Material Sciences
Vol.
10
No.
06
(
2020
), Article ID:
36236
,
11
pages
10.12677/MS.2020.106060
Different Confined Noble-Metal Nanoparticles Catalysts on Porous g-C3N4 and Enhanced Catalytic Activity
Min Deng, Fengyu Tian, Dongfang Hou*, Dongsheng Li
Key Laboratory of Inorganic Nonmetallic Crystalline and Energy Conversion Materials, Hubei Provincial Collaborative Innovation Center for New Energy Microgrid, College of Materials and Chemical Engineering, China Three Gorges University, Yichang Hubei

Received: Jun. 3rd, 2020; accepted: Jun. 17th, 2020; published: Jun. 24th, 2020

ABSTRACT
Noble-metal nanoparticles (Au, Ag, Pd, Pt) with the same nominal loading amounts were immobilized on porous g-C3N4 (pg-C3N4) using a facile photochemical reduction route. The as-prepared M@pg-C3N4 (M = Au, Ag, Pd, Pt) nanocomposites were characterized by XRD, SEM, TEM, XPS, FT-IR and N2 physisorption measurements. The results indicated that the noble-metal nanoparticles were successfully grown on the pg-C3N4. The catalytic activities of the M@pg-C3N4 composites to the reduction of 4-nitrophenol (4-NP) with KBH4 were tracked by UV-visible spectroscopy. It was found that the M@pg-C3N4 nanocomposites exhibited enhanced catalytic performance toward the reduction of 4-NP. In particular, the highest rate constant reaches 1.386 min−1 in the dark over the Au@pg-C3N4, which exceed the catalytic activity of M@pg-C3N4 (M = Ag, Pd and Pt). It attributed to the integrative confining effect of porous structure of g-C3N4 and the super electron conductive properties of noble-metal nanoparticles.
Keywords:Noble-Metal Nanoparticles, Porous g-C3N4, 4-NP Reduction, Catalytic Activity

多孔g-C3N4负载不同受限贵金属纳米粒子及其催化活性研究
邓敏,田逢雨,侯东芳*,李东升
三峡大学材料与化工学院,湖北省新能源微电网协同创新中心,无机非金属晶态及能量转换材料重点实验室,湖北 宜昌

收稿日期:2020年6月3日;录用日期:2020年6月17日;发布日期:2020年6月24日

摘 要
通过简单的光化学还原方法,得到一系列具有相同负载量的贵金属(Au, Ag, Pd, Pt)纳米颗粒@多孔g-C3N4纳米复合材料。利用XRD、SEM、TEM、XPS、FT-IR和N2物理吸附等手段对样品的微观结构、形貌等性能进行了表征,并评价了M@pg-C3N4纳米复合材料对KBH4还原4-硝基苯酚(4-NP)的催化活性。其结果表明,贵金属纳米颗粒均匀负载在pg-C3N4上,与纯pg-C3N4相比,所有M@pg-C3N4纳米复合材料对4-NP催化还原性能明显增强。其中,Au@pg-C3N4的最高速率常数达到1.386 min−1,优于M@pg-C3N4的催化活性(M = Ag,Pd和Pt)。这归因于g-C3N4的多孔结构对不同贵金属纳米颗粒的整体限域效应和贵金属纳米粒子的优良的电子传输性能。本文为贵金属基复合催化剂的选择和应用提供了一定的实验依据。
关键词 :贵金属纳米颗粒,多孔g-C3N4,4-NP还原,催化活性

Copyright © 2020 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
在农药、医药和染料等工业制造过程中,4-硝基苯酚(4-NP)是一种经常使用的化工原料,由于硝基基团的存在不易降解且具有生物耐受性,在废液中被认为是一种有害污染物 [1] [2] [3]。在催化剂存在下利用硼氢化物还原4-NP可生成毒性较小的4-氨基苯酚(4-AP),是一种有效的去除4-NP毒性的方法 [4] [5] [6]。而且,对氨基苯酚是目前应用较广泛的一种精细有机化工中间体,在制药工业中得到了广泛利用,例如制造止痛药和退热药等过程 [7] [8] [9]。因此,直接还原4-NP不仅可以降低其对环境的毒害作用,还可以产生有用的4-AP满足市场的巨大需求,这是非常有意义的。
