Advances in Clinical Medicine
Vol.
11
No.
10
(
2021
), Article ID:
45713
,
8
pages
10.12677/ACM.2021.1110645
鼠骨髓间充质干细胞同种异体关节内和静脉内的移植研究
李娟1,张丽1,孙津津1,任虹旭2,荣春1*
1青岛大学附属医院,手术室,山东 青岛
2烟台毓璜顶医院,手术室,山东 烟台

收稿日期:2021年9月11日;录用日期:2021年10月2日;发布日期:2021年10月13日

摘要
目的:研究鼠骨髓间充质干细胞(Bone marrow mesenchymal stem cells, BMSCs)同种异体膝关节内移植,静脉移植后在膝关节内的存活与分布情况,为BMSCs在软骨损伤治疗中应用提供理论基础。方法:采用全骨髓培养法分离培养大鼠骨髓间充质干细胞,用5-溴-2-脱氧尿苷标记细胞,计算标记阳性率,通过关节腔注射或鼠尾静脉植入大鼠体内,免疫组化观察术后2 w、4 w标记细胞存活、分布,HE染色观察植入后组织的免疫反应。结果:培养48 h后,BMSCs贴壁大部分呈长梭形,个别为多角形,7 d后细胞融合达90%以上。细胞生长曲线显示细胞生长潜伏期较长,接种后第3~6 d进入对数生长期,第7 d后进入平台期,传代后细胞在24 h内贴壁,以均一的长梭形为主,且生长增殖速度比原代快。标记的BMSCs呈绿色,经计数标记率为95%。同种异体BMSCs植入后均表现出较强的迁移能力,进入关节软骨内。随时间延长,关节软骨内的标记细胞逐渐增多,在异体关节软骨中至少可以存活4 w,关节腔注射和鼠尾静脉植入BMSCs均未观察到明显的免疫反应,且两组在植入2 w和4 w后Brdu标记阳性的BMSCs表达差异无统计学意义(P > 0.05)。结论:异体BMSCs通过关节内注射和静脉移植对于治疗关节软骨损伤均具有一定的临床应用价值。
关键词
骨髓间充质干细胞,移植,5-溴-2-脱氧尿苷,软骨

Study on Intra-Articular and Intravenous Transplantation of Mouse Bone Marrow Mesenchymal Stem Cells
Juan Li1, Li Zhang1, Jinjin Sun1, Hongxu Ren2, Chun Rong1*
1Operation Room, The Affiliated Hospital of Qingdao University, Qingdao Shandong
2Operation Room, Yantai Yuhuangding Hospital, Yantai Shandong

Received: Sep. 11th, 2021; accepted: Oct. 2nd, 2021; published: Oct. 13th, 2021

ABSTRACT
Purpose: To study the survival and distribution of bone marrow mesenchymal stem cells (BMSCs) in the knee joint after allogeneic intra-articular knee transplantation, and intravenous transplantation, so as to provide theoretical basis for the application of BMSCs in the treatment of cartilage injury. Methods: Rat bone marrow mesenchymal stem cells were isolated and cultured by whole bone marrow culture method. 5-Bromo-2-deoxyuridine was used to label the cells. The positive rate of labeling was calculated. The survival and distribution of labeled cells were observed by immunohistochemistry at 2 and 4 weeks after operation, and the immune response of implanted tissues was observed by HE staining. Results: After 48 hours of culture, most of BMSCs adhered to the wall in a long spindle shape, and a few were polygonal. After 7 days of culture, more than 90% of the cells were fused. The cell growth curve showed that the incubation period of cell growth was longer. The logarithmic growth phase entered 3~6 days after inoculation, and entered the plateau stage after 7 days. After passage, the cells adhered to the wall within 24 hours, and the growth and proliferation rate was faster than that of the primary generation. The labeled BMSCs were green, and the labeling rate was 95%. Allogeneic BMSCs showed strong migration ability and entered into articular cartilage. The number of labeled cells in articular cartilage increased gradually with time, and could survive at least 4 weeks in allogeneic articular cartilage. No obvious immune response was observed in intra-articular injection and tail vein implantation of BMSCs. There was no significant difference in the expression of BrdU positive BMSCs between the two groups at 2 and 4 weeks after implantation (P < 0.05). Conclusion: Allogeneic BMSCs can be used in the treatment of articular cartilage injury by intra-articular injection and intravenous transplantation.
