Hans Journal of Surgery
Vol.
09
No.
02
(
2020
), Article ID:
34963
,
8
pages
10.12677/HJS.2020.92008
The Surgical Treatment of Deep Sternal Wound Infections after Thoracic Aortic Prosthesis Graft
Jifu Liu1,2, Yongshun Gao2, Feng Zhou1, Shou Huang1, Peng Wang1, Ming Yang1
1Department of Thoracic Surgery, Beijing ChaoYang Emergency Center of Integrated Traditional Chinese and Western Medicine, Beijing
2Department of Cardiothoracic Surgery, The Seventh Medical Center, General Hospital of PLA, Beijing

Received: Mar. 20th, 2020; accepted: Apr. 3rd, 2020; published: Apr. 10th, 2020

ABSTRACT
Object: To study surgical effects of deep sternal wound infections (DSWI) treated after thoracic aortic prosthesis graft (TAPG). Method: 25 patients were complicated with DSWI after TAPG, male 19 cases, female 6, age 24 - 69 y. The patients had aortic dissecting aneurysm, underwent ascending aorta and arterial arch replacement, and 8 patients underwent complex heart valve or coronary artery surgery. There were 18 patients with early DSWI, 7 with late. 6 cases of all patients were complicating systemic infection. The incision is suitable for the short-term use of vacuum sealing drainage (VSD) in patients before surgery. Surgical Method: Under general anesthesia, the sternal and mediastinal infection was debrided by the strict and thorough procedure flow sheet, and the dead space in the mediastinal was completely obliterated by the major muscle flaps to turn inward, by the special suture method without foreign matter remaining in the wound. Results: I stage wound healing by the surgery was in the 24 of 25 patients with DSWI after TAPG (96.0%). 22 patients completely recovered after the operation. The prosthesis ascending aortic fistula was found for 1 patient during late surgery and healed by reoperation. 2 cases died respectively from multiple organ failure and migratory intracranial abscess. 1 case worsened from migratory abscesses 4 months after surgery. Conclusion: The patients with TAPG complicated DSWI diagnosed early and operated actively, had high wound healing rate, and better cure rate.