目前,很多研究者们开发了报道了用于4-NP还原的多种催化剂 [5] [6] [7] [10]。其中,贵金属纳米颗粒(Au,Ag,Pt等)由于其优良的光电特性在催化领域得到了广泛应用 [11] [12] [13] [14]。无疑贵金属纳米颗粒(NPs)也已成为4-NP还原反应中最常见的有效催化剂 [15] [16] [17]。尽管贵金属NPs具有较好的催化性能,但由于表面能高,它们在生长过程中通常会发生严重的聚集,从而导致催化活性迅速下降而浪费稀有资源 [18] [19]。因此,人们设计了很多方法来解决这些问题。其中,利用合适的载体负载贵金属NPs的方法得到了研究者的注意 [20]。它不仅可以大大降低生产成本,而且由于载体材料本身的某些独特性能(如高的比表面积、机械强度和优异的电导率)可以进一步显著提高催化性能 [7] [21] [22] [23]。先前的一些研究也表明,合适的载体材料在4-NP还原过程中也发挥了非常积极的作用 [2] [8]。
近年来,二维(2D)材料在催化反应中的限域效应(物理或化学方面)引起了人们的广泛关注,因为它们会影响催化体系的形貌、电子结构、催化活性及其稳定性等 [24] [25] [26] [27]。众所周知,二维石墨相氮化碳(g-C3N4)在催化领域具有许多优势,包括无金属成分,良好的热稳定性,易于制备,完美的耐酸碱性能等 [28] [29] [30]。同时,g-C3N4也被证明是一种负载金属NPs有前途的载体材料,因为g-C3N4的重复三嗪单元可以提供大量的结合位点,这些结合位点可以用来稳定金属NPs [31] [32]。值得注意的是,作为聚合物的g-C3N4可以构筑许多特殊的结构而具有不同的功能,如一维纳米结构 [33]、多孔g-C3N4 [34] 和空心球 [35] 等。多孔g-C3N4(pg-C3N4)可以作为金属NPs负载合适的载体,因为其多孔结构具有更大的比表面积并有利于质量传输 [36] 从而促进催化反应的进行。更有趣的是,在多孔g-C3N4载体中也观察到了限域效应,界面间会形成新的电子态,最终主导催化性能 [25]。一些研究结果也表明,构建贵金属负载的g-C3N4复合材料是增强4-NP还原性能的行之有效的方法之一 [17] [31] [32] [37]。但是,很少有报道比较相同含量不同贵金属/g-C3N4复合催化剂对4-NP的还原性能。特别是多孔g-C3N4对不同的贵金属NPs呈现出不同的限域效应,从而影响对催化活性增强机理的理解。
基于此,本实验采用一种简单的光化学途径合成一系列分散良好的M@pg-C3N4 (M = Au, Ag, Pd, Pt)纳米复合材料。研究了其在黑暗中还原4-NP为4-AP的产物的催化活性。结果表明,负载Au NPs的pg-C3N4表现出较好的催化性能,表观速率常数为1.386 min−1。基于结构性能表征,进一步探讨了催化活性提高的作用机制以及相同负载量的不同贵金属的影响。
2. 实验部分
2.1. pg-C3N4制备
使用的所有化学药品均为分析级试剂,无需进一步纯化。通过使三聚氰胺与盐酸反应合成pg-C3N4。具体过程为,将三聚氰胺溶解于热蒸馏水中,并滴加适量的盐酸。搅拌30分钟后,将溶液转移到80℃烘箱中蒸干水份。最后,将产物置于有盖的坩埚中,在550℃马弗炉中加热2小时,加热速率为10℃ min−1,冷却收集样品。
2.2. 合成M@pg-C3N4纳米复合物(M = Au,Ag,Pd,Pt)
本制备了贵金属含量为4 wt%的M@pg-C3N4(M = Au,Ag,Pd,Pt)复合催化剂。首先将100 mg制备好的pg-C3N4超声分散于20 mL去离子水中,持续30分钟。然后,将一定量的HAuCl4水溶液缓慢加入上述悬浮液中,并搅拌30分钟。在搅拌下用汞灯(500 W)将悬浮液照射3小时,将得到的产物抽滤,并用去离子水洗涤3次,经80℃烘干,制得Au@pg-C3N4复合催化剂。按照类似的步骤合成了其他M@pg-C3N4(M = Ag,Pd,Pt)复合催化剂,而HAuCl4换为AgNO3,H2PdCl4和H2PtCl6。为了进行比较,对pg-C3N4进行了相同的处理,但未添加任何溶液。
2.3. 材料表征
用X射线衍射(XRD, Rigaku Ultima IV)和Cu Kα (λ = 0.15418 nm)表征了产物的晶体结构和物相。用X射线光电子能谱(XPS, PHI 5000)分析了样品的表面原子状态。用场发射扫描电镜(FE-SEM, JEOLJSM-7500F)和透射电镜(TEM, JEOLJEM-2100F)对样品的形貌和结构进行了表征。用Brunauer-Emmett-Teller (BET)方法得到了比表面积,并用Barret-Joyner-Halender (BJH)方法计算了孔径分布。
2.4. 催化活性测试
通过将贵金属M@pg-C3N4 (M = Au,Ag,Pd和Pt)纳米复合物应用于室温下对4-NP的催化还原来评价样品的活性性能。配制浓度为25 mg·L−1的4-NP溶液,取3 mL加入石英比色皿中,再加入3.0 mg KBH4,一定时间间隔后通过紫外分光光度计检测4-NP的浓度随时间的变化,直至反应终点。
3. 结果与讨论
3.1. 表征分析
pg-C3N4和M@pg-C3N4(M = Au,Ag,Pd,Pt)的XRD谱图如图1所示。从图中可以看出,纯pg-C3N4谱图显示位于27.4˚处出现一特征峰,对应于g-C3N4的(002)晶面,并且另一小峰在13.0˚处对应于(100)晶面。与pg-C3N4相比,Au@pg-C3N4样品,分别在38.1˚,44.3˚,64.6˚和77.6˚处观察到四个附加峰,分别归属于Au(111),(200),(220)和(311)晶面。对于Ag@ pg-C3N4复合材料,在38.1˚,44.2˚,64.4˚和77.3˚处出现四个弱峰,分别对应于Ag的(111),(200),(220)和(311)晶面。这些结果表明,光还原反应后,Ag和Au纳米颗粒成功地负载在pg-C3N4上。此外,在M@pg-C3N4中未观察到Pd和Pt的特征衍射。该结果可能是由于pg-C3N4表面上的Pd和Pt NPs的尺寸较小。XRD分析结果也显示出pg-C3N4对各种贵金属还原晶体生长的不同限制作用。
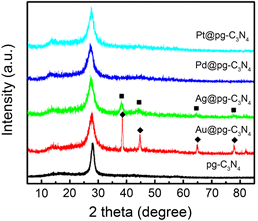
