Journal of Organic Chemistry Research
Vol.06 No.02(2018), Article ID:25395,8
pages
10.12677/JOCR.2018.62010
Research of Biginelli Three-Component Reaction Catalyzed by Brønsted Acidic Ionic Liquid [DC2O2IM][p-CH3PhSO3]
Haiyan Zhang, He Li, Chenjiang Liu*
The Key Laboratory of Oil and Gas Fine Chemicals, Ministry of Education & Xinjiang Uygur Autonomous Region, School of Chemistry and Chemical Engineering, Xinjiang University, Urumqi Xinjiang

Received: May 17th, 2018; accepted: Jun. 3rd, 2018; published: Jun. 13th, 2018

ABSTRACT
In this paper, a series of 3,4-hydropyrimidine-2(1H)-ones/thiones was synthesized via the Biginelli three-component reaction using the Brønsted acidic ionic liquid 1,3-dicarboxy methylimidazole p-toluenesulfonate as a green and environmentally friendly catalyst. The method has the advantages of mild conditions, high yield, and short reaction time. In addition, the catalyst ionic liquid can be recycled five times without significant reduction in catalytic activity.
Keywords:Ionic Liquid, Catalysis, Biginelli Reaction, 3,4-Dihydropyrimidin-2(1H)-Ones/Thiones
布朗斯特酸性离子液体[DC2O2IM][p-CH3PhSO3]催化Biginelli三组分反应的研究
张海燕,李贺,刘晨江*
新疆大学化学化工学院,石油天然气精细化工教育部&自治区重点实验室,新疆 乌鲁木齐
收稿日期:2018年5月17日;录用日期:2018年6月3日;发布日期:2018年6月13日

摘 要
本文报道了布朗斯特酸性离子液体1,3-二羧甲基咪唑对甲苯磺酸盐作为一种绿色、环境友好的催化剂,成功地催化Biginelli三组分反应合成了一系列3,4-二氢嘧啶-2(1H)-酮或硫酮化合物。该方法具有条件温和、产率高、反应时间短的优点。此外,催化剂离子液体可循环使用五次且催化活性没有明显降低。
关键词 :离子液体,催化,Biginelli反应,3,4-二氢嘧啶-2(1H)-酮/硫酮

Copyright © 2018 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
3,4-二氢嘧啶-2(1H)-酮及衍生物具有十分重要的生物和药理活性,在抗病毒、降血压、杀菌、抗癌等 [1] - [6] 领域具有广阔的应用前景,其合成受到化学家们的广泛关注。众所周知,Biginelli反应是合成3,4-二氢嘧啶-2-酮衍生物(DHPMs)的重要方法,酸 [7] [8] 、碱 [9] 、有机小分子 [10] 等作为催化剂已被应用于该反应。
