World Journal of Cancer Research
Vol.
12
No.
03
(
2022
), Article ID:
53528
,
11
pages
10.12677/WJCR.2022.123019
经多肽修饰后的联合化疗药及基因纳米复合物在靶向治疗喉癌干细胞的作用研究
许晓燕1,2,卫旭东1,2,3*
1甘肃中医药大学第一临床医学院,甘肃 兰州
2甘肃省人民医院耳鼻咽喉头颈外科,甘肃 兰州
3兰州大学第一临床医学院,甘肃 兰州
收稿日期:2022年6月17日;录用日期:2022年6月29日;发布日期:2022年7月12日

摘要
目的:靶向杀伤喉癌干细胞已是喉癌治疗的共识。方法:拟用前期已构建的共载PTX与Bmi-1RNAi的壳聚糖–聚乙二醇缓释纳米微粒(mPEG-CS-cRGD/Bmi-1RNAi-PTX),通过体外和体内试验证实mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒对喉癌Hep-2细胞和喉癌Hep-2细胞所建立的荷瘤裸鼠模型的增殖、凋亡能力的影响及其安全性。比较采用PTX和mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒作用前后CD133和C-myc基因的表达比例差异。结果:比单一应用PTX化疗相比,该纳米微粒联合化疗药物,在可以显著抑制喉癌Hep-2细胞增殖,促进凋亡,而且可以明显抑制移植瘤的生长,并且具有生物安全性。同时,在体外和体内分别可以降低喉癌干细胞的干性标记CD133和C-myc的表达量。结论:cRGD肽、紫杉醇与Bmi-1RNAi可以多重靶向杀伤喉癌干细胞,具有重叠增效机制。
关键词
纳米复合物,靶向治疗,喉癌

Effect of Peptide Modified Combined Chemotherapy Drugs and Gene Nanocomposites on Targeted Therapy of Laryngeal Cancer Stem Cells
Xiaoyan Xu1,2, Xudong Wei1,2,3*
1The First School of Clinical Medicine, Gansu University of Traditional Chinese Medicine, Lanzhou Gansu
2Department of E.N.T., Gansu Provincial People’s Hospital, Lanzhou Gansu
3The First School of Clinical Medicine, Lanzhou University, Lanzhou Gansu
Received: Jun. 17th, 2022; accepted: Jun. 29th, 2022; published: Jul. 12th, 2022

ABSTRACT
Objective: Targeted killing of laryngeal cancer stem cells has become a consensus in the treatment of laryngeal cancer. Methods: The chitosan-polyethylene glycol sustained-release nanoparticles (mPEG-CS-cRGD/Bmi-1RNAi-PTX) co-loaded with PTX and Bmi-1RNAi previously constructed were used. The effects of mPEG-CS-cRGD/Bmi-1RNAi-PTX nanoparticles on the proliferation and apoptosis of laryngeal carcinoma Hep-2 cells and laryngeal carcinoma Hep-2 cells in tumor-bearing nude mice models and their safety were confirmed by in vitro and in vivo experiments. The expression ratios of CD133 and C-myc genes before and after treatment with PTX and mPEG-CS-cRGD/Bmi-1RNAi-PTX nanoparticles were compared. Results: Compared with the single application of PTX chemotherapy, the nanoparticles combined with chemotherapy drugs can significantly inhibit the proliferation of laryngeal carcinoma Hep-2 cells, promote apoptosis, and significantly inhibit the growth of transplanted tumors, with biological safety. At the same time, the expression levels of CD133 and C-myc in laryngeal cancer stem cells can be reduced in vitro and in vivo, respectively. Conclusion: cRGD peptide, paclitaxel and Bmi-1RNAi can kill laryngeal cancer stem cells with multiple targets and have overlapping synergistic mechanism.