Figure 1. XRD patterns of pg-C3N4 and M@pg-C3N4 (M = Au, Ag, Pd, Pt). The peaks marked with ■ and ♦ belong to Au and Ag, respectively
图1. pg-C3N4和 M@pg-C3N4 (M = Au, Ag, Pd, Pt)样品的XRD谱图。■和♦分别对应于金属Au和Ag的XRD特征峰
利用FE-SEM初步研究了所有样品的典型微观形貌。图2所示为经盐酸处理的三聚氰胺热聚合合成的具有堆积褶皱的pg-C3N4负载贵金属Au、Ag、Pd和Pt的复合催化剂。可以看出,四个样品形貌相似,所有样品中没有观察到明显的贵金属NPs,这可能归因于较小的颗粒尺寸。
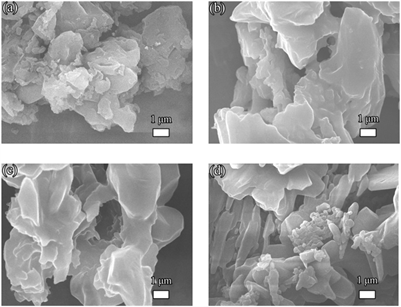
Figure 2. SEM images of the (a) Au@pg-C3N4, (b) Ag@pg-C3N4, (c) Pd@pg-C3N4, (d) Pt@pg-C3N4
图2. (a) Au@pg-C3N4、(b) Ag@pg-C3N4、(c) Pd@pg-C3N4和(d) Pt@pg-C3N4的SEM照片
为了更详细地研究基于pg-C3N4的纳米复合材料的微观结构,进行了TEM和高分辨透射电子显微镜(HRTEM)观察。如图3所示,可以清楚地看到pg-C3N4载体具有明显的多孔结构,并且可见贵金属NPs附着在pg-C3N4上(图3(a)、图3(d)、图3(g)和图3(j))。HRTEM图像也(插入TEM图像)清楚地显示出Au、Ag、Pd和Pt NP的特征晶格条纹分别为0.231、0.230、0.226和0.223 nm,对应于各自贵金属的(111)晶面 [38]。同时进行EDS分析以确认M@pg-C3N4的元素组成。图3(b)、图3(e)、图3(h)和图3(k)中的结果表明,M@pg-C3N4分别含有C、N和Au/Ag/Pd/Pt。图3(c),图3(f),图3(i)和图3(l)给出相应的元素mapping,显示C、N和Au/Ag/Pd/Pt的各元素分布。显然,C和N的分布是均匀且连续的,类似于pg-C3N4载体的形态。而元素Au (图3(c4))和Ag (图3(f4))呈现非均匀分布形态,表明Au纳米颗粒在pg-C3N4表面上有轻微的团聚。与Au和Ag相比,Pd (图3(i4))和Pt (图3(l4))的元素mapping图显示,Pd和Pt NPs的分布是弥散的,表明NPs在pg-C3N4的表面上分散良好。此结果与XRD结果一致。有趣的是,尽管M @ pg-C3N4纳米复合物(M = Au,Ag,Pd,Pt)的制备方法相似,但我们也可以看到这些贵金属的粒径彼此之间存在很大差异,这意味着pg-C3N4对不同的贵金属纳米粒子的限域效应存在差别。

Figure 3. TEM, HRTEM, EDS and element mapping images of the M@pg-C3N4 (M = Au, Ag, Pd, Pt)
图3. M@pg-C3N4 (M = Au, Ag, Pd, Pt)样品的TEM、HRTEM、EDS和元素mapping照片
图4为纯pg-C3N4和M@ pg-C3N4复合材料的红外光谱图(FT-IR)。810 cm−1处的特征峰对应于三嗪单元的振动模式。1200~1700 cm−1范围内出现的多峰,归属于C-N的典型伸缩振动 [13] [17]。M @ pg-C3N4的FT-IR光谱(M = Au,,Ag,Pd,Pt)在特征谱图中与pg-C3N4类似,这表明在负载Au、Ag、Pd和Pt NPs后,所有样品的pg-C3N4典型石墨相结构保持了很好的稳定性。
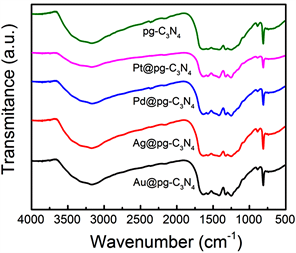
Figure 4. FT-IR spectra of pg-C3N4 and M@pg-C3N4 (M = Au, Ag, Pd, Pt) samples
图4. pg-C3N4和 M@pg-C3N4 (M = Au, Ag, Pd, Pt)样品的红外谱图
通过X射线光电子能谱(XPS)分析研究M@pg-C3N4复合材料的表面元素组成和化学状态。结合能的数据根据C1s(284.6 eV)进行了较准。图5对应于不同贵金属Au、Ag、Pd和Pt的高分辨XPS图谱。图5(a)是Au4f的高分辨XPS图谱,从图中看出结合能位于87.4和83.6 eV处的峰分别对应着Au4f5/2和Au4f7/2,表明pg-C3N4上Au的存在状态是金属Au0 [22]。类似地,可以将Ag 3d (图5(b))中结合能为373.8(Ag 3d3/2)和367.8 eV(Ag 3d5/2)的两峰对应于Ag0 [8];Pd 3d (图5(c))中的342.9(Pd 3d3/2)和337.5 eV(Pd 3d5/2)的特征峰对应于Pd0 [2];Pt 4f (图5(d))谱图中75.5和72.4 eV的两个特征峰,分别对应金属Pt0的Pt 4f5/2和Pt 4f7/2的结合能 [13]。XPS的结果进一步证明了M@pg-C3N4复合材料中贵金属的存在,这也与EDS结果相符。

Figure 5. XPS spectra of M@pg-C3N4 (M = Au, Ag, Pd, Pt)
图5. M@pg-C3N4 (M = Au, Ag, Pd, Pt)样品的XPS谱图
XRD,TEM和XPS结果证实,pg-C3N4表面的贵金属(Au,Ag,Pd,Pt)NPs以金属形式存在,没有明显的氧化发生。利用N2的吸附-脱吸等温线和BJH孔径分布曲线,进一了解M@pg-C3N4的表面及内部结构性质。如图6所示,所有样品的的等温曲线为IV型(BDDT分类),其相对压力P/P0在0.4~1.0范围内有明显的滞回环,显示了所制备M@pg-C3N4具有介孔结构。相应的孔径分布也证实了这种多孔结构(图6的插图),为反应物的表面迁移提供了更多传质路径。对于M@pg-C3N4(M = Au,Ag,Pd,Pt),BET比表面积经计算分别为13.5、16.1、14.1和12.6 m2∙g−1,比表面积的变化可能与不同贵金属颗粒的尺寸及存在状态有关系,有些pg-C3N4孔会被负载的贵金属NPs堵塞,使得比表面积略有下降。