Keywords:Bone Marrow Mesenchymal Stem Cells, Transplantation, 5-Bromo-2-Deoxyuridine, Cartilage

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 前言
间充质干细胞是来源于中胚层的成体干细胞,具有高度的自我更新能力和多种分化潜能,能够分化为各种类型的细胞,包括骨细胞、脂肪细胞、软骨细胞、肌细胞、心肌细胞和神经元,容易在体外大量获取和扩增而不丢失分化能力 [1] 。近二十年间充质干细胞在软骨组织工程中得到应用,动物实验显示了良好的软骨修复潜能,并应用到临床实践中 [2] [3] [4] 。但是目前还没有确切的办法跟踪植入的间充质干细胞,对于新形成的组织中是否含有植入的间充质干细胞不是很清楚。本研究将5-溴-2-脱氧尿苷(Brdu)标记的骨髓间充质干细胞(Bone marrow mesenchymal stem cells, BMSCs)植入鼠体内,观察其存活和分布,阐明其在软骨损伤治疗中的应用前景。
2. 材料与方法
2.1. 材料
DMEM培养基(美国Hyclone公司)、胎牛血清(杭州四季青公司)、胰蛋白酶(美国Sigma公司)、BrdU (美国Sigma公司)、兔抗鼠BrdU一抗(武汉博士德公司),山羊抗兔二抗(武汉博士德公司),免疫组化试剂盒(北京中杉公司),海藻酸钠(青岛南山公司),Whistar大鼠(购自青岛市药检所)。本试验已通过医院伦理委员会批准。
2.2. 方法
2.2.1. 大鼠骨髓间充质干细胞培养
大鼠麻醉后在75%的酒精中浸泡30 min,无菌条件下在培养箱中取双侧后腿股骨,用剪刀去除贴附在骨表面的肌肉,置于无菌培养皿中,用碘伏浸泡30 min,无菌生理盐水冲洗后剪断两端骨垢,用吸取DMEM (含10%胎牛血清)的5 ml注射器冲洗骨髓腔,将冲洗液导入25 cm2培养瓶中,每3 d换液一次。培养箱内参数设定:37℃、5% CO2。待细胞融合率达90%以上后,用0.25%的胰酶消化传代,以8 × 103/cm2的密度传代培养。
2.2.2. BMSCs生长曲线绘制
分别将1、3代BMSCs悬液100 mL加入到96孔板中,在培养箱中预培养12 h,分别于第1、2、3、4、5、6、7、8 d加入10 Ul CCK-8溶液,在450 nm波长下测定相应OD值,绘制时间为横坐标(X轴),OD值为纵坐标(Y轴)的细胞生长曲线。
2.2.3. BrdU标记BMSCs及免疫荧光检测标记率
在细胞培养液中加入10 μmol/L BrdU进行标记,48 h后将标记的BMSCs接种在有盖玻片的六孔板中,待BMSCs达70%融合率时,弃去培养基,PBS清洗三次,以40 g/L多聚甲醛固定30 min,0.5% Triton通透15 min,然后加入10 g/L牛血清白蛋白封闭30 min,PBS冲洗三次后加入兔抗鼠BrdU抗体,于37℃孵育2 h,避光加入山羊抗兔FITC荧光抗体,避光孵育1 h,PBS冲洗后甘油封片,荧光显微镜下观察细胞的即时标记情况。随机选取5个视野,分别计数暗视野下绿色荧光细胞数(阳性细胞数)和明视野下细胞数,按下列公式计算标记阳性率,标记阳性率 = 5个视野阳性细胞总数/5个视野细胞总数 × 100%。
2.2.4. BMSCs同种异体植入
选取9只只体重150~200 g的2月龄Whistar大鼠,随机分为对照组、关节注射组和静脉植入组。对照组、关节注射组大鼠经10%水合氯醛腹腔麻醉后,用碘酊和酒精消毒后腿的左膝关节。依据分组,在大鼠内侧膝关节间隙用1 ml注射器分别注射100 ml的生理盐水或100 ml 1 × 106的BMSCs悬液。静脉组大鼠用碘酊和酒精消毒尾部,用软针穿刺入尾静脉,注射1 × 106 BMSCs悬液。术后分笼常规饲养和观察并给予抗生素肌注预防感染。
2.2.5. 免疫组化及HE染色大鼠关节软骨
植入术后2、4 w处死老鼠,取左膝关节内的韧带和软骨。用40 g/L多聚甲醛固定24 h,石蜡包埋切片,用0.3% Triton 通透10 min,血清封闭液封闭15 min,直接倾去封闭液,加入BrdUI抗4℃过夜孵育;第二天加入II抗在室温下反应1 h,PBS漂洗后滴加DAB显色,二甲苯通透,中性树胶封片,光镜下观察BrdU阳性细胞。HE染色观察局部组织反应。
2.2.6. 统计学分析
应用SPSS 19.0统计软件进行统计学分析。所有定量变量以均数 ± 标准差(x ± s)表示。两组比较采用t检验,P < 0.05表示差异具有统计学意义。
3. 结果
3.1. 大鼠BMSCs的分离培养
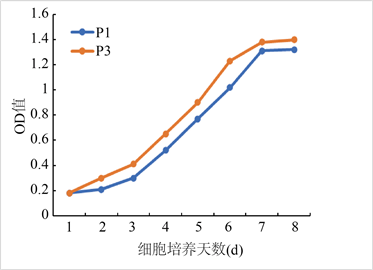