Keywords:Nanocomposites, Targeted Therapy, Laryngeal Cancer

Copyright © 2022 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
喉癌是仅次于肺癌的第二大呼吸道高发癌,在头颈部鳞状细胞癌(Head and Neck Squamous Cell Carcinoma, HNSCC)中位居第二 [1]。目前的治疗主要是手术切除,如全喉切除术和喉部分切除术不利于喉结构和功能的保持,对于大部分患者来说,造成心理负担和社会压力,最重要的是,治疗后的复发、转移、放疗抵抗及化疗耐受等问题,仍是患者致死的主要原因。因此,我们急需研发更加精准且副作用更小的新治疗方法。
紫杉醇(Paclitaxel, PTX)是一种从红豆杉属植物的树皮和针叶中分离的天然抗癌药物,属于细胞周期阻断剂,可以稳定微管,导致细胞生长抑制及凋亡,是常用的喉癌化疗药物之一。在全身化疗时,单一应用PTX存在全身毒副作用、水溶性差、靶向性差、治疗效率不高和容易出现多药耐药(Multidrug-Resistant, MDR)等问题 [2] [3]。
近年来,癌干细胞(Cancer Stem Cells, CSCs)理论认为,肿瘤中存在极少数具有无限增殖、多分化潜能的CSCs,是肿瘤无限增殖、复发和转移的根本原因 [4] [5]。CD133是一种分子量大小为120 KD的5次跨膜糖蛋白,被认为是正常造血干细胞的标志物 [6],研究认为,在喉癌Hep-2细胞中,CD133+细胞比CD133-细胞群有更高的自我更新、增殖、集落形成和体外成瘤能力,CD133是喉癌干细胞(Laryngeal Cancer Stem Cells, LCSCs)的生物标记之一 [7]。有研究报道,C-myc是CSCs相关的细胞因子,对于保持CSCs的干性具有重要作用。
纳米粒子(Nanoparticles, NPs)是微米级的胶体粒子,大小约10~100 nm。壳聚糖(Chitosan, CS)是近年来应用广泛的基因传输纳米载体,化学名为(1,4)-2-氨基-2-脱氧-B-D-葡聚糖,是甲壳素脱乙酰化的产物,其毒性极小甚至是无毒性、而且具有资源丰富、生物相容性较好和生物可降解性等优点,可同时连接化疗药物、多肽和目的基因等。但是CS具有疏水性,一旦进入体内,容易被网状内皮吞噬系统(Retieuloendothelial System, RES)所吞噬 [8] [9] [10] [11] [12]。聚乙二醇(Polyethylene Glycol, PEG)是一种以-CH2CH2O-为结构基础的支链或者直链大分子聚合物 [13]。CS经PEG修饰后,药物外排有效减少、渗透性和组织滞留性作用增强(Enhanced Permeation and Retention, EPR) [14] [15] [16]。
B细胞特异性MLV整合位点-1 (B Cell Specific MLV Integration Site-1, Bmi-1)是转录调节因子多梳基因(Polycomb Group Gene, PcG)家族的成员之一,它所涉及的信号系统参与了多种CSCs的自我更新 [17]。研究证实,Bmi-1与喉癌的淋巴转移和化疗耐药相关 [18] [19] [20],靶向下调Bmi-1基因可以有效抑制LCSCs的增殖和分化,使其对化疗药物PTX的敏感性增强 [21]。但在人体,未经包裹修饰的单纯Bmi-1 RNAi核苷酸片段易被体内核酸酶所降解,并且对肿瘤组织缺乏主动靶向性,不能精准有效地导入目的细胞 [22]。
精氨酸–甘氨酸–天冬氨酸(Glycine Aspartic Acid, RGD)肽是一类含有精氨酸–甘氨酸–天冬氨酸(ARG-GLy-ASP)序列的短肽,是整合素(Integrin, ITG) αvβ3 (ITG αvβ3)与其配体蛋白相互作用的识别位点。研究发现,ITG αv和ITG β3在喉癌细胞表面高表达,在肿瘤细胞表面有兼具黏附和信号传导功能的受体,基质蛋白RGD三肽序列能与整合素αvβ3特异性结合,在喉癌细胞的黏附、增殖、分化、侵袭、转移、凋亡等过程中起着重要的调控作用 [23] [24]。研究发现,环指结构域(Retieuloendothelial System, RF) RGD (cRGD)较线形RGD多肽有更高的受体结合特异性 [25] [26]。
基于以上理论基础阐述,本研究拟利用前期研究合成的Bmi-1RNAi重组质粒,构建cRGD肽修饰的共载PTX与Bmi-1RNAi的壳聚糖–聚乙二醇缓释纳米微粒(cRGD/CS-PEG-Bmi-1RNAi-PTX),检测其对喉癌杀伤作用和降低LCSCs的干性的作用研究。
2. 材料与方法
2.1. 细胞培养
Hep-2细胞(北京博奥森生物技术有限公司,北京,中国)培养在含10%小牛血清(FBS, Gibco, USA),100 U/mL的青链霉素的RPMI1640培养液(Hyclone, USA),于37℃、5% CO2培养箱中培养,每3~4天传代1次。
2.2. CCK-8试验
取生长对数期的喉癌Hep-2细胞,将细胞悬液铺于96孔细胞培养板,每孔对应细胞为1 × 103个 [27]。向96细胞培养板中加入PTX溶液(设置6个浓度:10、100、200、500、1000 和5000 ng/mL)共孵育(每孔对应体积为100 μL),每组分别设立6个重复孔。在相同培养条件下各培养24 h和48 h后,弃去旧培养液,向每孔分别加入10 μL cck-8溶液,孵育3 h,在酶标仪上检测450 nm波长的各孔吸收度(A值)。