Keywords:Thoracic Aortic Prosthesis Graft, Deep Sternal Wound Infection, Systemic Infection, Surgery

胸主动脉置换后胸骨切口深部感染的外科治疗
刘吉福1,2,高永顺2,周峰1,黄铄1,王鹏1,杨明1
1北京朝阳中西医结合急诊抢救中心胸外科,北京
2中国人民解放军总医院第七医学中心胸心外科,北京

收稿日期:2020年3月20日;录用日期:2020年4月3日;发布日期:2020年4月10日

摘 要
目的:探讨胸主动脉置换后胸骨切口深部感染的手术治疗效果。方法:25例胸主动脉置换术后并发胸骨深部感染,男19例,女6例;年龄24~69岁。患者为主动脉夹层动脉瘤,行升主动脉及动脉弓置换,8例复合心瓣膜或冠脉手术。早期感染18例,后期7例。25例中有6例并发全身感染。切口适宜做负压引流装置(VSD)患者术前短期使用。手术方法:全麻下行胸骨伤口和纵膈感染手术治疗,以严格、彻底的清创操作流程,用胸大肌瓣内翻消除纵膈人工血管周围和切口残腔,用特殊方式缝合切口,使伤口内无异物存留。结果:25例胸主动脉置换并发胸骨切口深部感染,术后24例切口愈合(96.0%);完全康复22例(88.0%)。后期升主动脉瘘1例,再手术治愈。2例因多器官衰竭和颅内迁徙性脓肿死亡;1例术后4月再现迁徙性脓肿病情恶化。结论:胸部大血管置换后伴胸骨深部感染的早期诊断和积极手术清除感染灶,切口I期愈合率高;总治愈率好。
关键词 :胸部大血管置换,胸骨切口深部感染,全身感染,外科治疗

Copyright © 2020 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
胸主动脉置换后胸骨切口深部感染(Deep Sternal Wound Infection, DSWI)比心脏术后发生感染的处理更困难、更复杂,原因是置换的人工血管在原位本身就是一种异物,病菌侵入血管壁,致感染灶难以消除,增加治疗的难度;而且随着时间的延长,细菌经人工血管壁侵入血流,形成全身感染(Systemic Infection, SI),既菌血症或脓毒血症,加重了对患者生命的威胁。自2017年6月至2019年10月收治心脏、大血管手术后胸骨切口深部感染患者共187例,其中胸部大动脉置换后发生感染25例,占13.4%。现将治疗结果和治疗难点做一报道。
2. 临床资料及方法
2.1. 一般资料
25例患者中,男19例,女6例,年龄24~69岁,平均50.8 ± 12.1。患者均为A型主动脉夹层动脉瘤,6例升主动脉置换;10例升主动脉和右半弓、头臂血管置换和左半弓腔内修复;复合心脏手术8例,其中联合心脏瓣膜置换或瓣膜成形6例,联合冠脉搭桥术2例;1例联合颈动脉置换和腹主动脉腔内修复术。25例术后均发生胸骨切口深部感染,早期感染18例,术后感染时间3天~120天,平均33.5 ± 30.5;切口哆开范围大小不等和胸骨异常活动;后期感染7例,术后感染时间5~48个月,平均14.7 ± 13.9月。胸骨均有窦道和胸骨旁压痛。确认胸骨感染至接受本方法治疗的时间5~24月,平均12.8 ± 8.3月。纵膈人工血管周围脓肿6例,最大脓肿12.0 × 10.0 × 8.0 cm,吸出脓液250 ml。1例有残留起搏导线1根。其中6例有全身感染(24.0%),2例有迁徙性脓肿,4例菌血症;其中1例大血管再次置换(见图1、图2)。3例做了PET-CT检查,显示感染范围及肝内迁徙性小脓肿。
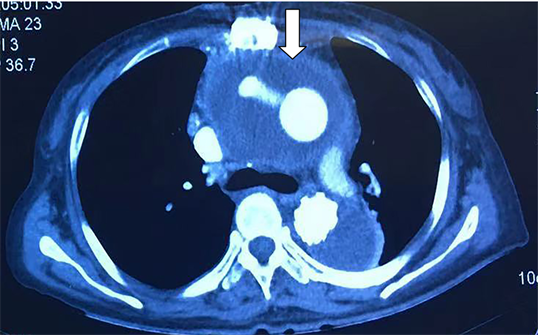
Figure 1. Enhanced chest CT, female, 50 years old, 3 months after ascending aortic replacement, dehiscence of the upper sternal incision, purulent discharge from the wound. Reduced area around the prosthetic aorta (arrow show)
图1. 胸部强化CT扫描,女性50岁,升主动脉置换术后3月,胸骨切口上段裂开,有脓性物流出。主动脉周围积液(箭头指的位置)
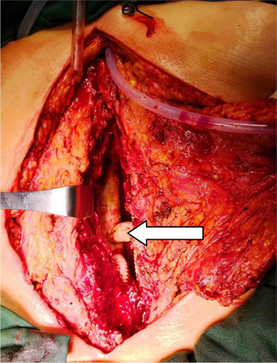
Figure 2. Intraoperative display: The giant pus cavity in the mediastinum, the prosthetic vessel immersed. After aspirate pus, visible the prosthetic aorta
