Advances in Clinical Medicine
Vol.
09
No.
11
(
2019
), Article ID:
32947
,
10
pages
10.12677/ACM.2019.911196
Clinical Application and Research Progress of Implantable Heart Monitor (ICM) in Unexplained Syncope and Palpitations
Yanfei Zhang, Jiefu Yang, Jiabin Tong, Jia Zhong, Junpeng Liu, Zhilei Wang, Xin Jin, Tong Zou
Department of Cardiology, National Gerontology Center, Beijing Hospital, Beijing

Received: Oct. 25th, 2019; accepted: Nov. 7th, 2019; published: Nov. 14th, 2019

ABSTRACT
Unexplained syncope and palpitation are the difficulties in the diagnosis and treatment of cardiovascular diseases. The rate of missed diagnosis and misdiagnosis is high, which leads to great clinical harm. At present, most patients are difficult to be diagnosed by routine examinations such as electrocardiogram. Studies have shown that 45% to 80% of unexplained syncope may be cardiogenic syncope, and many arrhythmias with occasional palpitations are difficult to diagnose. Therefore, the study of effective technical means to improve its diagnostic rate is of great significance. In recent years, with the development of implantable ECG monitor (ICM) technology and the publication of relevant research results, it has been proved that it can significantly improve the diagnostic rate of syncope and palpitations of unknown causes. This article reviews the application and development of implantable heart monitor (ICM) in syncope and palpitations.
Keywords:Implantable Heart Monitor, Unexplained Syncope, Palpitation, ICM
植入式心脏监测器(ICM)在不明原因晕厥 和心悸中的临床应用和研究进展
张雁飞,杨杰孚,佟佳宾,种甲,刘俊鹏,王志蕾,金鑫,邹彤
北京医院,国家老年医学中心,心内科,北京

收稿日期:2019年10月25日;录用日期:2019年11月7日;发布日期:2019年11月14日

摘 要
不明原因晕厥和心悸是心血管系统疾病诊疗中的难点,漏诊误诊率高,临床危害大。目前大多数患者很难通过心电图等常规检查确诊。研究显示,45%~80%的不明原因晕厥可能为心源性晕厥,同时,很多偶发心悸的心律失常患者很难确诊。因此,研究有效的技术手段以提高其诊断率意义重大。近年来,随着植入性心电监测仪(ICM)技术的升级以及相关研究结果的发表,证实其可以显著提高不明原因晕厥和心悸的诊断率。本文对植入式心脏监测器(ICM)在晕厥和心悸中的应用和发展进行概述。
关键词 :植入式心脏监测器,不明原因晕厥,心悸,ICM

Copyright © 2019 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 晕厥心悸的概述
晕厥及心悸是心血管内科门诊患者常见主诉,其发病原因很多 [1]。病理基础为一过性全脑低灌注,临床表现为突发短暂性意识丧失和姿势性张力丧失,时间一般小于20 s。晕厥是常见临床症状,具有突发、持续时间短、能自行完全恢复的特点,伴头晕、黑曚等症状。在美国每年有大约75万人因晕厥而就诊 [2]。Framingham研究显示,晕厥发生率为每年每千人6.2人次,70岁以后发病率急剧增加 [3]。预估晕厥终生累积发病率为30%~40% [4]。晕厥可以分为心源性晕厥、脑源性晕厥、反射性晕厥(包括血管迷走性晕厥、情境性晕厥、颈动脉窦过敏综合征)及其他类型晕厥。血管迷走性晕厥是最常见的病因,其次是心源性晕厥,分别占晕厥比例的21%和9%。其中心源性晕厥最为凶险,其主要原因是心脏流出道发生了较为严重的阻塞,或受到心律失常的影响,出现血流动力学的障碍,引发短暂性的意识丧失。心源性晕厥常在运动或情绪激动时发生,发病前无明显症状,发作时常伴心律失常、心力衰竭等症状。心源性晕厥患者一年内猝死率增加30%,有过心力衰竭病史的患者病死率更高 [5]。5年病死率接近50% [6]。有1/3的晕厥患者病因诊断不明 [7],病因诊断不明的晕厥患者比没有发生过晕厥的患者死亡率高30%。