2.3. 流式细胞术
取对数生长期的喉癌Hep-2细胞,按2 × 105个细胞的接种密度加入6孔细胞培养板,与PTX共培养48 h (对应PTX的浓度为90.11 ng/mL),之后更换成新鲜培养基继续培养24 h,重复3次上述步骤 [28]。随后用用mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米溶液处理48 h。细胞定量于每管1 × 106个细胞。按照1:100比例加入FITC标记的CD133抗体2 μL,上机检测,比较两组CD133的比例。
2.4. 实验动物
BALB/c nude裸鼠(中国北京,许可证号:SCXK(京) 2019-0010) (30只,均为雄性5~6周龄,体重18~21 g)根据国家动物福利要求,所有裸鼠均在兰州大学SPF级动物实验室(中国,兰州)喂养SPF级饲料和纯净水,室温维持在20℃~22℃。
2.5. 体内试验
BALB/c nude裸鼠被随机分为Free-PTX、mPEG-CS/PTX、mPEG-CS-cRGD/PTX和mPEG-CS-cRGD/Bmi-1RNAi-PTX五组,每组6只。取对数生长期的喉癌Hep-2细胞,将其制备成细胞悬液,按照每只5 × 106/mL,100 μL/只的剂量,进行颈背部皮下注射。接种后第二天开始测量肿瘤大小并称重,每天观察裸鼠健康状况。肿瘤生长计算公式为:V = (aXb2)/2 (a:长径,mm;b:短径,mm)。小鼠皮下注射喉癌Hep-2细胞第7天,肿瘤体积大约达到100 mm3后,隔两天在BALB/c nude裸鼠尾静脉按照组别分别注射PBS、Free-PTX、mPEG-CS-cRGD/PTX、mPEG-CS/PTX和mPEG-CS-cRGD/Bmi-1RNAi-PTX (PTX:5 mg/kg,Bmi-1RNAi:10 ug/只)各100 uL。每次给药前检测体重和肿瘤体积,绘制肿瘤生长曲线。control组肿瘤体积大约达到300 mm3后处死并剥离肿瘤,比较五组的移植肿瘤大小和小鼠体重改变。
2.6. 肿瘤组织苏木精和伊红染色(Stained with Hematoxylin and Eosin, H & E Staining)
用上述对应药物治疗19天后,取心脏、肝脏、肾脏,采用H&E染色进行组织学评价,将组织固定在4%多聚甲醛中,制作石蜡切片,按照文献提示及标准程序用苏木精和伊红染色后拍照 [29]。
2.7. 免疫组织化学
将肿瘤组织置于4%多聚甲醛固定后,脱水、透化和包埋,切成5 um的切片,将切片与一抗(C-myc, Mouse mAb, Proteintech, 1:200)孵育过夜后,与对应的辣根过氧化物酶相结合,最后染色、封片,拍照,用ImageJ软件定量阳性染色比例。
3. 结果
3.1. mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒对喉癌Hep-2细胞增殖能力的影响
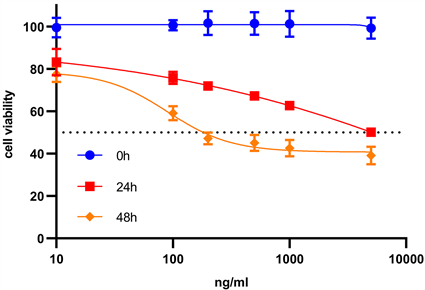
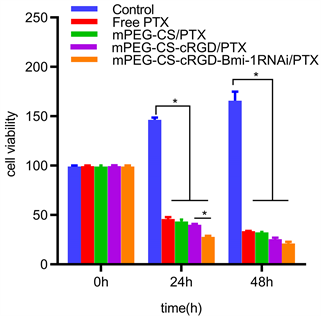
用cck-8增殖–毒性法检测mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒对喉癌Hep-2细胞增殖的影响。得到,PTX对喉癌Hep-2细胞的IC50为90.11 μg/mL (图1(a))。分别用Free PTX、mPEG-CS/PTX、mPEG-CS-cRGD/PTX和mPEG-CS-cRGD/Bmi-1RNAi-PTX溶液(对应PTX的终浓度为90.11 μg/mL)四组药物分别作用于喉癌Hep-2细胞24 h和48 h后,从增殖–毒性柱状图可以看出,对喉癌Hep-2细胞的毒性作用大小排列顺序为:Control < Free PTX < mPEG-CS/PTX < mPEG-CS-cRGD/PTX < mPEG-CS-cRGD/Bmi-1RNAi-PTX。mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒对喉癌Hep-2细胞的毒性最大,而且具有时间依赖性,即作用48 h后对应的细胞毒性高于24 h,细胞毒性随着作用时间延长而明显提高,Control组相比,其余四组均具有统计学差异(p < 0.001)。综上,我们可以得出,mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒可以发挥PTX、cRGD肽、Bmi-1RNAi的协同增效作用,而且比单用游离PTX相比,对喉癌Hep-2细胞有更显著的毒性。
 (a)
(a)
 (b)
(b)
Figure 1. CCK-8 proliferation-toxicity test. (a) The IC50 of PTX on laryngeal carcinoma Hep-2 cells was calculated; (b) The proliferation of laryngeal carcinoma Hep-2 cells was significantly inhibited by mPEG-CS-cRGD/Bmi-1RNAi-PTX nanoparticles
图1. CCK-8增殖–毒性试验。(a) 计算PTX对喉癌Hep-2细胞的IC50。(b) 观察mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒明显抑制喉癌Hep-2细胞的增殖能力