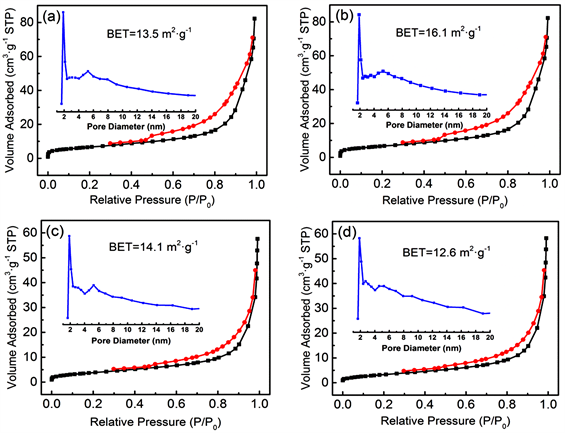
Figure 6. Pore size distribution curves and the corresponding nitrogen adsorption-desorption isotherms of M@pg-C3N4 (M = Au, Ag, Pd, Pt), respectively
图6. M@pg-C3N4 (M = Au, Ag, Pd, Pt)样品的孔径分布曲线及相应的氮气吸附–脱吸等温线
3.2. 催化活性评价
M@pg-C3N4(M = Au,Ag,Pd和Pt)纳米复合材料的催化性能通过在KBH4存在下将4-NP还原为4-AP来评价。众所周知,在中性或酸性条件下,4-NP溶液的最大吸收波长位于317 nm处,用KBH4处理后峰移至400 nm,揭示了碱性介质中对硝基酚盐的形成 [7] [15]。图7显示了加入M@pg-C3N4催化剂之后反应溶液的紫外-可见光谱随时间的变化规律,也揭示了4-NP还原为4-AP的过程。从图中可以看出,所有样品均对4-AP的还原具有明显的催化效果。如图7(a)所示在Au@pg-C3N4 NPs的作用下,400 nm处的峰迅速减小,而300 nm处的4-AP典型峰出现,并随时间增加而增强。400 nm处的初始峰在3分钟内消失,表明4-NP完全降解,这也可以由300 nm峰的稳定状态证实。对于Ag@pg-C3N4(图7(b)),还原反应需要6分钟反应物可以完全被反原为产物,在Pd@pg-C3N4(图7(c))作用下,完全转化需要7分钟。而对于Pt @ pg-C3N4(图7(d)),反应在13分钟后仍未完成。图7(e)和图7(f)绘出了C/C0和ln(C/C0)与反应时间的关系。可以看出,纯的pg-C3N4本身对4-NP的还原几乎没有催化活性,溶液浓度的轻微降低可能由于少量的吸附作用。值得注意的是,pg-C3N4负载AuNPs产生比Ag、Pd和Pt呈现最佳的催化性能。还原反应遵循一级动力学方程ln(C/C0) = −kt,其中t是反应时间,k是表观一级速率常数(min−1)。如图7(f)所示计算,也揭示了速率常数的变化,Au@pg-C3N4的最高速率常数为1.386 min−1。动力学速率常数遵循Au@pg-C3N4 > Ag @ pg-C3N4 > Pd @ pg-C3N4 > Pt @ pg-C3N4的顺序,可以看出在催化还原反应中,Au@pg-C3N4催化作用有着较明显的优势。这也表明,即使四种贵金属的含量相同,催化活性也高度依赖于贵金属的种类及其与载体pg-C3N4的相互作用。

Figure 7. Catalytic performance of M@pg-C3N4 composites. (a)-(d) UV-vis absorption spectra during the catalytic reduction of 4-NP; (e) C/C0 and (f) ln(C/C0) versus reaction time
图7. M@pg-C3N4样品的催化性能图 (a)-(d)即催化还原4-NP过程中的紫外可见吸收光谱,(e) 催化剂C/C0和(f) ln(C/C0)与t的催化速率图
3.3. 催化机制
催化剂被认为是电子给体BH4−和电子受体分子4-NP之间的电子转移媒介质,其电子转移效率和速率在催化还原过程中起着决定性的作用 [7]。Au@pg-C3N4纳米复合材料上的KBH4将4-NP催化还原为4-AP的机理如图8所示。负载在pg-C3N4上的贵金属Au NPs通过传递来自BH4−的电子给4-NP分子来传递催化过程,从而实现了还原反应。其中pg-C3N4的多孔结构有利于贵金属NPs的均匀生长和分布,并同时增强了对4-NP分子的有效吸附,这也有助于提高催化活性。
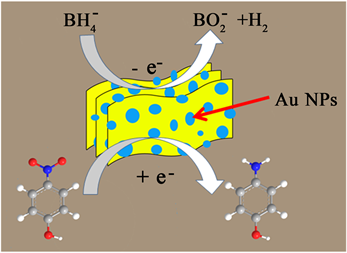
Figure 8. Schematic illustration of the mechanism for catalytic reduction of 4-NP to 4-AP by the Au@pg-C3N4
图8. Au@pg-C3N4样品催化4-NP还原为4-AP过程中的反应机理示意图
通常,贵金属NPs与载体材料之间的接触会在界面处形成肖特基势垒,这会使电子传输更加有效。在本工作中,贵金属NPs对催化活性几乎没有显示出明显的尺寸效应。因为Au NPs具有更大的尺寸,但在4-NP还原中表现出更加优异的活性,这不仅可以归因于Au的固有物理性质,还可以归因于作为4-NP还原的有效活性位点的比例较高 [39]。此外,在载体材料pg-C3N4的作用下,原有贵金属催化的反应能,机理和动力学都会发生一定程度的改变。此处的限域效应应该是物理和化学因素的共同结果,这些因素会很大程度影响催化性能。Au@pg-C3N4纳米复合材料优异的催化活性应该来源于为了进一步了解详细的机制,还需要进行更多更深入的研究工作。
4. 结论
论文本文通过一种简便的光化学方法成功地制备了一系列贵金属M@pg-C3N4 (M = Au, Ag, Pd, Pt)纳米复合材料,并将其应用于4-NP的还原反应中,考察不同贵金属负载对催化剂的催化活性的影响。与M@pg-C3N4 (M = Ag,Pd,Pt)相比,Au@pg-C3N4纳米复合材料表现出显著增强的催化性能,这归因于g-C3N4的多孔结构的整体限域效应和金纳米粒子优异的物理性能。此项工作可为贵金属基纳米复合催化剂的选择和应用提供思路和参考。
基金项目
这项工作得到了中国国家自然科学基金会(No.:51572152、21673127、21671119和21805165),教育部多金属氧酸盐科学重点实验室开放项目,湖北省111项目的资助(2019-8-1)以及教育部的111项目(D20015)。
文章引用
邓 敏,田逢雨,侯东芳,李东升. 多孔g-C3N4负载不同受限贵金属纳米粒子及其催化活性研究
Different Confined Noble-Metal Nanoparticles Catalysts on Porous g-C3N4 and Enhanced Catalytic Activity[J]. 材料科学, 2020, 10(06): 495-505. https://doi.org/10.12677/MS.2020.106060