心脏流出道阻塞可以通过心脏彩超判断,心律失常可通过心电图、Holter等手段确诊。但晕厥不可预测,绝大部分患者很难捕捉到症状发生时的心电图。所以,植入式心电监测仪对于明确晕厥或心悸患者的病因具有重要作用 [8] [9]。目前多个指南推荐应用体外或植入式心电监测仪对无法明确病因的晕厥患者进行心律失常筛查 [10] [11]。不明原因晕厥反复发作,产生了巨大的医疗资源消耗,在美国每年用于诊断评估晕厥的医疗费用高达24亿美元 [12]。
首版欧洲心脏病学会(European Society of Cardiology, ESC)在2001年、2004年和2009年分别发布了三版晕厥诊断与管理指南,2015年实践指南委员会进行了修订。2017年由美国心脏病学会(ACC)、美国心脏协会(AHA)与美国心律协会(HRS)联合颁布了晕厥诊断与处理指南 [13],对于危险因素进行了综合和更新,针对患者的临床表现和危险分层提出了有针对性的诊断检查方式,其中患者若初始评估提示心血管异常,可根据晕厥发作的频率和特征选用心脏监测(I类推荐),包括植入式心电监测(II a类推荐)和动态体外心电监测(II a类推荐)。2018年3月19日,ESC颁布了新版晕厥诊断和管理指南 [14]。我国于2014年由中国心律学会、中国老年学会心血管病专业委会员出台了晕厥的诊断与治疗中国专家共识。
2. 植入式心脏监测器(ICM)的发展情况
随着医学信息技术的快速发展,对于疾病诊断和治疗的相关设备也在不断的研发更新,应用于临床。这些智能化的设备将智能技术与软件作为载体,使用大数据与云端交互技术,将使用者的医疗检测数据进行存储,并进行动态的检测,采用互联网信息传输和处理技术,将搜集到的信息进行在线分析和离线记录等智能管理和使用。植入式心电事件监测器(insertabile cardiac monitor, ICM)是无线皮下植入设备,能持续监测心律并记录在监测时间内发生事件时心电图,主要为不明原因心悸、晕厥的患者提供病因依据(图1)。
 Source: Medtronic, Inc. Reveal LINQ LNQ11 Insertable Cardiac Monitor. Clinician Manual. 2014.
Source: Medtronic, Inc. Reveal LINQ LNQ11 Insertable Cardiac Monitor. Clinician Manual. 2014.
Figure 1. Medtronic’s latest generation of implantable monitors, is the size of a paperclip, 1.2 CC, 2.5 G
图1. 美敦力最新一代植入式监测器(Reveal LINQ),只有曲别针大小,1.2 CC,2.5 G
ICM的起源是对心脏进行长程的监测,1947年Norman J. Holter设计并应用的心脏监测系统仅能记录几个小时的数据,随着技术进步,从短期(24~72 h)动态心电图监测发展到到长达1个月的心脏事件记录仪,移动心脏事件遥测仪 [15] 也逐渐开发和应用起来。20世纪90年代第一代ICM研制成功,并在不明原因晕厥患者中进行应用。随着技术更新,到2017年发展成为体积小、电池寿命长、植入方法简单易操作、兼容磁共振检查、增加了“P波感知”,能更好地识别记录房颤(图2)。
目前主要ICM产品包括美敦力公司的Reveal LINQ、圣犹达公司的Confirm、百多力公司的Bio Monitor 2等。2014年2月19日,美敦力公司发布了Reveal LINQ可插入式心脏监测器(ICM)系统,该设备在美国和欧洲监管机构明确表明允许使用,是当前世界上最小的可植入心脏监测设备,属于植入式心电记录器系列的第六代产品(图3,图4)。这一设备明显的特征就是体积小,仅为7 × 45 × 4 mm,重约2.5 g,可提供长达3年的持续不间断监测,并且植入ICM并不影响磁共振检查,可安全接受1.5 T和3.0 T的磁共振扫描,同时具有氮化钛涂层电极高保真信号,除了连续和无线监测功能,还可以通过Carelink®网络进行远程监控,可以提高事件检出及临床处理的效率 [16]。通过注射植入,无需制作囊袋,创伤微小,操作简单。其适应证如下:1) 有临床症状或状况,处于心律失常风险增加状态的患者;2) 经历过短暂性症状,可能提示有心律失常的患者。临床研究表明该装置信息精准,对心律失常检出率高,可准确记录房颤、室速、心动过缓、心脏停搏等事件,储存59 min心电图资料并记录保存心律失常关键信息。
 Source: Medtronic, Inc. Reveal LINQ LNQ11 Insertable Cardiac Monitor. Clinician Manual. 2014.