离子液体(ILs)因具有蒸汽压低、毒性低、可循环利用、热稳定性好和溶解性好等优点被应用于Biginelli反应 [11] [12] [13] [14] [15] 。基于本课题组在离子液体合成和催化应用领域所积累的经验 [16] [17] ,我们发展了一种布朗斯特酸性离子液体[DC2O2IM][p-CH3PhSO3]催化Biginelli反应合成3,4-二氢嘧啶-2(1H)-酮/硫酮的方法。分别考察了催化剂的种类和用量、反应溶剂、反应时间和温度等因素对反应产率的影响,同时对反应底物的普适性进行了研究。催化剂离子液体至少可以循环使用5次催化活性无明显下降。
2. 实验部分
2.1. 仪器与试剂
瑞士Büchi B-560型熔点仪;德国Bruker Equinox 55红外光谱仪(KBr压片);Varian inova-400型核磁共振仪(400 MHz),美国HP1100液相色谱质谱仪。所用药品及试剂均为市售分析纯,用前未经处理。1,3-二丁基咪唑氯盐的合成参照文献 [18] 、1-乙基-3-羧甲基咪唑氯盐和1,3-二羧甲基咪唑氯盐的合成参照文献 [19] 。
2.2. 离子液体IL1-IL3的合成
离子液体1,3-二丁基咪唑对甲苯磺酸盐[DBIM][p-CH3PhSO3] (IL1)、1-乙基-3-羧甲基咪唑对甲苯磺酸盐[C2O2EIM][p-CH3PhSO3] (IL2)和1,3-二羧甲基咪唑对甲苯磺酸盐[DC2O2IM][p-CH3PhSO3] (IL3)的合成如式1所示。分别将0.05 mol1,3-二丁基咪唑氯盐、1-乙基-3-羧甲基咪唑氯盐、1,3-二羧甲基咪唑氯盐和0.05 mol对甲苯磺酸在90℃下回流72 h,反应结束后用乙醚洗涤产物,随后减压除去乙醚,然后将产物置于90℃真空干燥至恒重即得离子液体IL1-IL3。
离子液体IL1-IL3的表征如下:
离子液体1,3-二丁基咪唑对甲苯磺酸盐[DBIM][p-CH3PhSO3]:棕黄色液体:1H NMR (400 MHz, D2O),δ:0.84 (t, J = 7.2 Hz, 6 H, 2 × CH3),1.19~1.24 (m, 4 H, 2 × CH2),1.71~1.79 (m, 4 H, 2 × CH2),2.31 (s, 3 H, CH3),4.09 (t, J = 7.2 Hz, 4 H, 2 × CH2),7.28 (d, J = 8.4 Hz, 2 H, ArH),7.38 (d, J = 4.0 Hz, 2 H, ArH),7.60 (d, J = 6.4 Hz, 2 H, ArH),8.68 (s, 1 H, ArH);13C NMR (100 MHz, D2O),δ:142.98,140.31,135.65,130.08,126.03,122.97,49.96,31.91,21.18,19.44,13.32;IR (KBr, v/cm−1):3400,3140,3088,2962,2935,2874,1711,1601,1564,463,1301,1239,1165,1122,1031,1006,818,754,681,565;ESI-MS:m/z (%) = 181.2 (100) [M+],171.0 (100) [M−]。
离子液体1-乙基-3-羧甲基咪唑对甲苯磺酸盐[C2O2EIM][p-CH3PhSO3]:棕黄色固体:m.p. 123℃~127℃;1H NMR (400 MHz, D2O),δ:1.44 (t, J = 11.2 Hz, 3 H, CH3),2.33 (s, 3 H, CH3),4.16~4.22 (m, 2 H, CH2),5.00 (s, 2 H, CH2),7.29~7.30 (m, 2 H, ArH),7.41 (s, 1 H, ArH),7.47 (s, 1 H, ArH),7.62~7.64 (m, 2 H, ArH),8.76 (s, 1 H, ArH);13C NMR (100 MHz, D2O),δ:170.76,143.09,140.24,136.91,130.12,126.03,124.23,122.61,50.64,45.77,21.17,14.99;IR (KBr, v/cm−1):3144,3109,3059,2985,2953,2863,2797,2712,2617,2524,2134,1891,1735,1570,1494,1454,1415,1332,1229,1162,1119,1034,1007,972,895,811,779,683,652,568,433;ESI-MS:m/z (%) = 155.1 (100) [M+],171.0 (100) [M−]。