参考文献
- 1. Zhang, J., Chen, G., Chaker, M., Rosei, F. and Ma, D. (2013) Gold Nanoparticle Decorated Ceria Nanotubes with Significantly High Catalytic Activity for the Reduction of Nitrophenol and Mechanism Study. Applied Catalysis B: Environmental, 132-133, 107-115. https://doi.org/10.1016/j.apcatb.2012.11.030
- 2. Sun, J., Fu, Y., He, G., Sun, X. and Wang, X. (2014) Catalytic Hydrogenation of Nitrophenols and Nitrotoluenes over a Palladium/Graphene Nanocomposite. Catalysis Science & Technology, 4, 1742-1748. https://doi.org/10.1039/C4CY00048J
- 3. Shen, W., Qu, Y., Pei, X., Li, S., You, S., Wang, J., Zhang, Z. and Zhou, J. (2017) Catalytic Reduction of 4-Nitro- phenol Using Gold Nanoparticles Biosynthesized by Cell-Free Extracts of Aspergillus sp. WL-Au. Journal of Hazardous Materials, 321, 299-306. https://doi.org/10.1016/j.jhazmat.2016.07.051
- 4. Ma, Y., Wu, X. and Zhang, G. (2017) Core-Shell Ag@Pt Nanoparticles Supported on Sepiolite Nanofibers for the Catalytic Reduction of Nitrophenols in Water: Enhanced Catalytic Performance and DFT Study. Applied Catalysis B: Environmental, 205, 262-270. https://doi.org/10.1016/j.apcatb.2016.12.025
- 5. Lu, H., Yin, H., Liu, Y., Jiang, T. and Yu, L. (2008) Influence of Support on Catalytic Activity of Ni Catalysts in p-Nitrophenol Hydrogenation to p-Aminophenol. Catalysis Communications, 10, 313-316. https://doi.org/10.1016/j.catcom.2008.09.015
- 6. Chen, R., Zhang, Q., Gu, Y., Tang, L., Li, C. and Zhang, Z. (2015) One-Pot Green Synthesis of Prussian Blue Nanocubes Decorated Reduced Graphene Oxide Using Mushroom Extract for Efficient 4-Nitrophenol Reduction. Analytica Chimica Acta, 853, 579-587. https://doi.org/10.1016/j.aca.2014.10.049
- 7. Zhang, P., Shao, C., Zhang, Z., Zhang, M., Mu, J., Guo, Z. and Liu, Y. (2011) In Situ Assembly of Well-Dispersed Ag Nanoparticles (Ag NPs) on Electrospun Carbon Nanofibers (CNFs) for Catalytic Reduction of 4-Nitrophenol. Nanoscale, 3, 3357-3363. https://doi.org/10.1039/c1nr10405e
- 8. Li, Y., Cao, Y., Xie, J., Jia, D., Qin, H. and Liang, Z. (2015) Facile Solid-State Synthesis of Ag/Graphene Oxide Nanocomposites as Highly Active and Stable Catalyst for the Reduction of 4-Nitrophenol. Catalysis Communications, 58, 21-25. https://doi.org/10.1016/j.catcom.2014.08.022
- 9. Feng, J., Su, L., Ma, Y., Ren, C., Guo, Q. and Chen, X. (2013) CuFe2O4 Magnetic Nanoparticles: A Simple and Efficient Catalyst for the Reduction of Nitrophenol. Chemical Engineering Journal, 221, 16-24. https://doi.org/10.1016/j.cej.2013.02.009