Source: Medtronic, Inc. Reveal LINQ LNQ11 Insertable Cardiac Monitor. Clinician Manual. 2014.
Figure 2. The optimal position of the instrument was a 45 degree Angle (v2-v3 electrode orientation) in the 4th intercostal space, and the upper end of the instrument was positioned about 2 cm to the left of the sternum margin
图2. 最佳器械位置在第4肋间隙的胸骨角呈45度角(V2-V3电极方位),器械上端定位在胸骨缘左侧位大约2 cm处
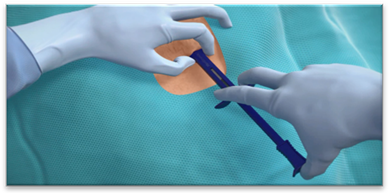 Source: Medtronic, Inc. Reveal LINQ LNQ11 Insertable Cardiac Monitor. Clinician Manual. 2014.
Source: Medtronic, Inc. Reveal LINQ LNQ11 Insertable Cardiac Monitor. Clinician Manual. 2014.
Figure 3. The implantation method is simple; injection implantation is adopted
图3. 植入方式简便,采取注射植入
 Source: Medtronic, Inc. Reveal LINQ LNQ11 Insertable Cardiac Monitor. Clinician Manual. 2014.
Source: Medtronic, Inc. Reveal LINQ LNQ11 Insertable Cardiac Monitor. Clinician Manual. 2014.
Figure 4. Placed in the subcutaneous
图4. 置于皮下
3. 植入式心脏监测器(ICM)在晕厥及心悸中的应用
3.1. 在晕厥中的应用
目前,对于怀疑心律失常原因导致的晕厥,推荐进行心电监测,包括ICM。2017年美国心脏协会(AHA)晕厥管理指南 [17] 指出传统常规检查方法对于不明原因的晕厥的诊断率较低,其中心电图的诊断率为2%~12%,动态心电图的诊断率为11%,电生理检查诊断率为11%,神经相关检查仅为4%。并提出,对不明原因晕厥患者应进行专科会诊,对于晕厥达到高风险标准的患者,建议尽早植入ICM (Ia类推荐)。这提示ICM在诊断不明原因晕厥有独特优势。目前,ICM已在国内外进行了大量应用。
多中心研究PICTURE对570例反复晕厥或晕厥先兆的患者,植入ICM,12个月内78%的再次晕厥患者得以明确诊断,其中,75%明确为心源性晕厥 [18]。
观察性研究FRESH通过对78例晕厥患者的随访,发现ICM对于晕厥患者有明确诊断及指导治疗的作用 [19]。对照研究RAST选择了60例不明原因晕厥患者,结果表明,应用ICM可较传统检查组有更高的晕厥病因检出率(52% vs 20%, P = 0.012) [20]。伊斯特本晕厥评估研究(EaSyAS)中,对246例晕厥患者给予经过相关检查未见明显异常的频繁发作晕厥患者植入ICM,随访约(4.4 ± 4.2)个月时,约56%的患者通过ICM明确心律失常诊断 [21]。Ea SyAS II研究显示,在植入心电记录器的246例晕厥患者中,检出缓慢性心律失常并需要起搏器治疗的比例比传统治疗组患者显著增高,且晕厥复发比例显著降低,可见,植入式心脏监测仪通过明确晕厥患者病因诊断使更多患者获益 [22] [23]。心源性晕厥的患者中有52%植入了起搏器,6%接受了植入型心律转复除颤器治疗。提示ICM不但可以提高晕厥的诊断率,而且可以指导治疗,改善患者生活质量,并具有较好的性价比,降低总医疗花费 [24] [25]。
ICM可以帮助患者明确心律失常诊断,为临床医生提供可靠诊断证据,尤其适合不明原因晕厥患者的筛查诊断、卒中患者阵发性房颤筛查和隐源性卒中的患者,相比于常规检查手段具有明显优势,可在患者没有麻醉的情况下注入到皮肤下面,只需90 s。其便捷的应用受到了心脏学家们的欢迎,国外临床已将该装置应用于不明原因晕厥和脑卒中诊断 [26] [27] [28]。对于不明原因晕厥的患者,其诊断率为心电图的6.5倍,可以帮助78%的患者明确诊断,该款植入性心脏监测器已经在31个国家和地区上市,超过10万例患者使用。随着国外临床应用证明该装置对不明原因晕厥和心脏性猝死诊断的重要临床意义,2015年9月开始我国医疗机构陆续将ICM应用于临床 [16]。
3.2. 在心悸中的应用
经临床应用发现该装置在诊断房颤、改善有卒中风险患者的预后和其他心律失常方面有重要作用 [29]。