图2. 术中见纵膈内巨大脓肿,人工血管浸泡其中,清除脓液后可见人工血管(箭头指位置)
14例患者住院前曾经接受切口清创手术1~4次,平均1.3次。
2.2. 胸骨切口术前负压引流装置(VSD)处理
11例早期患者适宜使用负压引流装置(Vacuum Sealing Drainage, VSD),切口哆开,胸骨缝合钢丝将劈开的胸骨一半切割,胸骨异常活动,伤口分泌物多。VSD术前短期使用,稀释的碘伏液冲洗,每日一次。
2.3. 胸骨切口深部感染的手术方法 [1]
全身麻醉下,采用严格、彻底的清创流程后,后期患者注意清除坏死的胸骨及肋软骨,使伤口达到无菌或低菌落状态;不使用任何材料固定胸骨;据胸骨切口和纵膈残腔的大小,需用一侧或两侧胸大肌瓣。将皮下与胸大肌筋膜充分游离后,在腋前线切断胸大肌,保留肋间穿支血供制备成胸大肌瓣,使其向内翻转至胸骨切口及前纵膈内,消灭纵膈、人工血管周围残腔,并成形胸廓;以特殊的减张缝合方式将肌瓣与皮下、皮肤固定,切口皮肤皮下全层缝合。拆除缝线后使切口内无任何异物残留。
2.4. 微生物检测及抗生素的选择
在获得细菌培养结果之前,多以经验性使用抗生素;如获得2次以上细菌培养一致的结果,据药敏结果使用抗生素。本组12例伤口分泌物细菌培养获得病源菌,其中6例血培养获得与伤口病菌一致,有关培养菌种及耐药情况(见表1)。
Table 1. Pathogens detected in sternum wound and blood
表1. 12例伤口分泌物和血培养细菌的结果
表注:表中“列”是实际例数;“行”有6例血和伤口分泌物培养是相同菌种,是同一例。多重耐药:三类以上抗生素耐药;敏感:头孢类、喹诺酮类、氨基糖类抗生素等均敏感。
术后规范性抗生素使用时间,一般无血行感染、伤口愈合良好者,连续使用2~3周;有血行感染者清创、胸廓成形术后需用4~6周,必要时延长。
2.5. 随访指标
患者出院后,每3~6月随访1次;采用门诊复诊或视频通话、影像资料随访。随访时间3~28月,平均17.2 ± 8.7月;随访完全。
3. 结果
11例术前短期使用VSD装置,消除了胸骨异常活动,改善患者活动能力、精神状态,促进伤口分泌物引流。
25例中,19例无血行感染者手术清创成形后一次性治愈,无复发;其中1例术后(5个月)置换的人工血管发生瘘形成假性动脉瘤,再次予以手术修补治愈(见图3)。6例有血行感染的患者治疗后胸骨切口I期愈合和残留窦道各3例。3例残留窦道者,2例术后仍有菌血症,其中1例因置换血管内感染血栓,再次血管置换,另1例再清创、肌瓣成形治愈。
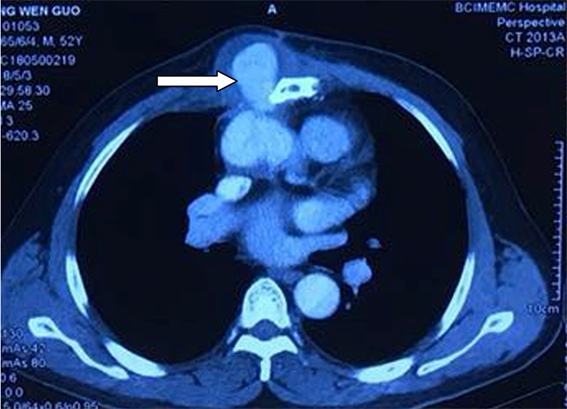
Figure 3. Enhanced chest CT, male 52 years old, 4 years after ascending aortic replacement, 5 months after sternum debridement, finding parasternal pseudoaneurysm (arrow show)
图3. 胸部强化CT轴位扫描,52岁男性,升主动脉置换后4年,胸骨感染清创术后5个月,胸骨旁出现假性动脉瘤(箭头位置)
切口愈合率96.0% (24/25例),其中一次性手术治愈22例,再手术治愈2例。
术后痊愈22例(占88%);病情恶化1例,术后3个月再出现纵膈、腰椎旁迁徙性脓肿;2例死亡,多器官衰竭和术后迁徙性颅内脓肿各1例(见表2)。
再现迁徙性脓肿2例,术后均拒绝规范抗生素治疗。
Table 2. Surgical results of TPGI with DSWI for 25 patients
表2. 25例大血管置换后胸骨深部切口感染
注释:1.B (Bentall’s) = 奔突式术;2. E (Endovascular repair) = 血管腔内修复术;3. D = 狄蓓科(Debeiky)手术;4. CABG (Coronary artery bypass graft) = 冠脉搭桥术;5. MVR=二尖瓣置换;6. AVR=主动脉瓣置换;7. AVP = 主动脉瓣成形;8. MOF = 多器官衰竭;9. AF = 主动脉瘘;10. MA (Migratory abscess) = 迁徙性脓肿;11. PG (prosthesis graft) = 人工血管再置换。
25例中12例获致病菌,6例血和伤口分泌物病源菌相同;11例菌种为多重耐药(91.7%) (见表1)。预后不良的患者均为多重耐药菌感染。
4. 讨论