3.2. 流式细胞仪检测mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒对CD133比例的影响
流式细胞仪检测结果显示,喉癌Hep-2细胞中CD133中只有微量表达,其表面膜抗原表达量仅有2.797% ± 1.017% (图2(a))。结果表明,单一应用PTX于细胞,能使细胞表面CD133表达量升高,表达率为13.167% ± 1.677%,这是出现耐药现象的原因之一。而mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒能有效靶向LCSCs,降低CD133表达量,表达率为3.456% ± 0.299%,使得LCSCs干性降低(图2(b))。
3.3. mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒对裸鼠喉癌移植瘤的抑制作用
以BALB/c-nude裸鼠制作喉癌动物模型,观察纳米药物微粒对喉癌细胞的体内抑制作用。给药处理后19天后发现,肿瘤体积大小的排列顺序为:Control > Free PTX > mPEG-CS/PTX > mPEG-CS-cRGD/PTX > mPEG-CS-cRGD/Bmi-1RNAi-PTX。mPEG-CS-cRGD/Bmi-1RNAi-PTX组的肿瘤体积呈下降趋势,趋势显著,而且与Control组相比都有显著性差异(p < 0.001) (图3(a), 图3(b))。
3.4. mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒作用于裸鼠体内的安全性评价
通过H&E组织染色进一步分析给药后裸鼠的脏器组织切片情况。发现各组的心脏、肾脏、肝脏均未见明显炎症、细胞坏死和凋亡。表明mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒在一定药物浓度范围内(PTX:5 mg/kg,Bmi-1RNAi 10 μg/只))对裸鼠具有生物安全性(图4)。
3.5. mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒在裸鼠体内减少C-myc的表达
为进一步验证mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒可以在体内通过降低LCSCs相关干性基因C-myc表达,用C-myc抗体对喉癌异体种植瘤进行免疫组织化学分析显示,Free PTX组相比于Control组,其C-myc表达量稍高,分别为0.797 ± 0.033和0.767 ± 0.582,但是无统计学意义(p = 0.536),可能与PTX作用的时间短,尚无在异位移植瘤中出现大量LCSCs有关,但是mPEG-CS/PTX组、mPEG-CS-cRGD/PTX组和mPEG-CS-cRGD/Bmi-1RNAi-PTX组3组的C-myc表达量均降低(p < 0.001),分别有0.503 ± 0.09、0.333 ± 0.052、0.174 ± 0.010 (图5(b))。实验结果提示,mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒在裸鼠体内能靶向到LCSCs,通过减少肿瘤细胞中C-myc的表达量,从而降低LCSCs的干性表达。
 (a)
(a)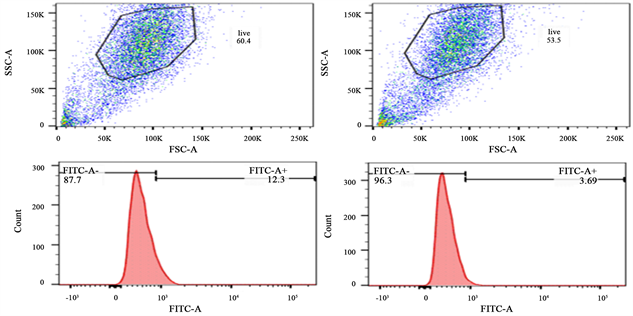 (b)
(b)
Figure 2. Flow cytometry. (a) Detection of CD133 expression in laryngeal carcinoma Hep-2 cells by flow cytometry; (b) mPEG-CS-cRGD/Bmi-1RNAi-PTX nanoparticles significantly decreased CD133 expression
图2. 流式细胞术。(a) 流式细胞术检测喉癌Hep-2细胞的CD133表达量;(b) mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒明显降低CD133表达量
 (a)
(a)
 (b)
(b)
Figure 3. Nude mice in vivo. (a) mPEG-CS-cRGD/Bmi-1RNAi-PTX nanoparticles had significant tumor inhibitory effect in nude mice; (b) Statistical analysis of tumor volume, *represents p < 0.05, **represents p < 0.01, ***represents p < 0.001, where n = 6
图3. 体内裸鼠试验。(a) mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒在裸鼠体内有明显的抑瘤作用;(b) 对肿瘤体积进行分析统计,*代表p < 0.05,**代表p < 0.01,***代表p < 0.001,其中n = 6
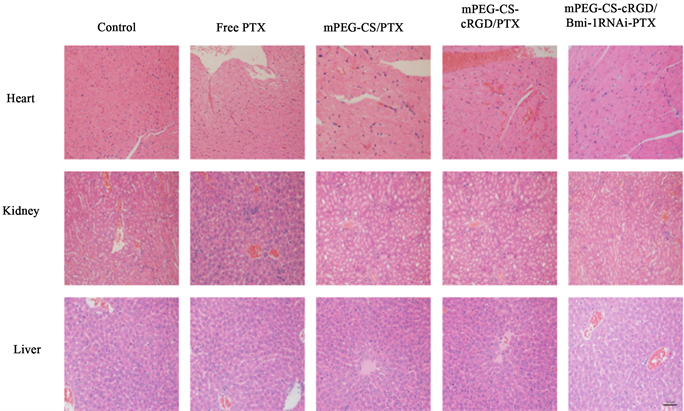
Figure 4. The mPEG-CS-cRGD/Bmi-1RNAi-PTX nanoparticles have biological safety with a scale of 50 μm