心动过速,尤其是房颤的筛查,目前亦是ICM应用的热点,对于明确隐源性脑卒中病因以及房颤治疗尤其是导管消融后房颤的监测,发挥着不可替代的作用。房颤(atrial fibrillation)是临床上最常见的持续性心律失常,随着老年人口的增长,房颤患者数量不断增加。目前男性的房颤年发病率大约为0.78‰、女性为0.60‰ [30]。ICM可持续进行心律监测2~3年,并将心电监测情况远程传输给计算机进行整理分析,大大提高了房颤的检出率。ICM在房颤患者的管理中主要用于测定节律控制的疗效、导管消融术后房颤的评估以及隐源性脑卒中患者房颤的检测。有研究显示,2580例无房颤病史的患者存在大量的无症状性房颤发生,这些患者体内植入了ICMs的前3个月内,10.1%的患者发现了房颤 [31]。与常规抗凝患者相比,ICM实时监测房颤的发生,对于临床抗凝治疗有指导作用,可将抗凝药的使用时间减少94%,证实了ICM引导下新型抗凝药物间歇抗凝治疗低血栓栓塞风险人群的可行性 [32]。
前瞻性、对照研究ISSUE对35例有风险的显性心脏病患者应用了植入式循环记录仪。合并器质性心脏病电生理检查阴性的晕厥患者中,40%通过ICM记录到晕厥或晕厥先兆发作时心电图 [33]。合并左束支阻滞电生理检查阴性的晕厥患者随访过程中再发晕厥者77%通过ICM明确病因 [34]。无明显器质性心脏病、倾斜试验阴性的不明原因晕厥患者中85.7%记录到晕厥事件心电图,而倾斜试验阳性者也有80%记录到晕厥事件心电图 [35]。
前瞻性DISCERN房颤研究针对50例发生房颤的患者,利用Reveal XTICM评价房颤射频消融术前后房颤负荷,结果显示消融后房颤负荷降低86% (P < 0.001),但消融后无症状性房颤发作比例较术前显著升高。CRYSTAL-AF选择208例脑卒中后发生房颤患者进行分组,比较了ICM和传统的心电监测系统在隐源性脑卒中患者中发现AF的效果,结果发现ICM长程事件记录仪发现房颤的能力明显优于传统的心电监测装置 [36]。随着临床上植入心脏起搏器或者除颤仪的患者逐渐增多,有关这些仪器对心律失常的检测的研究也被人们所重视。YANO等 [26] 对植入心脏起搏器的患者的每日心电情况进行了分析,结果发现患者在植入装置后的第14、28、56、112、365d检测到的快速性房性心律失常的比例为10%、15%、21%、28%、50%。
4. 植入式心脏监测器(ICM)在其他心血管疾病中的应用
早期植人ICM可减少相关心脑血管检查,且因其诊断率较传统方法高,还可减少住院及相关检查费用。对不明性脑卒中患者应用插入式心电监测器(ICM) Reveal XT可明显改善心房颤动(房颤)的检出率,估计3年房颤检出率约为30%,而Holter的检出率仅3% [28]。近期,Li等 [37] 对Reveal LINQ的临床应用进行了研究,目前尚无ICM Reveal LINQ在原因不明性脑卒中患者中的临床应用研究。Purerfellner等研究在原因不明性脑卒中患者中全部全部成功植入Reveal LINQ,无明显并发症,与既往临床研究结果类似 [38]。
2013年Rittea等 [39] 发表的观察性结果证实,对于不明原因脑卒中患者进行连续7 d动态心电图监测。与植入ICM对比,动态心电图组仅检出1.7%的房颤患者,而ICM组在2周时房颤检出率为17%,检出第1次房颤发作平均耗时64 d。ICM对房颤的检出率是动态心电图监测的10倍。随后,SURPRISE大型研究发表了类似的观察性研究结果,发现首次检测到房颤发作均在脑卒中30 d以后,这对当前指南给予了指导性补充意见 [40]。除此之外,2017年ARREST研究显示,射血分数保留的急性心肌梗死患者,植入ICM后随访2年,检出房颤是最常见的心律失常,其中93%的房颤发生时无症状 [41]。
Mascarenha等 [42] 对70例阵发性房颤脑卒中高风险(CHAZDSi VASc评分 ≥ 2)同时合并高出血风险(HAS-BLED评分 ≥ 3)的患者植入ICM。对于维持窦性节律或房颤负荷低的患者停用抗凝药物,在近2年的随访时间里,76%的未服用抗凝药物的患者,未发生脑卒中、TIA等事件,更没有出现出血事件。而没有停用杭凝药物的患者中,1/4因发生严重出血事件而再次入院。Zuern [42] 等对房颤消融术后患者的观察中,观察到了类似的结果,常规消融术后3个月,65例CHADSZ脑卒中评分在1~3分的患者植入ICM,并停用抗凝药物。随访近3年,63%的房颤负荷低(每天持续发作时间 < 1 h)患者,停用抗凝药物后并未见栓塞事件的发生。这些研究均为以后ICM指导抗凝药物使用方面提供了有益的探索。以上研究中,ICM不再局限于最初用于晕厥和房颤的识别,在临床的多个领域能帮助医务人员提高对各种疾病的准确认知。
5. 国内对于植入式心脏监测仪的研究
北京医院心内科邹彤教授团队联合中国医学科学院阜外医院,首都医科大学附属北京安贞医院,普仁医院4家医院开展了北京市科委老年重大课题子项目:“植入器械的移动及远程监测功能对老年人常见心脑血管事件的早期预警价值及干预效果研究”,已完成中国临床试验注册中心注册,注册号ChiCTR1800017222,项目预计完成时间为2021年12月。实验流程图如图5所示。
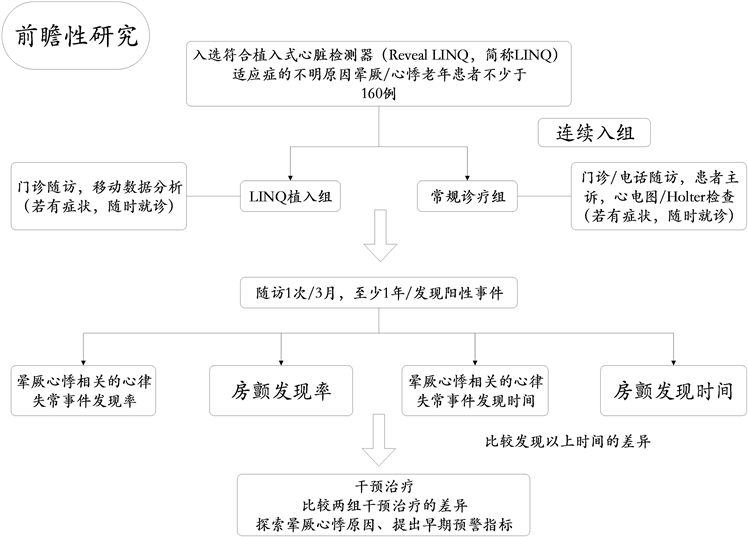
Figure 5. The flow chart of the clinical trial
图5. 临床试验流程图
目前,北京医院将植入式心脏监测仪(Reveal LINQ)应用于临床,并将其纳入医保,计划入组160例符合植入式心脏监测仪(Reveal LINQ)适应症的不明原因晕厥/心悸患者。自2016年2月至2019年10月北京医院心内科已植入心脏监测器(Reveal LINQ) 80例,有10例患者配备了CareLink远程数据传输设备,居国内第一。并创新性的纳入对照组,于基线开始,每三个月进行随访。
