Reactive Protein and Interleukin-6 in Post-Wake Stroke[J]. 临床医学进展, 2022, 12(07): 6455-6463. https://doi.org/10.12677/ACM.2022.127931
参考文献
- 1. Wiśniewski, A. (2021) Safety and Efficacy of Intravenous Thrombolytic Treatment in Wake-Up Stroke: Experiences from a Single Center. Brain and Behavior, 11, e02152. https://doi.org/10.1002/brb3.2152
- 2. 黄文立. WUS经机械取栓1例并文献复习[J]. 中华全科医学, 2019, 17(4): 702-704.
- 3. Kim, J.S., Kim, S., Lee, S.H., et al. (2018) Increased Risk of Ischemic Stroke during Sleep in Apneic Patients. Journal of Clinical Neurology, 14, 174-178. https://doi.org/10.3988/jcn.2018.14.2.174
- 4. Orrù, G., Storari, M., Scano, A., et al. (2020) Obstructive Sleep Apnea, Oxidative Stress, Inflammation and Endothelial Dysfunction—An Overview of Predictive Laboratory Bi-omarkers. European Review for Medical and Pharmacological Sciences, 24, 6939-6948.
- 5. McCabe, J.J., O’Reilly, E., Coveney, S., et al. (2021) Interleukin-6, C-Reactive Protein, Fibrinogen, and Risk of Recurrence after Ischaemic Stroke: Systematic Review and Meta-Analysis. The European Stroke Journal, 6, 62-71. https://doi.org/10.1177/2396987320984003
- 6. Georgakis, M.K., Malik, R., Gill, D., et al. (2020) Interleukin-6 Signaling Effects on Ischemic Stroke and Other Cardiovascular Outcomes: A Mendelian Randomization Study. Circula-tion: Genomic and Precision Medicine, 13, e002872. https://doi.org/10.1101/19007682
- 7. 中华医学会神经病学分会, 中华医学会神经病学分会脑血管病学组. 中国急性缺血性脑卒中诊治指南2018 [J]. 中华神经科杂志, 2018, 51(9): 666-682.
- 8. Kwah, L.K. and Diong, J. (2014) National Institutes of Health Stroke Scale (NIHSS). Journal of Physiotherapy, 60, 61. https://doi.org/10.1016/j.jphys.2013.12.012
- 9. Adams, H.P., Bendixen, B.H., Kappelle, L.J., et al. (1993) Clas-sification of Subtype of Acute Ischemic Stroke. Definitions for Use in a Multicenter Clinical Trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke, 24, 35-41. https://doi.org/10.1161/01.STR.24.1.35
- 10. Herpich, F. and Rincon, F. (2020) Management of Acute Ischemic Stroke. Critical Care Medicine, 48, 1654-1663. https://doi.org/10.1097/CCM.0000000000004597
- 11. McDermott, M., Brown, D.L. and Chervin, R.D. (2018) Sleep Disorders and the Risk of Stroke. Expert Review of Neurotherapeutics, 18, 523-531. https://doi.org/10.1080/14737175.2018.1489239
- 12. Khot, S.P. and Morgenstern, L.B. (2019) Sleep and Stroke. Stroke, 50, 1612-1617. https://doi.org/10.1161/STROKEAHA.118.023553
- 13. Otto-Yáñez, M., Torres-Castro, R., Nieto-Pino, J., et al. (2018) Obstructive Sleep Apnea-Hypopnea and Stroke. Medicina (B Aires), 78, 427-435.
- 14. Hasumi, K. and Suzuki, E. (2021) Impact of SMTP Targeting Plasminogen and Soluble Epoxide Hydrolase on Thrombolysis, Inflammation, and Ischemic Stroke. International Journal of Molecular Sciences, 22, 954. https://doi.org/10.3390/ijms22020954
- 15. Liu, X., Ma, Y., Ouyang, R., et al. (2020) The Relationship between In-flammation and Neurocognitive Dysfunction in Obstructive Sleep Apnea Syndrome. Journal of Neuroinflammation, 17, 229. https://doi.org/10.1186/s12974-020-01905-2
- 16. Moutachakkir, M., Lamrani Hanchi, A., Baraou, A., et al. (2017) Immunoanalytical Characteristics of C-Reactive Protein and High Sensitivity C-Reactive Protein. Annales de Biologie Clinique (Paris), 75, 225-229. https://doi.org/10.1684/abc.2017.1232
- 17. Moriya, J. (2019) Critical Roles of Inflammation in Atherosclerosis. Journal of Cardiology, 73, 22-27. https://doi.org/10.1016/j.jjcc.2018.05.010
- 18. Mazaheri, S., Reisi, E., Poorolajal, J., et al. (2018) C-Reactive Pro-tein Levels and Clinical Outcomes in Stroke Patients: A Prospective Cohort Study. Archives of Iranian Medicine, 21, 8-12.
- 19. Chen, V.G., Fonseca, V.M.G.D., Amaral, J.B., et al. (2020) Inflammatory Markers in Palatine Tonsils of Children with Obstructive Sleep Apnea Syndrome. Brazilian Journal of Otorhinolaryngology, 86, 23-29. https://doi.org/10.1016/j.bjorl.2018.08.001
- 20. Aydin, Ş., Özdemir, C., Küçükali, C.I., et al. (2018) Reduced Pe-ripheral Blood Mononuclear Cell ROCK1 and ROCK2 Levels in Obstructive Sleep Apnea Syndrome. In Vivo, 32, 319-325. https://doi.org/10.21873/invivo.11240
- 21. Hou, Y., Shang, Y., Cheng, R., et al. (2014) Obstructive Sleep Apnea-Hypopnea Results in Significant Variations in Cerebral Hemodynamics Detected by Diffuse Optical Spec-troscopies. Physiological Measurement, 35, 2135⁃2148. https://doi.org/10.1088/0967-3334/35/10/2135
- 22. Rose-John, S. (2018) Interleukin-6 Family Cytokines. Cold Spring Harbor Perspectives in Biology, 10, a028415. https://doi.org/10.1101/cshperspect.a028415
- 23. Tanaka, T., Narazaki, M. and Kishimoto, T. (2018) Interleukin (IL-6) Immunotherapy. Cold Spring Harbor Perspectives in Biology, 10, a028456. https://doi.org/10.1101/cshperspect.a028456
- 24. Tyrrell, D.J. and Goldstein, D.R. (2021) Ageing and Atheroscle-rosis: Vascular Intrinsic and Extrinsic Factors and Potential Role of IL-6. Nature Reviews Cardiology, 18, 58-68. https://doi.org/10.1038/s41569-020-0431-7
- 25. Zhang, J., Wu, Y. and Gao, Z. (2019) Correlations of C-Reactive Protein (CRP), Interleukin-6 (IL-6), and Insulin Resistance with Cerebral Infarction in Hypertensive Patients. Medical Science Monitor, 25, 1506-1511. https://doi.org/10.12659/MSM.912898
- 26. Kheirandish-Gozal, L. and Gozal, D. (2019) Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. International Journal of Molecular Sciences, 20, 459. https://doi.org/10.3390/ijms20030459
- 27. Armstead, W.M., Hekierski, H., Pastor, P., et al. (2019) Release of IL-6 after Stroke Contributes to Impaired Cerebral Autoregulation and Hippocampal Neuronal Necrosis through NMDA Re-ceptor Activation and Upregulation of ET-1 and JNK. Translational Stroke Research, 10, 104-111. https://doi.org/10.1007/s12975-018-0617-z
NOTES
*通讯作者。