图4. mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒具有生物安全性,标尺 = 50 μm
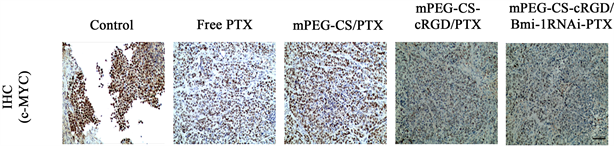 (a)
(a)
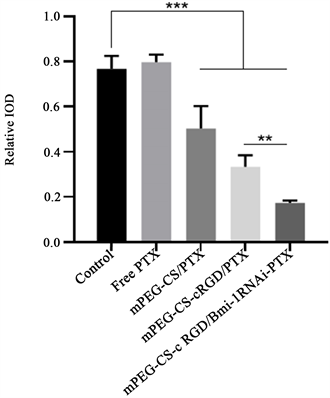 (b)
(b)
Figure 5. Immunohistochemical staining. (a): Immunohistochemical analysis was performed on the tumor sections of nude mice in each group, with a scale of 50 μm; (b): mPEG-CS-cRGD/Bmi-1RNAi-PTX nanoparticles can effectively reduce the expression of C-myc in tumor cells, **represents p < 0.01, **represents p < 0.001, where n = 6
图5. 免疫组织化学染色。(a):对各组裸鼠的肿瘤切片进行免疫组织化学分析,标尺 = 50 μm;(b):mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒可以有效减少肿瘤细胞C-myc的表达量,**代表p < 0.01,***代表p < 0.001,其中n = 6
4. 讨论
本实验在前期已合成的一种能多重靶向LCSCs的纳米微粒,即mPEG-CS-cRGD/Bmi-1RNAi-PTX,已证明,该纳米药物微粒形态规整,粒径小,包封率高,载药量大,具有低细胞毒性,是理想的基因药物治疗微粒。聚合纳米颗粒是一种应用于纳米医学的纳米技术 [30]。而且纳米孔或者纳米通道容易受外界PH因素刺激,导致离子传输性质的改变,即PH响应性,mPEG-CS NPs属于PH响应型NPs [31] [32] [33],肿瘤细胞膜带负电荷,合成的mPEG-CS纳米粒子带正电,在一定程度上实现了对所携带物质的可控性释放 [34] [35],同时增强了肿瘤细胞和组织摄取目的分子的能力 [36] [37]。正常组织的血管的间隙小,微血管内皮细胞间隙不到10 nm,紧密连接,而肿瘤组织的新生血管间隙较大,具有“小孔”,表现为一定的渗漏性,直径一般在380~780 nm之间,课题组前期对mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米微粒进行表征评价,观察到其近似球形,分布较为均匀,测量其大小约为182 nm ± 30.6 nm [38],因此可以被动靶向穿透肿瘤细胞膜,将目的分子和药物从细胞靶向递送到实体肿瘤组织。与单一PTX化疗治疗相比,转染纳米药物复合物后的喉癌细胞,体外增殖及分化能力下降,细胞凋亡增多,体内成瘤性明显减低,抑瘤显著。利用cRGD肽与LCSCs表面整合素αvβ3特异性结合的特点,可将外源性RGD肽与纳米载体偶联构建靶向纳米载体,提高纳米药物的主动靶向性,与纳米粒子的被动靶向性相结合,可将效应分子精确的导入肿瘤部位,发挥抑瘤作用,有效避开肿瘤治疗中对正常组织细胞的损害。纳米复合物在体内及体外均可高效靶向转染LCSCs,沉默Bmi-1基因,实现PTX的精准化疗。同时通过敲除Bmi-1基因、联合PTX精准化疗、cRGD靶向肽三者结合,纳米复合物微粒能靶向LCSCs,减少CD133和C-myc的基因表达量,降低LCSCs的干性表达,是较为理想的喉癌靶向药物之一。但是在本纳米复合物在体内可能受体液,酸碱度等影响,粒径改变,可能出现析出现象,影响作用效果,总体的制备工艺尚需改正,综述所述,该纳米复合物总体上有望成为一种新型的、安全有效的、通过多重靶向LCSCs来杀伤喉癌的纳米药物。
致谢
首先感谢国家自然基金委员会对这篇文章的支持与帮助(No. 81860475)。
文章引用
许晓燕,卫旭东. 经多肽修饰后的联合化疗药及基因纳米复合物在靶向治疗喉癌干细胞的作用研究
Effect of Peptide Modified Combined Chemotherapy Drugs and Gene Nanocomposites on Targeted Therapy of Laryngeal Cancer Stem Cells[J]. 世界肿瘤研究, 2022, 12(03): 136-146. https://doi.org/10.12677/WJCR.2022.123019
参考文献
- 1. Steinbichler, T.B., Dudás, J., Skvortsov, S., et al. (2018) Therapy Resistance Mediated by Cancer Stem Cells. Seminars in Cancer Biology, 53, 156-167.
https://doi.org/10.1016/j.semcancer.2018.11.006 - 2. Jia, H.R., Jiang, Y.W., Zhu, Y.X., et al. (2017) Plasma Membrane Activatable Polymeric Nanotheranostics with Self- Enhanced Light-Triggered Photosensitizer Cellular Influx for Photodynamic Cancer Therapy. Journal of Controlled Release, 255, 231-241.