另外,北京医院有专门的患者关爱团队和技术团队共同来管理数据,可以很及时地对报警事件作出反馈,方便临床医生及时进行个体化的治疗,从而帮助临床医生及时对患者进行管理和干预。
6. 结语
不明原因晕厥和心悸患者诊断的焦点在于对发病情况进行长期监测,针对不同病人进行精准治疗。但目前,诊断流程较松散,诊断准确性也不高,技术应用很受限。以北京医院为首的国内三甲医院加强了对ICM的研究,这对于晕厥和心悸的诊断具有积极作用,能够大大提升晕厥的诊断率,增加心律失常的检出率,改善晕厥、心悸患者的诊断和治疗,对于心脏性猝死具有积极的预防作用。
基金项目
北京市科学技术委员会(D181100000218005)。
文章引用
张雁飞,杨杰孚,佟佳宾,种 甲,刘俊鹏,王志蕾,金 鑫,邹 彤. 植入式心脏监测器(ICM)在不明原因晕厥和心悸中的临床应用和研究进展
Clinical Application and Research Progress of Implantable Heart Monitor (ICM) in Unexplained Syncope and Palpitations[J]. 临床医学进展, 2019, 09(11): 1266-1275. https://doi.org/10.12677/ACM.2019.911196
参考文献
- 1. Raviele, A., Giada, F., Bergfeldt, L., et al. (2011) Management of Patients with Palpitations: A Position Paper from the European Heart Rhythm Association. Europace, 13, 920-934. https://doi.org/10.1093/europace/eur130
- 2. Tomson, T.T. and Passman, R. (2017) Current and Emerging Uses of Insertable Cardiac Monitors: Evaluation of Syncope and Monitoring for Atrial Fibrillation. Cardiology in Review, 25, 22-29. https://doi.org/10.1097/CRD.0000000000000129
- 3. Soteriades, E.S., Evans, J.C., Larson, M.G., et al. (2002) Incidence and Prognosis of Syncope. The New England Journal of Medicine, 347, 878-885. https://doi.org/10.1056/NEJMoa012407
- 4. Ganzeboom, K.S., Mairuhu, G., Reitsma, J.B., Linzer, M., Wieling, W. and van Dijk, N. (2006) Lifetime Cumulative Incidence of Syncope in the General Population: A Study of 549 Dutch Subjects Aged 35-60 Years. Journal of Cardiovascular Electrophysiology, 17, 1172-1176. https://doi.org/10.1111/j.1540-8167.2006.00595.x
- 5. Patel, P.R. and Quinn, J.V. (2015) Syncope: A Review of Emergency Department Management and Disposition. Clinical and Experimental Emergency Medicine, 2, 67-74. https://doi.org/10.15441/ceem.14.049
- 6. Kuhne, M., Schaer, B., Sticherling, C. and Osswald, S. (2011) Holter Monitoring in Syncope: Diagnostic Yield in Octogenarians. Journal of the American Geriatrics Society, 59, 1293-1298. https://doi.org/10.1111/j.1532-5415.2011.03486.x
- 7. Edvardsson, N., Garutti, C., Rieger, G. and Linker, N.J. (2014) Unexplained Syncope: Implications of Age and Gender on Patient Characteristics and Evaluation, the Diagnostic Yield of an Implantable Loop Recorder, and the Subsequent Treatment. Clinical Cardiology, 37, 618-625. https://doi.org/10.1002/clc.22300