离子液体1,3-二羧甲基咪唑对甲苯磺酸盐[DC2O2IM][p-CH3PhSO3]:棕黄色固体:m.p. 158℃~162℃;1H NMR (400 MHz, D2O),δ:2.32 (s, 3 H, CH3),5.08 (s, 4 H, 2 × CH2),7.28 (d, J = 8 Hz, 2 H, ArH),7.49~7.50 (m, 2 H, ArH),7.62 (d, J = 8.4 Hz, 2 H, ArH),8.86 (s, 1 H, ArH);13C NMR (100 MHz, D2O),δ:170.32,143.10,140.20,138.84,130.11,126.01,124.15,50.77,21.15;IR (KBr, v/cm−1):3164,3137,2965,2625,1734,1591,1565,1500,1449,1414,1347,1244,1220,1177,1142,1032,1006,974,899,821,768,737,680,644,566,496,443;ESI-MS:m/z (%) = 185.1 (100) [M+],171.0 (100) [M−]。
2.3. 目标化合物4a-4n的合成
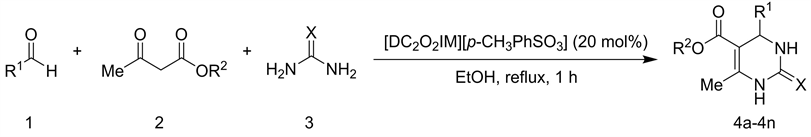
化合物4a-4n的合成反应如式2所示。将0.2 mmol芳香醛、0.2 mmol 1,3-二羰基化合物、0.3 mmol脲或硫脲和20 mol%的离子液体1,3-二羧甲基咪唑对甲苯磺酸盐[DC2O2IM][p-CH3PhSO3]在乙醇中回流反应1 h。反应结束后,向反应体系中加入大量碎冰,室温下充分搅拌,过滤,产物经大量冰水洗涤,即得纯净的产物4a-4n。
3. 结果与讨论
3.1. 反应条件优化
以苯甲醛、乙酰乙酸乙酯和脲三组分为模型反应,考察了离子液体的种类及其用量、溶剂、反应时间、反应温度等对反应的影响。首先,考察了不同咪唑离子液体对反应的影响,发现当IL3作为催化剂时产物产率可以达到93%,即为最优催化剂(表1,entries 1-3)。其次,考察了不同催化剂用量对反应的
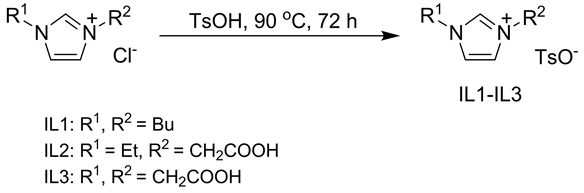
Scheme 1. The synthesis of ionic liquid
式1. 离子液体的合成
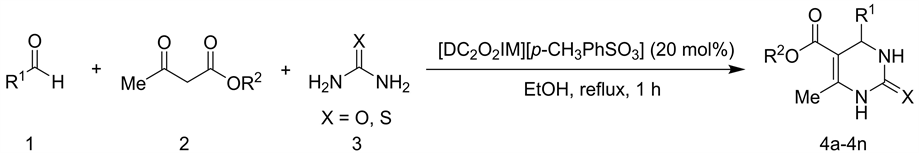
Scheme 2. Synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones(4a-4n)
式2. 3,4-二氢嘧啶-2(1H)-酮/硫酮(4a-4n)的合成
Table 1. Optimization of reaction conditionsa
表1. 反应条件的优化a

a反应条件:苯甲醛(0.2 mmol),乙酰乙酸乙酯(0.2 mmol),脲(0.3 mmol),离子液体,80℃;b分离产率;c反应温度50℃。
影响,当催化剂用量为10 mol%时,反应产率明显降低,当催化剂用量为30 mol%时,反应产率没有明显增加,因此催化剂的最佳用量为20 mol% (表1,entries 3-5)。然后考察了反应时间对反应的影响,当反应时间为0.5 h时,反应产率降低至89%,当反应为1.5 h和2 h时,反应产率没有明显提高,因此最佳反应时间为1 h (表1,entries 3, 6-8)。反应温度对反应的影响研究发现,当反应温度降到50℃时只能以45%的产率得到相应的目标化合物(表1,entries 3, 9)。最后,分别考察了反应溶剂为乙醇、乙腈、1,2-二氯乙烷、乙酸乙酯和水对反应的影响,结果表明当反应溶剂为乙醇时反应效果最好(表1,entries 3, 10-13)。综上,该反应的最佳条件为:离子液体1,3-二羧甲基咪唑对甲苯磺酸盐[DC2O2IM][p-CH3PhSO3]的用量为20 mol%,反应溶剂为乙醇,回流反应1 h。
3.2. 底物普适性研究