心脏大血管术后并发胸骨切口深部感染,是一种灾难性并发症,对胸心外科医师是一种挑战。人工血管植入感染(Prosthetic Graft Infection, PGI)率1%~6%,与心脏术后切口感染率相似 [2]。因胸骨正中切口、纵膈感染易侵蚀置换的大血管,致血管内感染性血栓形成和全身血行感染和血管破裂的危险;人工血管异物的存在,增加了感染伤口治疗困难和效果。有关这种并发症的治疗报道较少,现将本组诊治要点做相关讨论。
早期诊断胸主动脉置换后胸骨切口深部感染(DSWI)是治愈感染的重要环节 当DSWI的细菌尚未侵入置换血管壁的深部,血管内感染性血栓未形成前更容易治愈。有依据表明PGI几乎都是手术部位感染所致 [3]。早期诊断要临床、影像、细菌学为关注点。注意切口有局部压痛或脓性分泌物,伴体温和血象增高;要及时胸部CT检查。影像重点关注皮下、劈开的胸骨间、前纵隔有否积液;置换的血管周围状况。必要时可在影像引导下穿刺,易获得诊断依据。WHO疾控中心(CDC)分类,符合下列标准之一可诊断PGI:①从患者移植血管周围区域无菌采集的活检或拭子标本中培养有微生物;②有感染累及移植物和周围组织的病理学或放射学证据;或③患者有血管移植物,没有其他感染灶者,而有连续菌血症 [4]。本组患者入院时,早期患者均有不同程度的伤口裂开,胸骨异常活动,至此已错过了早期诊断;其中4例感染伴有纵膈残存脓肿形成,1例脓腔巨大,如能得到早期诊断,可能免去后患。胸部人工血管置换后出现不明原因发热或持续菌血症,应该考虑大血管感染的可能 [5]。PET-CT检查有助于诊断感染范围、置换的血管状况、迁徙性小脓肿的发现,本组结果与文献报道一致 [6] [7]。
胸部大血管置换后感染分类,Tossios等 [8] 按时间分为早期和后期感染两类,以4个月为界限,4个月内和以后为早期和后期。本组患者早期18例,切口哆开,常显露出人工血管;有发热、血象高等全身症状;晚期切口多呈窦道状,感染已累及胸骨和肋软骨并延及人工血管。
胸骨及纵膈感染灶的清除是治愈的关键措施 一旦明确诊断,手术部位感染灶的尽早、尽快消除十分重要。随着时间的延长,人工血管被侵蚀,管腔内感染血栓形成,是感染难以控制、全身播散的重要源灶。积极手术治疗,采用本团队手术技术方法,可显著提高治愈机率。有报道在发生感染3~5天和1周后手术治疗,其住院天数和医疗花费显著不同 [9]。与我们的思路和结果基本一致。本组一次性手术治愈率达88.0%,与Suzuki等 [10] 报道植入血管再置换和非手术治疗相比,其治愈率显著增高。
清创治疗后再出现迁徙性脓肿与抗生素使用密切相关 迁徙性脓肿是全身感染脓毒血症的重要临床表现,更易威胁生命。本组2例手术3个月后出现迁徙性脓肿,均为肺炎克雷伯菌感染,多重耐药,只有替加环素敏感,治疗3周后患者拒绝再进一步治疗。本组结果更支持术后抗生素使用时间要足够长(6周左右)。
DSWI恰当使用VSD可以改善患者伤口及全身状况VSD可减少细菌生物被膜的形成、降低创面细菌菌落已被实验证实 [11]。DSWI有关应用VSD的报道主要作为切口纵膈感染术前准备,但达到伤口细菌培养阴性则耗时长,而治愈率也仅66.7% [12];低于本组88.0%的结果。亦有报道VSD可导致出血、纵膈组织损伤等并发症 [13]。本组VSD使用仅作为术前感染创口和全身条件的初步准备,对于切口哆开、胸骨不稳定,切口分泌物多者,可显著改善患者全身状况和消除切口裂开给患者带来的精神焦虑,但不宜长时间使用,以免延误有利的手术时机。术前12小时应去除VSD封膜,避免切口周围皮肤红肿、糜烂,影响术后切口愈合。
本组患者检测出感染细菌杆菌类多于球菌,与报道球菌为主不一致 [14],这可能是多次辗转医疗机构的影响,仅代表当前伤口细菌感染的状况,不一定代表手术后感染的菌种,但绝大多数菌种是多重耐药,显著增加治疗的难度。对血行、多重耐药菌感染,多数预后不良。应该指出的是术前、后规范性使用抗生素是治愈的重要环节。
5. 结论
胸部人工血管置换后伴胸骨深部感染要早期发现,一旦诊断明确,应积极手术治疗,清除感染源,胸肌瓣成形,切口愈合率仍然很高;伴有全身感染者手术治疗仍是首选。同时有针对性选用抗生素、规范治疗时间不可或缺。
文章引用
刘吉福,高永顺,周 峰,黄 铄,王 鹏,杨 明. 胸主动脉置换后胸骨切口深部感染的外科治疗
The Surgical Treatment of Deep Sternal Wound Infections after Thoracic Aortic Prosthesis Graft[J]. 外科, 2020, 09(02): 50-57. https://doi.org/10.12677/HJS.2020.92008
参考文献
- 1. 刘吉福, 高永顺, 李宝成, 等. 肌瓣成形新技术治疗胸骨切口深部感染23例[J]. 中国胸心血管外科临床杂志, 2018, 25(4): 321-324.