- 8. Edvardsson, N., Frykman, V., van Mechelen, R., et al. (2011) Use of an Implantable Loop Recorder to Increase the Diagnostic Yield in Unexplained Syncope: Results from the PICTURE Regis-try. Europace, 13, 262-269.
- 9. Linker, N.J., Voulgaraki, D., Garutti, C., Rieger, G. and Edvardsson, N. (2013) Early versus Delayed Implantation of a Loop Recorder in Patients with Unexplained Syncope—Effects on Care Pathway and Diagnostic Yield. International Journal of Cardiology, 170, 146-151. https://doi.org/10.1016/j.ijcard.2013.10.025
- 10. Varosy, P.D., Chen, L.Y., Miller, A.L., Noseworthy, P.A., Slot-winer, D.J. and Thiruganasambandamoorthy, V. (2017) Pacing as a Treatment for Reflex-Mediated (Vasovagal, Situa-tional, or Carotid Sinus Hypersensitivity) Syncope: A Systematic Review for the 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients with Syncope: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm, 14, e255-255e269. https://doi.org/10.1161/CIR.0000000000000500
- 11. Brignole, M., et al. (2018) 2018 ESC Guidelines for the Di-agnosis and Management of Syncope. Revista Española de Cardiología, 71, 837. https://doi.org/10.1016/j.rec.2018.09.002
- 12. Sun, B.C. (2013) Quality-of-Life, Health Service Use, and Costs Associated with Syncope. Progress in Cardiovascular Diseases, 55, 370-375. https://doi.org/10.1016/j.pcad.2012.10.009
- 13. Golovina, G.A. and Duplyakov, D.V. (2018) Key Points of the 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients with Syncope. Kardiologiia, No. 8, 89-100. https://doi.org/10.18087/cardio.2018.8.10151
- 14. Brignole, M., Moya, A., de Lange, F.J., et al. (2018) 2018 ESC Guidelines for the Diagnosis and Management of Syncope. European Heart Journal, 39, 1883-1948. https://doi.org/10.1093/eurheartj/ehy210
- 15. Tomson, T.T. and Passman, R. (2015) The Reveal LINQ Insertable Cardiac Monitor. Expert Review of Medical Devices, 12, 7-18. https://doi.org/10.1586/17434440.2014.953059
- 16. Maines, M., Zorzi, A., Tomasi, G., et al. (2018) Clinical Im-pact, Safety, and Accuracy of the Remotely Monitored Implantable Loop Recorder Medtronic Reveal LINQTM. Euro-pace, 20, 1050-1057. https://doi.org/10.1093/europace/eux187
- 17. Shen, W.K., Sheldon, R.S., Benditt, D.G., et al. (2017) 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients with Syncope: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm, 14, e218-218e254.