https://doi.org/10.1016/j.jconrel.2017.04.030 - 3. Wathoni, N., Rusdin, A., Febriani, E., et al. (2019) Formulation and Characterization of α-Mangostin in Chitosan Nanoparticles Coated by Sodium Alginate, Sodium Silicate, and Polyethylene Glycol. Journal of Pharmacy and Bioallied Sciences, 11, S619-S627.
https://doi.org/10.4103/jpbs.JPBS_206_19 - 4. Strączek, T., Fiejdasz, S., Rybicki, D., et al. (2019) Dynamics of Superparamagnetic IronOxide Nanoparticles with Various Polymeric Coatings. Materials, 12, Article No. 1793.
https://doi.org/10.3390/ma12111793 - 5. Ait Bachir, Z., Huang, Y., He, M., et al. (2018) Effects of PEG Surface Density and Chain Length on the Pharmacokinetics and Biodistribution of Methotrexate-Loaded Chitosan Nanoparticles. International Journal of Nanomedicine, 13, 5657-5671.
https://doi.org/10.2147/IJN.S167443 - 6. Chu, L., Zhang, Y., Feng, Z., et al. (2019) Synthesis and Application of a Series of Amphipathic Chitosan Derivatives and the Corresponding Magnetic Nanoparticle-Embedded Polymeric Micelles. Carbohydrate Polymers, 223, Article ID: 114966.
https://doi.org/10.1016/j.carbpol.2019.06.005 - 7. Fang, X., Jiang, W., Huang, Y., et al. (2017) Size Changeable Nanosystems for Precise Drug Controlled Release and Efficient Overcoming of Cancer Multidrug Resistance. Journal of Materials Chemistry B, 5, 944-952.
https://doi.org/10.1039/C6TB02361D - 8. Suo, A., Qian, J., Zhang, Y., Liu, R., et al. (2016) Comb-Like Amphiphilic Polypeptide-Based Copolymer Nanomicelles for Co-Delivery of Doxorubicin and P-gp siRNA into MCF-7 Cells. Materials Science & Engineering C: Materials for Biological Applications, 62, 564-573.
https://doi.org/10.1016/j.msec.2016.02.007 - 9. Amani, A., Dustparast, M., Noruzpour, M., et al. (2021) Design and in Vitro Characterization of Green Synthesized Magnetic Nanoparticles Conjugated with Multitargeted Poly Lactic Acid Copolymers for Co-Delivery of siRNA and Paclitaxel. European Journal of Pharmaceutical Sciences, 167, Article ID: 106007.
https://doi.org/10.1016/j.ejps.2021.106007 - 10. Chung, S. and Zhang, M. (2021) Microwave-Assisted Synthesis of Carbon Dot-Iron Oxide Nanoparticles for Fluorescence Imaging and Therapy. Frontiers in Bioengineering and Biotechnology, 9, Article ID: 711534.
https://doi.org/10.3389/fbioe.2021.711534 - 11. 吕睿. mPEG-CS-cRGD/Bmi-1RNAi-PTX纳米缓释微粒的构建及体外杀伤喉癌细胞作用研究[D]: [硕士学位论文].兰州: 甘肃中医药大学, 2020.