在确定最佳反应条件后,对底物的普适性进行了研究,即选用不同的芳香醛,不同的1,3-二羰基化合物与脲或硫脲参与反应,得到了一系列相应的3,4-二氢嘧啶-2(1H)-酮或硫酮,结果见表2。从中可以看出所有反应都以良好到优秀的产率得到相应的目标化合物。取代苯甲醛的取代基无论是吸电子基团如2-氟、3-氟、4-氟、2-氯、2-溴还是给电子基团如4-甲基、3-甲氧基都能以优秀的产率得到相应产物,取代
Table 2. Research of substratescopea
表2. 底物的普适性研究a
a反应条件:芳香醛(0.2 mmol),1,3-二羰基化合物(0.2 mmol),脲/硫脲(0.3 mmol),催化剂[DC2O2IM][p-CH3PhSO3] 20 mol%,乙醇,回流反应1 h;b分离产率。
基团的位置对产物的产率影响也不大(表2,entries 4b-4h)。基于3,4-二氢嘧啶-2(1H)-硫酮的生物活性,硫脲代替脲被用于Biginelli三组分反应,成功地以86%~89%的产率合成了产物4i-4k,显示硫脲与脲的反应特性相似。乙酰乙酸甲酯作为代替乙酰乙酸乙酯的一种1,3-二羰基化合物也顺利地参与了反应(表2,entries 4l-4n)。因此,离子液体1,3-二羧甲基咪唑对甲苯磺酸盐催化合成二氢嘧啶-2(1H)-酮/硫酮化合物具有很好的底物普适性。
3.3. 离子液体循环使用性
将离子液体1,3-二羧甲基咪唑对甲苯磺酸盐[DC2O2IM][p-CH3PhSO3]用于催化苯甲醛、乙酰乙酸乙酯和脲三组分反应之后进行再生循环使用的研究。具体方法为:两相分离后,将水相用乙酸乙酯萃取除去有机物残留物,再经旋转蒸发除水,残余物在90℃真空干燥至恒重后即得回收的离子液体,直接加入原料应用于三组分循环反应的研究,结果如图1所示。可以看出,离子液体循环五次后,目标化合物3,4-二氢嘧啶-2(1H)-酮的产率仍然高达88%。
4. 总结
我们发展了一种布朗斯特酸性新型离子液体1,3-二羧甲基咪唑对甲苯磺酸盐作为催化剂成功催化
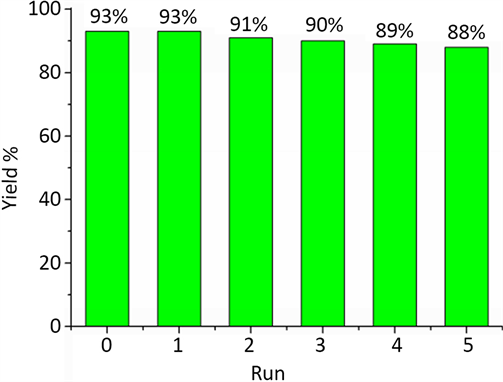
Figure 1. Recycling research of ionic liquid [DC2O2IM][p-CH3PhSO3]
图1. 离子液体[DC2O2IM][p-CH3PhSO3]的循环性研究
Biginelli反应合成一系列3,4-二氢嘧啶-2(1H)-酮和硫酮的方法。该方法具有条件绿色、温和、产率较高、反应时间较短等优点。催化剂离子液体至少可循环使用五次并且活性没有明显降低。因此,该方法是离子液体催化Biginelli三组分缩合反应的一个重要补充。
基金项目
“万人计划”后备人选培养项目(No. wr2016cx0145);国家自然科学基金(No. 21572195)。
文章引用
张海燕,李 贺,刘晨江. 布朗斯特酸性离子液体[DC2O2IM][p-CH3PhSO3]催化Biginelli三组分反应的研究
Research of Biginelli Three-Component Reaction Catalyzed by Br?nsted Acidic Ionic Liquid [DC2O2IM][p-CH3PhSO3][J]. 有机化学研究, 2018, 06(02): 61-68. https://doi.org/10.12677/JOCR.2018.62010
参考文献
- 1. Atwalk, K.S., Rovnyak, G.C., Kimball, S.D., Floyd, D.M., Moreland, S., Swanson, B.N., Gougoutas, J.Z., Schwartz, J., Smillie, K.M. and Malley, M.F. (1990) Dihydropyrimidine Calcium Channel Blockers. II.3-Substituted-4-aryl-dihydro-6-methyl-5- pyrimidine Carboxylic Acid Esters as Potent Mimics of Dihydropyridines. Journal of Medical Chemistry, 33, 2629-2635.