原代培养48 h可见细胞贴壁大部分呈长梭形,个别为多角形,以后细胞呈克隆样生长,血细胞随换液而逐渐清除,7 d后细胞融合达90%以上(图1)。细胞生长曲线显示细胞生长潜伏期较长,接种后第3~6 d进入对数生长期,第7 d后进入平台期,传代后细胞在24 h内贴壁,以均一的长梭形为主,且生长增殖速度比原代快。
 (a)
(a)
 (b)
(b)
Figure 1. Isolation and culture of rat BMSCs. (a) Observation of adherent rat bone marrow mesenchymal stem cells under inverted microscope (×200 fold); (b) Cell growth curve
图1. 大鼠BMSCs的分离培养。(a) 倒置显微镜下观察贴壁的大鼠骨髓间充质干细胞(×200倍);(b) 细胞生长曲线
3.2. BrdU标记BMSCs阳性率
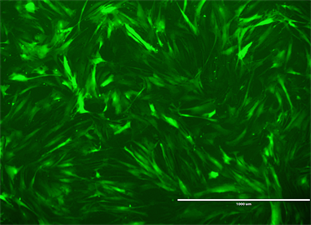
标记的BMSCs在倒置荧光显微镜下呈绿色(图2),经计数标记率为95%。
 (a)
(a)
 (b)
(b)
Figure 2. Bone marrow mesenchymal stem cells treated with BrdU were observed under fluorescence microscope. (a) Bone marrow mesenchymal stem cells under white light; (b) Bone marrow mesenchymal stem cells under fluorescence microscope
图2. 荧光显微镜下观察BrdU处理的骨髓间充质干细胞。(a) 白光下骨髓间充质干细胞;(b) 荧光显微镜下骨髓间充质干细胞
3.3. 植入后大鼠关节软骨BrdU阳性细胞免疫组化及HE染色
术后全部动物正常存活,注射部位均未出现红肿和分泌物,动物精神可,饮食,活动状况良好,无异常死亡。异体细胞植入术后2 w,在关节内注射组和静脉注射组,免疫组化染色显示在软骨内存在少量的BrdU阳性细胞,且阳性细胞数两者无统计学差异(P > 0.05);对照组中没有BrdU阳性细胞表达,HE染色均未见到明显炎性反应。术后4 w,在关节组和静脉组软骨组织中,免疫组化结果显示软骨内出现BrdU阳性细胞比第2 w时增多,且两组BrdU阳性细胞表达差异无统计学意义(P > 0.05);对照组没有BrdU阳性细胞表达(图3),HE染色均未见到明显炎性反应(图4)。
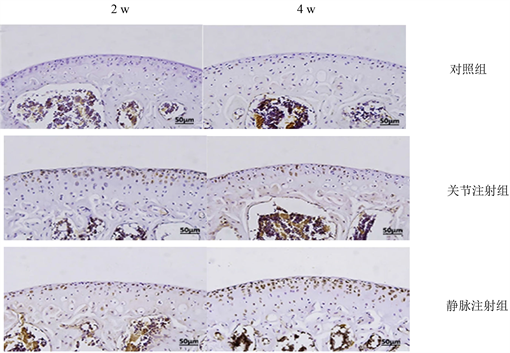
Figure 3. BrdU positive cells in articular cartilage of the three groups were immunohistochemical
图3. 三组免疫组化关节软骨中BrdU阳性细胞
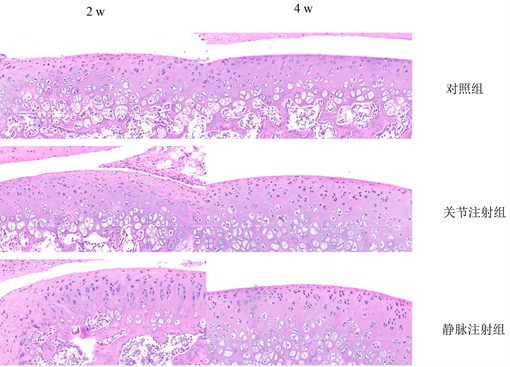
Figure 4. The immune response was observed by HE staining in the three groups
图4. 三组HE染色观察免疫反应
4. 讨论
1966年Friedenstein等首先从骨髓中分离培养出骨髓间充质干细胞。研究显示间充质干细胞可以从骨髓、脂肪和脐血中得到 [5] [6] ,具有自我更新和多向分化潜能,容易获取及体外扩增培养,是骨、软骨等组织工程较理想的种子细胞、基因转染的适宜细胞载体,动物实验显示了良好的软骨修复潜能 [7] [8] 。骨髓间充质干细胞取材容易,对机体损伤小,其分离和培养方法有密度梯度离心法、磁珠分选法和流式细胞仪筛选法。Percoll密度梯度离心法被认为是最经典的方法,但操作较为复杂。免疫磁珠或流式细胞仪分选法则价格昂贵。将骨髓直接稀释后接种培养,保留了血液中的多种细胞及营养因子,利用细胞分泌的各种因子间接促进BMSCs的生长,增殖效果较好 [9] [10] 。但存在需多次半量换液、观察时间长、杂类细胞接触抑制等问题。本实验采用全骨髓培养法培养出间充质干细胞,简单易行,随换液除去未贴壁的细胞,可以扩增得到所需要的细胞数量,然后进行标记,研究植入的BMSCs在关节软骨内的分布和存活情况。
研究细胞移植后在体内的分布、存活,需要对细胞进行标记并检测。由于机体组织和细胞中无内源性BrdU存在,Brdu作为DNA前体胸腺嘧啶核苷的类似物,通过与胸腺嘧啶竞争,特异地掺入S期(DNA合成期)细胞单链DNA核苷酸序列里,可用来标记处于增殖期的细胞 [11] ,用抗BrdU单克隆抗体进行免疫细胞化学检测即可观察到S期细胞在组织中的定位与分布,定量评估细胞合成DNA的水平 [12] 。研究证实BrdU标记和检测的准确性高、标记率高,方法简便、迅速、安全,是反映细胞增殖及示踪监测移植细胞指标 [13] 。此实验采用了BrdU标记大鼠BMSCs并进行异体移植研究,检测标记率为75%。