- 18. Krahn, A.D., Klein, G.J., Norris, C. and Yee, R. (1995) The Etiology of Syncope in Patients with Negative Tilt Table and Electrophysiological Testing. Circulation, 92, 1819-1824. https://doi.org/10.1161/01.CIR.92.7.1819
- 19. Krahn, A.D., Klein, G.J., Yee, R. and Skanes, A.C. (2001) Randomized Assessment of Syncope Trial: Conventional Diagnostic Testing versus a Prolonged Monitoring Strategy. Circulation, 104, 46-51. https://doi.org/10.1161/01.CIR.104.1.46
- 20. Sulke, N., Sugihara, C., Hong, P., Patel, N. and Freemantle, N. (2016) The Benefit of a Remotely Monitored Implantable Loop Recorder as a First Line Investigation in Unexplained Syncope: The EaSyAS II Trial. Europace, 18, 912-918. https://doi.org/10.1093/europace/euv228
- 21. Podoleanu, C., DaCosta, A., Defaye, P., et al. (2014) Early Use of an Implantable Loop Recorder in Syncope Evaluation: A Randomized Study in the Context of the French Healthcare System (FRESH Study). Archives of Cardiovascular Diseases, 107, 546-552. https://doi.org/10.1016/j.acvd.2014.05.009
- 22. Davis, S., Westby, M., Petkar, S. and Pitcher, D. (2013) Tilt Test-ing Is More Cost-Effective than Implantable Loop Recorder Monitoring as a Means of Directing Pacing Therapy in Peo-ple with Recurrent Episodes of Suspected Vasovagal Syncope That Affect Their Quality of Life or Present a High Risk of Injury. Heart, 99, 805-810. https://doi.org/10.1136/heartjnl-2012-302851
- 23. Farwell, D.J., Freemantle, N. and Sulke, N. (2006) The Clinical Impact of Implantable Loop Recorders in Patients with Syncope. European Heart Journal, 27, 351-356. https://doi.org/10.1093/eurheartj/ehi602
- 24. Davis, S., Westby, M., Pitcher, D. and Petkar, S. (2012) Implantable Loop Recorders Are Cost-Effective When Used to Investigate Transient Loss of Consciousness Which Is Either Sus-pected to Be Arrhythmic or Remains Unexplained. Europace, 14, 402-409. https://doi.org/10.1093/europace/eur343
- 25. Reinsch, N., Ruprecht, U., Buchholz, J., Diehl, R.R., Kalsch, H. and Neven, K. (2018) The BioMonitor 2 Insertable Cardiac Monitor: Clinical Experience with a Novel Implantable Cardiac Monitor. Journal of Electrocardiology, 51, 751-755. https://doi.org/10.1016/j.jelectrocard.2018.05.017
- 26. Sanna, T., Diener, H.C., Passman, R.S., et al. (2014) Cryptogenic Stroke and Underlying Atrial Fibrillation. The New England Journal of Medicine, 370, 2478-2486. https://doi.org/10.1056/NEJMoa1313600
- 27. Nakano, Y. and Wataru, S. (2017) Syncope in Patients with Inherited Arrhythmias. Journal of Arrhythmia, 33, 572-578. https://doi.org/10.1016/j.joa.2017.07.007
- 28. Li, Y., Nantsupawat, T., Olson, M., et al. (2018) A Single Center Experience on the Clinical Utility Evaluation of an Insertable Cardiac Monitor. Journal of Electrocardiology, 51, 583-587. https://doi.org/10.1016/j.jelectrocard.2018.05.002
- 29. Salvatori, V., Becattini, C., Laureti, S., et al. (2015) Holter Monitoring to Detect Silent Atrial Fibrillation in High-Risk Subjects: The Perugia General Practitioner Study. Internal and Emergency Medicine, 10, 595-601. https://doi.org/10.1007/s11739-015-1241-5
- 30. Healey, J.S., Connolly, S.J., Gold, M.R., et al. (2012) Subclinical Atrial Fibrillation and the Risk of Stroke. The New England Journal of Medicine, 366, 120-129. https://doi.org/10.1056/NEJMoa1105575