https://doi.org/10.1021/jm00171a044 - 2. Kappe, C.O. (1993) 100 Years of the Biginelli Dihydropyrimidine Syn-thesis. Teterahedron, 49, 6937-6963.
https://doi.org/10.1016/S0040-4020(01)87971-0 - 3. Overman, L.E., Rabinowitz, M.H. and Renhowe, P.A. (1995) Enantioselective Total Synthesis of (-)-Ptilomycalin A. Journal of the American Chemical Society, 117, 2657-2658.
https://doi.org/10.1021/ja00114a034 - 4. Zhang, X.L., Li, Y.P., Liu, C.J. and Wang, J.D. (2006) An Efficient Synthesis of 4-Substituted Pyrazolyl-3,4-Dihydropy- rimidin-2(1H)-(Thio) Ones Catalyzed by Mg(ClO4)2 un-der Ultrasound Irradiation. Journal of Molecular Catalysis A: Chemical, 253, 207-211.
https://doi.org/10.1016/j.molcata.2006.03.018 - 5. Kappe, C.O., Fabian, W.M.F. and Semones, M.A. (1997) Conformational Analysis of 4-Aryl-Dihydropyrimidine Calcium Channel Modulator. A Comparison of abinitio, Sem-iempirical and X-Ray Crystallographic Studies. Teterahedron, 53, 2803-2816.
https://doi.org/10.1016/S0040-4020(97)00022-7 - 6. Yang, Z.Y. and Guo, H.Y. (2011) One-Pots Synthesis of 3,4-Dihydropyrimidin-2-(1H)-Ones Catalyzed by Acidic Ionic Liquid under Solvent-Free Conditions. Journal of Zhejiang University of Technology, 39, 512-515.
- 7. Ranu, B.C., Hajra, A. and Jana, U. (2000) Indium(III) Chlo-ride-Catalyzed One-Pot Synthesis of Dihydropyrimidinones by a Three-Component Coupling of 1,3-Dicarbonyl Com-pounds, Aldehydes, and Urea: An Improved Procedure for the Biginelli Reaction. The Journal of Organic Chemistry, 65, 6270-6272.
https://doi.org/10.1021/jo000711f - 8. Rahmatpour, A. (2012) Polyvinylsulfonic Acid: An Efficient, Water-Soluble and Reusable Brønsted Acid Catalyst for the Three-Component Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones/Thiones in Water and Ethanol. Catalysis Letters, 142, 1505-1511.
https://doi.org/10.1007/s10562-012-0873-6 - 9. Debache, A., Amimour, M., Belfaitah, A., Rhouati, S. and Carboni, B. (2008) A One-Pot Biginelli Synthesis of 3, 4-Dihydropyrimidin-2-(1H)-Ones/Thiones Catalyzed by Tri-phenylphosphine as Lewis Base. Tetrahedron Letters, 49, 6119-6121.