本实验通过BrdU标记BMSCs并用关节注射和静脉体内移植,结果发现异体BMSCs可以进入到正常的关节软骨中且细胞的数量随着时间延长而逐渐增多,至少可以存活4周,表明异体BMSCs具有较强的迁移能力,可以进入关节软骨内,两组之间的细胞数量没有明显差别,细胞植入后关节切片的HE染色观察发现,术后植入区域没有出现明显的炎性细胞浸润。表明两种植入细胞的方法均可以应用为治疗软骨损伤,且没有明显的免疫反应。已经有动物试验证实通过关节内直接注射BMSC治疗软骨损伤显示良好的效果。有研究者利用荧光染料进行细胞示踪,也发现羊自体MSCs与明胶海绵复合后植入关节软骨缺损处,1 w后植入区域BMSCs数量明显减少,而附近骨组织中出现较多植入细胞 [14] ,表明植入的自体MSCs具有明显的迁移行为,但由于荧光染料在细胞内逐渐衰减,而难于对细胞进行长时间示踪。从山羊自体骨髓获取自体间充质干细胞扩增后转染绿色荧光蛋白。在山羊骨性关节炎膝关节内直接注射含有间充质干细胞的透明质酸钠溶液,细胞治疗组内侧半月板明显再生,在新形成的组织中发现植入的间充质干细胞,关节软骨的退变及软骨下骨硬化减轻。国外学者将骨髓间充质干细胞从幼稚的雄性的绿色荧光蛋白转基因大鼠中抽吸,并在单层培养。在制造骨软骨缺损后4 w,18只老鼠分为三个组:对照组(在关节内只注射磷酸盐缓冲生理盐水)、钻孔组(关节内注射磷酸盐缓冲生理盐水,并应用骨髓刺激技术)和骨髓间充质干细胞组(关节内注射骨髓间充质干细胞,并应用骨髓刺激技术)。在4、8、12 w杀死老鼠。在所有的时间点,间充质干细胞处理组组织学评分比对照组和钻孔组明显的增高。在治疗后4 w可以在样本内发现绿色荧光蛋白阳性细胞 [15] 。研究报道在成体迷你猪的股骨内侧髁制造软骨缺损,从同种猪的髂棘骨髓获取间充质干细胞,直接关节内注射,结果显示软骨在6 w和12 w愈合,并在新形成的软骨中检测到植入的荧光标记的BMSCs,证实了间充质干细胞治疗软骨损伤是可以选择的治疗方法 [16] 。
大多数动物试验是制造软骨缺损模型,以修复软骨缺损检测修复效果 [17] [18] [19] 。对于其机制,认为本实验将BMSC植入到鼠的正常的关节腔内,同样可以在软骨内发现标记的细胞,对其机制需要进一步的研究。同时静脉移植实验也发现细胞迁移到软骨内的现象,为静脉移植治疗软骨损伤具有一定的提示和潜在的应用基础,进一步证实了间充质干细胞的性质。
本实验采用BrdU示踪技术,确定了鼠骨髓MSCs同种异体关节内注射和静脉植入后,可以转移到关节软骨组织中至少存活4 w,且具有迁移能力,为进一步研究应用BMSCs异体移植应用治疗软骨损伤奠定了良好基础。未来将进一步研究异体BMSCs在体内迁移的机制和能否转化为具有正常性质的软骨细胞。
文章引用
李 娟,张 丽,孙津津,任虹旭,荣 春. 鼠骨髓间充质干细胞同种异体关节内和静脉内的移植研究
Study on Intra-Articular and Intravenous Transplantation of Mouse Bone Marrow Mesenchymal Stem Cells[J]. 临床医学进展, 2021, 11(10): 4401-4408. https://doi.org/10.12677/ACM.2021.1110645
参考文献
- 1. Ding, D.C., Shyu, W.C. and Lin, S.Z. (2011) Mesenchymal Stem Cells. Cell Transplant, 20, 5-14. https://doi.org/10.3727/096368910X
- 2. Dao, T.T., Vu, N.B., Pham, L.H., et al. (2019) In Vitro Production of Cartilage Tissue from Rabbit Bone Marrow-Derived Mesenchymal Stem Cells and Polycaprolactone Scaffold. Advances in Experimental Medicine and Biology, 1084, 45-60. https://doi.org/10.1007/5584_2017_133
- 3. Richardson, S.M., Kalamegam, G., Pushparaj, P.N., et al. (2016) Mesenchymal Stem Cells in Regenerative Medicine: Focus on Articular Cartilage and Intervertebral Disc Regeneration. Methods, 99, 69-80. https://doi.org/10.1016/j.ymeth.2015.09.015