- 10. Dai, Y., Yu, P., Zhang, X. and Zhuo, R. (2016) Gold Nanoparticles Stabilized by Amphiphilic Hyperbranched Polymers for Catalytic Reduction of 4-Nitrophenol. Journal of Catalysis, 337, 65-71. https://doi.org/10.1016/j.jcat.2016.01.014
- 11. Herves, P., Perez-Lorenzo, M.L., Liz-Marzan, M., Dzubiella, J., Lu, Y. and Ballauff, M. (2012) Catalysis by Metallic Nanoparticles in Aqueous Solution: Model Reactions. Chemical Society Reviews, 41, 5577-5587. https://doi.org/10.1039/c2cs35029g
- 12. Zhang, J., Hou, C., Huang, H., Zhang, L., Jiang, Z., Chen, G., Jia, Y., Kuang, Q., Xie, Z. and Zheng, L. (2013) Surfactant-Concentration-Dependent Shape Evolution of Au-Pd Alloy Nanocrystals from Rhombic Dodecahedron to Trisoctahedron and Hexoctahedron. Small, 9, 538-544. https://doi.org/10.1002/smll.201202013
- 13. Gao, G., Jiao, Y., Waclawik, E.R. and Du, A. (2016) Single Atom (Pd/Pt) Supported on Graphitic Carbon Nitride as an Efficient Photocatalyst for Visible-Light Reduction of Carbon Dioxide. Journal of the American Chemical Society, 138, 6292-6297. https://doi.org/10.1021/jacs.6b02692
- 14. Bulushev, D.A., Zacharska, M., Lisitsyn, A.S., Podyacheva, O.Y., Hage, F.S., Ramasse, Q.M., Bangert, U. and Bulusheva, L.G. (2016) Single Atoms of Pt-Group Metals Stabilized by N-Doped Carbon Nanofibers for Efficient Hydrogen Production from Formic Acid. ACS Catalysis, 6, 3442-3451. https://doi.org/10.1021/acscatal.6b00476
- 15. Liao, G., Chen, J., Zeng, W., Yu, C., Yi, C. and Xu, Z. (2016) Facile Preparation of Uniform Nanocomposite Spheres with Loading Silver Nanoparticles on Polystyrene-Methyl Acrylic Acid Spheres for Catalytic Reduction of 4-Nitrophenol. The Journal of Physical Chemistry C, 120, 25935-25944. https://doi.org/10.1021/acs.jpcc.6b09356
- 16. Shi, Y., Zhang, X., Zhu, Y., Tan, H., Chen, X. and Lu, Z.H. (2016) Core-Shell Structured Nanocomposites Ag@CeO2 as Catalysts for Hydrogenation of 4-Nitrophenol and 2-Nitroaniline. RSC Advances, 6, 47966-47973. https://doi.org/10.1039/C6RA00631K
- 17. Fu, Y., Huang, T., Jia, B., Zhu, J. and Wang, X. (2017) Reduction of Nitrophenols to Aminophenols under Concerted Catalysis by Au/g-C3N4 Contact System. Applied Catalysis B: Environmental, 202, 430-437. https://doi.org/10.1016/j.apcatb.2016.09.051
- 18. Saha, S., Pal, A., Kundu, S., Basu, S. and Pal, T. (2010) Photochemical Green Synthesis of Calcium-Alginate-Stabi- lized Ag and Au Nanoparticles and Their Catalytic Application to 4-Nitrophenol Reduction. Langmuir, 26, 2885-2893. https://doi.org/10.1021/la902950x
- 19. Liu, H., Wang, J., Feng, Z., Lin, Y., Zhang, L. and Su, D. (2015) Facile Synthesis of Au Nanoparticles Embedded in an Ultrathin Hollow Graphenenanoshell with Robust Catalytic Performance. Small, 11, 5059-5064. https://doi.org/10.1002/smll.201500635