- 12. Wei, K.R., Zheng, R.S., Liang, Z.H., et al. (2018) Incidence and Mortality of Laryngeal Cancer in China, 2008-2012. Chinese Journal of Cancer Research, 30, 299-306.
https://doi.org/10.21147/j.issn.1000-9604.2018.03.02 - 13. Liu, H.T., Xia, T., You, Y.W., et al. (2021) Characteristics and Clinical Significance of Polyploid Giant Cancer Cells in Laryngeal Carcinoma. Laryngoscope Investigative Otolaryngology, 6, 1228-1234.
https://doi.org/10.1002/lio2.667 - 14. Ghofrani, M., Shirmard, L.R., Dehghankelishadi, P., et al. (2019) Development of Octreotide-Loaded Chitosan and Heparin Nanoparticles: Evaluation of Surface Modification Effect on Physicochemical Properties and Macrophage Uptake. Journal of Pharmaceutical Sciences, 108, 3036-3045.
https://doi.org/10.1016/j.xphs.2019.05.002 - 15. Ayob, A.Z. and Ramasamy, T.S. (2018) Cancer Stem Cells as Key Drivers of Tumour Progression. Journal of Biomedical Science, 25, Article No. 20.
https://doi.org/10.1186/s12929-018-0426-4 - 16. Kuşoğlu, A. and Biray Avcı, Ç. (2019) Cancer Stem Cells: A Brief Review of the Current Status. Gene, 681, 80-85.
https://doi.org/10.1016/j.gene.2018.09.052 - 17. Arndt, K., Grinenko, T., Mende, N., et al. (2013) CD133 Is a Modifier of Hematopoietic Progenitor Frequencies but Is Dispensable for the Maintenance of Mouse Hematopoietic Stem Cells. Proceedings of the National Academy of Sciences of the United States of America, 110, 5582-5587.
https://doi.org/10.1073/pnas.1215438110 - 18. Zhou, L., Wei, X., Cheng, L., et al. (2007) CD133, One of the Markers of Cancer Stem Cells in Hep-2 Cell Line. Laryngoscope, 117, 455-460.
https://doi.org/10.1097/01.mlg.0000251586.15299.35 - 19. Rizeq, B.R., Younes, N.N., Rasool, K., et al. (2019) Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan- Based Nanoparticles. International Journal of Molecular Sciences, 20, Article No. 5776.
https://doi.org/10.3390/ijms20225776 - 20. Amirani E., Hallajzadeh J., Asemi Z., et al. (2020) Effects of Chitosan and Oligochitosans on the Phosphatidylinositol 3-Kinase-AKT Pathway in Cancer Therapy. International Journal of Biological Macromolecules, 164, 456-467.
https://doi.org/10.1016/j.ijbiomac.2020.07.137 - 21. Helmi, O., Elshishiny, F. and Mamdouh, W. (2021) Targeted Doxorubicin Delivery and Release within Breast Cancer Environment Using PEGylated Chitosan Nanoparticles Labeled with Monoclonal Antibodies. International Journal of Biological Macromolecules, 184, 325-338.
https://doi.org/10.1016/j.ijbiomac.2021.06.014 - 22. Veiseh, O., Kievit, F.M., Fang, C., et al. (2010) Chlorotoxin Bound Magnetic Nanovector Tailored for Cancer Cell Targeting, Imaging, and siRNA Delivery. Biomaterials, 31, 8032-8042.
https://doi.org/10.1016/j.biomaterials.2010.07.016 - 23. Arya, G., Das, M. and Sahoo, S.K. (2018) Evaluation of Curcumin Loaded Chitosan/PEG Blended PLGA Nanoparticles for Effective Treatment of Pancreatic Cancer. Biomedicine & Pharmacotherapy, 102, 555-566.
https://doi.org/10.1016/j.biopha.2018.03.101 - 24. Fang, X., Wang, X., Li, G., et al. (2018) SS-mPEG Chemical Modification of Recombinant Phospholipase C for Enhanced Thermal Stability and Catalytic Efficiency. International Journal of Biological Macromolecules, 111, 1032- 1039.
https://doi.org/10.1016/j.ijbiomac.2018.01.134 - 25. Sharifi, F., Jahangiri, M. and Ebrahimnejad, P. (2021) Ebrahimnejad. Synthesis of Novel Polymeric Nanoparticles (Methoxy-Polyethylene Glycol-Chitosan/Hyaluronic Acid) Containing 7-Ethyl-10-Hydroxycamptothecin for Colon Cancer Therapy: In Vitro, ex Vivo and in Vivo Investigation. Artificial Cells, Nanomedicine and Biotechnology, 49, 367-380.