- 4. Panadero, J.A., Lanceros-Mendez, S. and Ribelles, J.L. (2016) Differentiation of Mesenchymal Stem Cells for Cartilage Tissue Engineering: Individual and Synergetic Effects of Three-Dimensional Environment and Mechanical Loading. Acta Biomaterialia, 33, 1-12. https://doi.org/10.1016/j.actbio.2016.01.037
- 5. Ding, D.C., Chang, Y.H., Shyu, W.C., et al. (2015) Human Umbilical Cord Mesenchymal Stem Cells: A New Era for Stem Cell Therapy. Cell Transplantation, 24, 339-347. https://doi.org/10.3727/096368915X686841
- 6. Berebichez-Fridman, R. and Montero-Olvera, P.R. (2018) Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-Art Review. Sultan Qaboos University Medical Journal, 18, e264-e277. https://doi.org/10.18295/squmj.2018.18.03.002
- 7. Mortada, I. and Mortada, R. (2018) Epigenetic Changes in Mesenchymal Stem Cells Differentiation. European Journal of Medical Genetics, 61, 114-118. https://doi.org/10.1016/j.ejmg.2017.10.015
- 8. Kim, D.H., Kim, B.Y., Kim, D.H., et al. (2019) Rabbit Palatum-Derived Mesenchymal Progenitor Cells Tri-Lineage Differentiation on 2D Substrates and 3D Printed Constructs. Journal of Applied Biomaterials & Functional Materials, 17. https://doi.org/10.1177/2280800019834520
- 9. Ferrin, I., Beloqui, I., Zabaleta, L., et al. (2017) Isolation, Culture, and Expansion of Mesenchymal Stem Cells. Methods in Molecular Biology, 1590, 177-190. https://doi.org/10.1007/978-1-4939-6921-0_13
- 10. Chu, D.T., Phuong, T.N.T., Tien, N.L.B., et al. (2020) An Update on the Progress of Isolation, Culture, Storage, and Clinical Application of Human Bone Marrow Mesenchymal Stem/Stromal Cells. International Journal of Molecular Sciences, 21, 708. https://doi.org/10.3390/ijms21030708
- 11. Harris, L., Zalucki, O. and Piper, M. (2018) BrdU/EdU Dual Labeling to Determine the Cell-Cycle Dynamics of Defined Cellular Subpopulations. Journal of Molecular Histology, 49, 229-234. https://doi.org/10.1007/s10735-018-9761-8
- 12. Cappella, P., Gasparri, F., Pulici, M., et al. (2015) Cell Proliferation Method: Click Chemistry Based on BrdU Coupling for Multiplex Antibody Staining. Current Protocols in Cytometry, 72, 7.34.31-37.34.17. https://doi.org/10.1002/0471142956.cy0734s72