https://doi.org/10.1016/j.tetlet.2008.08.016 - 10. Xu, D.Z., Li, H. and Wang, Y. (2012) Highly Enantioselective Biginelli Reaction Catalyzed by a Simple Chiral Primary Amine Catalyst: Asymmetric Synthesis of Dihydropyrimidines. Tetrahedron, 68, 7867-7872.
https://doi.org/10.1016/j.tet.2012.07.027 - 11. Mohammadi, K., Shirini, F. and Yahyazadeh, A. (2015) 1,3-Disulfonic Acid Imidazolium Hydrogen Sulfate: A Reusable and Efficient Ionic Liquid for the One-Pot Mul-ti-Component Synthesis of Pyrimido[4,5-b]Quinoline Derivatives. RSC Advances, 5, 23586-23590.
https://doi.org/10.1039/C5RA02198G - 12. Elhamifar, D., Nasr-Esfahani, M., Karimi, B., Moshkelgosha, R. and Shábani, A. (2016) Ionic Liquid and Sulfonic Acid Based Bifunctional Periodic Mesoporous Organosilica (BPMO-IL-SO3H) as a Highly Efficient and Reusable Nanocatalyst for the Biginelli Reaction. ChemCatChem, 6, 2593-2599.
https://doi.org/10.1002/cctc.201402162 - 13. Gholap, A.R., Venkatesan, K., Daniel, T., Lahoti, R. and Srinivasan, K. (2004) Ionic Liquid Promoted Novel and Efficient One Pot Synthesis of 3,4-Dihydropyrimidin-2-(1H)-Ones at Ambient Temperature under Ultrasound Irradiation. Green Chemistry, 6, 147-150.
https://doi.org/10.1039/b314015f - 14. 赵新海, 刘晨江, 李燕萍. 超声波促进离子液体中Biginelli一锅法合成苯并咪唑并[2,1-b]喹啉-6-酮[J]. 高等学校化学学报, 2010, 31(9): 1769-1773.
- 15. Sharma, N., Sharma, U.K., Kumar, R. and Sinha, A.K. (2012) Green and Recyclable Glycine Nitrate (GlyNO3) Ionic Liquid Triggered Mul-ticomponent Biginelli Reaction for the Efficient Synthesis of Dihydropyrimidinones. RSC Advances, 2, 10648-10651.
https://doi.org/10.1039/c2ra22037g - 16. Cao, D.W., Zhang, Y.H., Liu, C.J., Wang, B., Sun, Y.D., Abdukadera, A., Hu, H.Y. and Liu, Q. (2016) Ionic Liquidpromoted Diazenylation of N-Heterocyclic Compounds with Aryltriazenes under Mild Conditions. Organic Letters, 18, 2000-2003.
https://doi.org/10.1021/acs.orglett.6b00605 - 17. Li, H., Liu, C.J., Zhang, Y.H., Sun, Y.D., Wang, B. and Liu, W.B. (2015) Green Method for the Synthesis of Chromeno [2,3-c]Pyrazol-4(1H) Ones through Ionic Liquid Promoted Directed Annulation of 5-(Aryloxy)-1H-Pyrazole- 4-Carbaldehydes in Aqueous Media. Organic Letters, 17, 932-935.
https://doi.org/10.1021/acs.orglett.5b00033 - 18. Nadaf, R.N., Siddiqui, S.A., Daniel, T., Lahoti, R.J. and Srini-vasan, K.V.(2015) Room Temperature Ionic Liquid Promoted Regioselective Synthesis of 2-Aryl Benzimidazoles, Benzoxazoles and Benzthiazoles under Ambient Conditions. Journal of Molecular Catalysis A: Chemical, 214, 155-160.
https://doi.org/10.1016/j.molcata.2003.10.064 - 19. Fei, Z., Zhao, D., Geldbach, T.J., Scopelliti, R. and Dyson, P.J. (2004) Brønsted Acidic Ionic Liquids and Their Zwitterions: Synthesis, Characterization and Pka Determination. Chemistry—A European Journal, 10, 4886-4893.