- 2. Legout, L., Sarraz-Bournet, B., D’Elia, P.V., Devos, P., Pasquet, A., et al. (2012) Characteristics and Prognosis in Patients with Prosthetic Vascular Graft Infection: A Prospective Observational Cohort Study. Clinical Microbiology and Infection, 18, 352-358. https://doi.org/10.1111/j.1469-0691.2011.03618.x
- 3. Fujii, T. and Watanabe, Y. (2015) Multidisciplinary Treatment Approach for Prosthetic Vascular Graft Infection in the Thoracic Aortic Area. Annals of Thoracic and Cardiovascular Surgery, 21, 418-427. https://doi.org/10.5761/atcs.ra.15-00187
- 4. Horan, T.C., Andrus, M. and Dudeck, M.A. (2008) CDC/NHSN Surveillance Definition of Health Care-Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting. American Journal of Infection Control, 36, 309-333. https://doi.org/10.1016/j.ajic.2008.03.002
- 5. Nakamura, T., Daimon, T., Mouri, N., et al. (2014) Staphylo-coccus aureus and Repeat Bacteremia in Febrile Patients as Early Signs of Sternal Wound Infection after Cardiac Surgery. Journal of Cardiothoracic Surgery, 9, 80-86. https://doi.org/10.1186/1749-8090-9-80
- 6. Tayama, E., Hori, H., Ueda, T., et al. (2014) Usefulness of F-FDG-PET/CT in Aortic Graft Infection: Two Cases. Journal of Cardiothoracic Surgery, 9, 42-47. https://doi.org/10.1186/1749-8090-9-42
- 7. Keidar, Z., Engel, A., Hoffman, A., et al. (2007) Prosthetic Vascular Graft Infection: The Role of 18F-FDG PET/CT. Journal of Nuclear Medicine, 48, 1230-1236. https://doi.org/10.2967/jnumed.107.040253
- 8. Tossios, P., Karatzopoulos, A., Tsagakis, K., et al. (2014) Treatment of Infected Thoracic Aortic Prosthetic Grafts with the In Situ Preservation Strategy: A Review of Its History, Surgical Technique, and Results. Heart, Lung and Circulation, 23, 24-31. https://doi.org/10.1016/j.hlc.2013.09.001
- 9. Wu, L., Chung, K.C., Waljee, J.F., et al. (2016) A National Study of the Impact of Initial Debridement Timing on Outcomes for Patients with Deep Sternal Wound Infection. Plastic and Reconstructive Surgery, 137, 414e-423e. https://doi.org/10.1097/01.prs.0000475785.14328.b2
- 10. Suzuki, T., Kawamoto, S., Motoyoshi, N., et al. (2015) Contemporary Outcome of the Surgical Management of Prosthetic Graft Infection after a Thoracic Aortic Replacement: Is There a Room to Consider Vacuum-Assisted Wound Closure as an Alternative? General Thoracic and Cardiovascular Surgery, 63, 86-92.
- 11. Li, T.T., Zhang, L.H., Han, L., et al. (2016) Early Application of Negative Pressure Wound Therapy to Acute Wounds Contaminated with Staphylococcus aureus: An Effective Approach to Preventing Biofilm Formation. Experimental and Therapeutic Medicine, 11, 769-776. https://doi.org/10.3892/etm.2016.3008
- 12. Deniz, H., Gokaslan, G., Arslanoglu, Y., et al. (2012) Treatment Outcomes of Postoperative Mediastinitis in Cardiac Surgery; Negative Pressure Wound Therapy versus Cenventional Treatment. Journal of Cardiothoracic Surgery, 7, 67-74. https://doi.org/10.1186/1749-8090-7-67
- 13. Rupprecht, L. and Schmid, C. (2013) Deep Sternal Wound Complications: An Overview of Old and New Therapeutic Options. Open Journal of Cardiovascular Surgery, 6, 9-19. https://doi.org/10.4137/OJCS.S11199
- 14. Zhang, Y.-G., Guo, X.-L., Song, Y., et al. (2015) Diagnosis and Treatment of Vascular Surgery Related Infection. The Open Biomedical Engineering Journal, 9, 250-255. https://doi.org/10.2174/1874120701509010250