- 20. Mitsudome, T., Arita, S., Mori, H., Mizugaki, T., Jitsukawa, K. and Kaneda, K. (2008) Supported Silver-Nanoparticle- Catalyzed Highly Efficient Aqueous Oxidation of Phenylsilanes to Silanols. Angewandte Chemie International Edition, 47, 7938-7940. https://doi.org/10.1002/anie.200802761
- 21. Zhen, W., Ma, J. and Lu, G. (2016) Small-Sized Ni (111) Particles in Metal-Organic Frameworks with Low Over-Po- tential for Visible Photocatalytic Hydrogen Generation. Applied Catalysis B: Environmental, 190, 12-25. https://doi.org/10.1016/j.apcatb.2016.02.061
- 22. Hao, Y., Shao, X., Li, B., Hu, L. and Wang, T. (2015) Mesoporous TiO2 Nanofibers with Controllable Au Loadings for Catalytic Reduction of 4-Nitrophenol. Materials Science in Semiconductor Processing, 40, 621-630. https://doi.org/10.1016/j.mssp.2015.07.026
- 23. Steffan, M., Jakob, A., Claus, P. and Lang, H. (2009) Silica Supported Silver Nanoparticles from a Silver (I) Carboxylate: Highly Active Catalyst for Regioselective Hydrogenation. Catalysis Communications, 10, 437-441. https://doi.org/10.1016/j.catcom.2008.10.003
- 24. Gerber, I.C. and Serp, P. (2020) A Theory/Experience Description of Support Effects in Carbon-Supported Catalysts. Chemical Reviews, 120, 1250-1349. https://doi.org/10.1021/acs.chemrev.9b00209
- 25. Wang, Y., Mao, J., Meng, X., Yu, L., Deng, D. and Bao, X. (2019) Catalysis with Two-Dimensional Materials Confining Single Atoms: Concept, Design, and Applications. Chemical Reviews, 119, 1806-1854. https://doi.org/10.1021/acs.chemrev.8b00501
- 26. Fu, Q. and Bao, X. (2017) Surface Chemistry and Catalysis Confined under Two-Dimensional Materials. Chemical Society Reviews, 46, 1842-1874. https://doi.org/10.1039/C6CS00424E
- 27. Fu, Q. and Bao, X. (2019) Confined Microenvironment for Catalysis Control. Nature Catalysis, 2, 834-836. https://doi.org/10.1038/s41929-019-0354-z
- 28. Kuriki, R., Sekizawa, K., Ishitani, O. and Maeda, K. (2015) Vis-ible-Light-Driven CO2 Reduction with Carbon Nitride: Enhancing the Activity of Ruthenium Catalysts. Angewandte Chemie International Edition, 54, 2406-2409. https://doi.org/10.1002/anie.201411170
- 29. Chen, X., Liu, Q., Wu, Q., Du, P., Zhu, J., Dai, S. and Yang, S. (2016) Incorporating Graphitic Carbon Nitride (g-C3N4) Quantum Dots into Bulk-Heterojunction Polymer Solar Cells Leads to Efficiency Enhancement. Advanced Functional Materials, 26, 1719-1728. https://doi.org/10.1002/adfm.201505321
- 30. Cao, S., Low, J., Yu, J. and Jaroniec, M. (2015) Polymeric Photo-catalysts Based on Graphitic Carbon Nitride. Advanced Materials, 27, 2150-2176. https://doi.org/10.1002/adma.201500033