https://doi.org/10.1002/chem.200400145 - 20. Rao, G.D., Acharya, B., Verma, S. and Kaushik, M.P. (2011) N, N′-Dichlorobis (2,4,6-Trichlorophenyl) Urea (CC-2) as a New Reagent for the Synthesis of Pyrimidone and Pyrimidine Derivatives via Biginelli Reaction. Tetrahedron Letters, 52, 809-812.
https://doi.org/10.1016/j.tetlet.2010.12.039 - 21. Boumoud, T., Boumoud, B., Mosset, P. and Debache, A. (2011) Gypsum-Catalyzed One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H) under Solvent-Free Conditions. Journal of Chemistry, 8, 312-318.
https://doi.org/10.1155/2011/780271 - 22. Yadav, J.S., Reddy, B.V.S., Sridhar, P., Reddy, J.S., Nagaiah, K., Lingaiah, N. and Saiprasad, P.S. (2004) Green Protocol for the Biginelli Three-Component Reaction: Ag3PW12O40 as a Novel, Water-Tolerant Heteropolyacid for the Synthesis of 3,4-Dihydropyrimidinones. European Journal of Organic Chemistry, 3, 552-557.
https://doi.org/10.1002/ejoc.200300559 - 23. Li, W., Zhou, G. and Zhang, P. (2011) One-Pot Synthesis of Dihy-dropyrimidiones via Environmentally Friendly Enzyme-Catalyzed Biginelli Reaction. Heterocycles, 83, 2067-2077.
https://doi.org/10.3987/COM-11-12267 - 24. Murata, H., Ishitani, H. and Iwamoto, M. (2010) Synthesis of Bigi-nelli Dihydropyrimidinone Derivatives with Various Substituents on Aluminium-Planted Mesoporous Silica Catalyst. Organic & Biomolecular Chemistry, 8, 1202-1211.
https://doi.org/10.1039/b920821f - 25. Silvada, D.L., Fernandes, S.A., Sabino, A.A. and Fatima, A.D. (2011) p-Sulfonic Acid Calixarenes as Efficient and Reusable Organocatalysts for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones/-Thiones. Tetrahedron Letters, 52, 6328-6330.
https://doi.org/10.1016/j.tetlet.2011.08.175 - 26. Kulangiappar, K., Anbukulandainathan, M. and Raju, T. (2014) An Ecofriendly and Efficient Catalyst for One-Pot Synthesis of 3,4-Dihydropyrimidine-2(1H)-Ones under Solvent-Free Conditions. Synthetic Communications, 1, 2494-2502.
https://doi.org/10.1080/00397911.2014.905599 - 27. Chandak, H.S., Lad, N.P. and Upare, P.P. (2009) Recyclable Amberlyst-70 as a Catalyst for Biginelli Reaction: An Efficient One-Pot Green Protocol for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones. Catalysis Letters, 131, 469-473.
https://doi.org/10.1007/s10562-009-9896-z - 28. Khabazzadeh, H., Saidi, K. and Sheibani, H. (2008) Micro-wave-Assisted Synthesis of Dihydropyrimidin-2(1H)-Ones Using Graphite Supported Lanthanum Chloride as AMild and Efficient Catalyst. Bioorganic & Medicinal Chemistry letters, 18, 278-280.
https://doi.org/10.1016/j.bmcl.2007.10.087 - 29. Dallinger, D. and Kappe, C.O. (2007) Automated Generation of a Dihydropyrimidine Compound Library Using Microwave-Assisted Processing. Nature Protocols, 2, 1713-1721.
https://doi.org/10.1038/nprot.2007.224 - 30. Dilmaghani, K.A., Zeynizadeh, B. and Yari, M. (2009) One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones and Their Sulfur Derivatives with H2SO4 Supported on Silica Gel or Alumina. Phosphorus, Sulfur, and Silicon and the Related Elements, 184, 1722-1728.
https://doi.org/10.1080/10426500802293153