- 13. Ligasová, A., Konečný, P., Frydrych, I., et al. (2017) Looking for Ugly Ducklings: The Role of the Stability of BrdU-Antibody Complex and the Improved Method of the Detection of DNA Replication. PLoS ONE, 12, e0174893. https://doi.org/10.1371/journal.pone.0174893
- 14. Gallego, L., Pérez-Basterrechea, M., García-Consuegra, L., et al. (2015) Repair of Segmental Mandibular Bone Defects in Sheep Using Bone Marrow Stromal Cells and Autologous Serum Scaffold: A Pilot Study. Journal of Clinical Periodontology, 42, 1143-1151. https://doi.org/10.1111/jcpe.12480
- 15. Nishimori, M., Deie, M., Kanaya, A., et al. (2006) Repair of Chronic Osteochondral Defects in the Rat. A Bone Marrow-Stimulating Procedure Enhanced by Cultured Allogenic Bone Marrow Mesenchymal Stromal Cells. The Journal of Bone and Joint Surgery. British Volume, 88, 1236-1244. https://doi.org/10.1302/0301-620X.88B9.17810
- 16. Chang, C.H., Kuo, T.F., Lin, F.H., et al. (2011) Tissue Engineering-Based Cartilage Repair with Mesenchymal Stem Cells in a Porcine Model. Journal of Orthopaedic Research, 29, 1874-1880. https://doi.org/10.1002/jor.21461
- 17. Enomoto, T., Akagi, R., Ogawa, Y., et al. (2020) Timing of Intra-Articular Injection of Synovial Mesenchymal Stem Cells Affects Cartilage Restoration in a Partial Thickness Cartilage Defect Model in Rats. Cartilage, 11, 122-129. https://doi.org/10.1177/1947603518786542
- 18. Muttigi, M.S., Kim, B.J., Choi, B., et al. (2018) Matrilin-3 Codelivery with Adipose-Derived Mesenchymal Stem Cells Promotes Articular Cartilage Regeneration in a Rat Osteochondral Defect Model. Journal of Tissue Engineering and Regenerative Medicine, 12, 667-675. https://doi.org/10.1002/term.2485
- 19. Dahlin, R.L., Kinard, L.A., Lam, J., et al. (2014) Articular Chondrocytes and Mesenchymal Stem Cells Seeded on Biodegradable Scaffolds for the Repair of Cartilage in a Rat Osteochondral Defect Model. Biomaterials, 35, 7460-7469. https://doi.org/10.1016/j.biomaterials.2014.05.055
NOTES
*通讯作者。