- 31. Li, X.H., Wang, X. and Antonietti, M. (2013) Mesoporous g-C3N4 Nanorods as Multifunctional Supports of Ultrafine Metal Nanoparticles: Hydrogen Generation from Water and Reduction of Nitrophenol with Tandem Catalysis in One Step. Chemical Science, 3, 2170-2174. https://doi.org/10.1039/c2sc20289a
- 32. Bhowmik, T., Kundu, M.K. and Barman, S. (2015) Ultra Small Gold Nanoparticles-Graphitic Carbon Nitride Composite: An Efficient Catalyst for Ultrafast Reduction of 4-Nitrophenol and Removal of Organic Dyes from Water. RSC Advances, 5, 38760-38773. https://doi.org/10.1039/C5RA04913J
- 33. Bai, X., Wang, L., Zong, R. and Zhu, Y. (2013) Photocatalytic Activity Enhanced via g-C3N4 Nanoplates to Nanorods. The Journal of Physical Chemistry C, 117, 9952-9961. https://doi.org/10.1021/jp402062d
- 34. Liang, Q., Li, Z., Yu, X., Huang, Z.H., Kang, F. and Yang, Q.H. (2015) Macroscopic 3D Porous Graphitic Carbon Nitride Monolith for Enhanced Photocatalytic Hydrogen Evolution. Advanced Materials, 27, 4634-4639. https://doi.org/10.1002/adma.201502057
- 35. Jun, Y.S., Park, J., Lee, S.U., Thomas, A., Hong, W.H. and Stucky, G.D. (2013) Three-Dimensional Macroscopic Assemblies of Low-Dimensional Carbon Nitrides for Enhanced Hydrogen Evolution. Angewandte Chemie International Edition, 52, 11083-11087. https://doi.org/10.1002/anie.201304034
- 36. Huang, Z.F., Song, J., Pan, L., Wang, Z., Zhang, X., Zou, J.J., Mi, W., Zhang, X. and Wang, L. (2015) Carbon Nitride with Simultaneous Porous Network and O-Doping for Efficient Solar-Energy-Driven Hydrogen Evolution. Nano Energy, 12, 646-656. https://doi.org/10.1016/j.nanoen.2015.01.043
- 37. Fageria, P., Uppala, S., Nazir, R., Gangopadhyay, S., Chang, C.H., Basu, M. and Pande, S. (2016) Synthesis of Mono-(Au & Pd) and Bimetallic (AuPd) Nanoparticles Using Carbon Nitride (C3N4) Quantum Dot via Photochemical Route for Nitrophenol Reduction. Langmuir, 32, 10054-10064. https://doi.org/10.1021/acs.langmuir.6b02375
- 38. Chen, S.F., Li, J.P., Qian, K., Xu, W.P., Lu, Y., Huang, W.X. and Yu, S.H. (2010) Large Scale Photochemical Synthesis of M@TiO2 Nanocomposites (M = Ag, Pd, Au, Pt) and Their Optical Properties, CO Oxidation Performance, and Antibacterial Effect. Nano Research, 3, 244-255. https://doi.org/10.1007/s12274-010-1027-z
- 39. Dong, C., Lian, C., Hu, S., Deng, Z., Gong, J., Li, M., Liu, H., Xing, M. and Zhang, J. (2018) Size-Dependent Activity and Selectivity of Carbon Dioxide Photocatalytic Reduction over Platinum Nanoparticles. Nature Communications, 9, 1252-1263. https://doi.org/10.1038/s41467-018-03666-2
NOTES
*通讯作者。