https://doi.org/10.1080/21691401.2021.1907393 - 26. Ramnandan, D., Mokhosi, S., Daniels, A., et al. (2021) Polyethylene Glycol and Polyvinyl Alcohol Modified MgFe2O4 Ferrite Magnetic Nanoparticles in Doxorubicin Delivery: A Comparative Study in Vitro. Molecules, 26, Article No. 3893.
https://doi.org/10.3390/molecules26133893 - 27. Kang, J.W., Cho, H.J., Lee, H.J., et al. (2019) Polyethylene Glycol-Decorated Doxorubicin/Carboxymethyl Chitosan/ Gold Nanocomplex for Reducing Drug Efflux in Cancer Cells and Extending Circulation in Blood Stream. International Journal of Biological Macromolecules, 125, 61-71.
https://doi.org/10.1016/j.ijbiomac.2018.12.028 - 28. Zhang, X., Guo, W., Wang, X., et al. (2016) Antitumor Activity and Inhibitory Effects on Cancer Stem Cell-Like Properties of Adeno-Associated Virus (AAV)-Mediated Bmi-1 Interference Driven by Bmi-1 Promoter for Gastric Cancer. Oncotarget, 7, 22733-22745.
https://doi.org/10.18632/oncotarget.8174 - 29. Allegra, E., Puzzo, L., Zuccalà, V., et al. (2012) Nuclear BMI-1 Expression in Laryngeal Carcinoma Correlates with Lymph Node Pathological Status. World Journal of Surgical Oncology, 10, Article No. 206.
https://doi.org/10.1186/1477-7819-10-206 - 30. Yu, D., Liu, Y., Yang, J., et al. (2015) Clinical Implications of BMI-1 in Cancer Stem Cells of Laryngeal Carcinoma. Cell Biochemistry and Biophysics, 71, 261-269.
https://doi.org/10.1007/s12013-014-0194-z - 31. Qin, L., Zhang, X., Zhang, L., et al. (2008) Downregulation of BMI-1 Enhances 5-Fluorouracil-Induced Apoptosis in Nasopharyngeal Carcinoma Cells. Biochemical and Biophysical Research Communications, 371, 531-535.
https://doi.org/10.1016/j.bbrc.2008.04.117 - 32. Wei, X., He, J., Wang, J., et al. (2015) Bmi-1 Is Essential for the Oncogenic Potential in CD133+ Human Laryngeal Cancer Cells. Tumor Biology, 36, 8931-8942.
https://doi.org/10.1007/s13277-015-3541-9 - 33. Liu, L., Li, H., Zhang, M., et al. (2015) Effects of Targeted Nano-Delivery Systems Combined with hTERT-siRNA and Bmi-1-siRNA on MCF-7 Cells. International Journal of Clinical and Experimental Pathology, 8, 6674-6682.
- 34. Jin, Y., Lv, L., Ning, S.X., et al. (2015) The Anti-Tumor Activity and Mechanisms of rLj-RGD3 on Human Laryngeal Squamous Carcinoma Hep2 Cells. Anti-Cancer Agents in Medicinal Chemistry, 19, 2108-2119.
https://doi.org/10.2174/1871520619666191022160024 - 35. Wang, J.T., Liu, Y., Kan, X., et al. (2014) Cilengitide, a Small Molecule Antagonist, Targeted to Integrin αν Inhibits Proliferation and Induces Apoptosis of Laryngeal Cancer Cells in Vitro. European Archives of Oto-Rhino-Laryngology, 271, 2233-2240.
https://doi.org/10.1007/s00405-014-2918-5 - 36. Gajbhiye, K.R., Gajbhiye, V., Siddiqui, I.A., et al. (2018) cRGD Functionalised Nanocarriers for Targeted Delivery of Bioactives. Journal of Drug Targeting, 27, 111-124.
https://doi.org/10.1080/1061186X.2018.1473409 - 37. Shao, F., Lv, M., Zheng, Y., et al. (2015) The Anti-Tumour Activity of rLj-RGD4, an RGD Toxin Protein from Lampetra japonica, on Human Laryngeal Squamous Carcinoma Hep-2 Cells in Nude Mice. Biochimie, 119, 183-191.
https://doi.org/10.1016/j.biochi.2015.11.004 - 38. Wang, J., Fan, J., Gao, W., et al. (2020) LY6D as a Chemoresistance Marker Gene and Therapeutic Target for Laryngeal Squamous Cell Carcinoma. Stem Cells and Development, 29, 774-785.
https://doi.org/10.1089/scd.2019.0210
NOTES
*通讯作者。