- 31. Passman, R., Leong-Sit, P., Andrei, A.C., et al. (2016) Targeted Anticoagulation for Atrial Fibrillation Guided by Continuous Rhythm Assessment with an Insertable Cardiac Monitor: The Rhythm Evaluation for Anticoagulation with Continuous Monitoring (REACT.COM) Pilot Study. Journal of Car-diovascular Electrophysiology, 27, 264-270. https://doi.org/10.1111/jce.12864
- 32. Menozzi, C., Brignole, M., Garcia-Civera, R., et al. (2002) Mechanism of Syncope in Patients with Heart Disease and Negative Electrophysiologic Test. Circulation, 105, 2741-2745. https://doi.org/10.1161/01.CIR.0000018125.31973.87
- 33. Brignole, M., Menozzi, C., Moya, A., et al. (2001) Mechanism of Syncope in Patients with Bundle Branch Block and Negative Electrophysiological Test. Circulation, 104, 2045-2050. https://doi.org/10.1161/hc4201.097837
- 34. Moya, A., Brignole, M., Menozzi, C., et al. (2001) Mechanism of Syncope in Patients with Isolated Syncope and in Patients with Tilt-Positive Syncope. Circulation, 104, 1261-1267. https://doi.org/10.1161/hc3601.095708
- 35. Verma, A., Champagne, J., Sapp, J., et al. (2013) Dis-cerning the Incidence of Symptomatic and Asymptomatic Episodes of Atrial Fibrillation before and after Catheter Abla-tion (DISCERN AF): A Prospective, Multicenter Study. JAMA Internal Medicine, 173, 149-156. https://doi.org/10.1001/jamainternmed.2013.1561
- 36. Yano, Y., Greenland, P., Lloyd-Jones, D.M., Daoud, E.G., Koehler, J.L. and Ziegler, P.D. (2016) Simulation of Daily Snapshot Rhythm Monitoring to Identify Atrial Fibrillation in Continuously Monitored Patients with Stroke Risk Factors. PLoS ONE, 11, e0148914. https://doi.org/10.1371/journal.pone.0148914
- 37. Purerfellner, H., Sanders, P., Pokushalov, E., Di, B.M., Berge-mann, T. and Dekker, L.R. (2015) Miniaturized Reveal LINQ Insertable Cardiac Monitoring System: First-in-Human Experience. Heart Rhythm, 12, 1113-1119. https://doi.org/10.1016/j.hrthm.2015.02.030
- 38. Ritter, M.A., Kochhauser, S., Duning, T., et al. (2013) Occult Atrial Fibrillation in Cryptogenic Stroke: Detection by 7-Day Electrocardiogram versus Implantable Cardiac Monitors. Stroke, 44, 1449-1452. https://doi.org/10.1161/STROKEAHA.111.676189
- 39. Christensen, L.M., Krieger, D.W., Hojberg, S., et al. (2014) Paroxysmal Atrial Fibrillation Occurs Often in Cryptogenic Ischaemic Stroke. Final Results from the SURPRISE Study. European Journal of Neurology, 21, 884-889. https://doi.org/10.1111/ene.12400
- 40. Romanov, A., Martinek, M., Purerfellner, H., et al. (2018) Incidence of Atrial Fibrillation Detected by Continuous Rhythm Monitoring after Acute Myocardial Infarction in Patients with Pre-served Left Ventricular Ejection Fraction: Results of the ARREST Study. Europace, 20, 263-270. https://doi.org/10.1093/europace/euw344
- 41. Mascarenhas, D.A., Farooq, M.U., Ziegler, P.D. and Kantharia, B.K. (2016) Role of Insertable Cardiac Monitors in Anticoagulation Therapy in Patients with Atrial Fibrillation at High Risk of Bleeding. Europace, 18, 799-806. https://doi.org/10.1093/europace/euv350
- 42. Zuern, C.S., Kilias, A., Berlitz, P., et al. (2015) Anticoagulation after Catheter Ablation of Atrial Fibrillation Guided by Implantable Cardiac Monitors. Pacing and Clinical Electrophysiology: PACE, 38, 688-693. https://doi.org/10.1111/pace.12625