Hans Journal of Ophthalmology
Vol.
08
No.
01
(
2019
), Article ID:
29434
,
11
pages
10.12677/HJO.2019.81008
Effect of Artesunate on the Expression of ICAM-1 and MMP-9 in Vascular Endothelial Cells under High Glucose Condition
Pengfei Ge, Tao Jiang*, Yao Zong*, Xuejiao Yang, Yuna Ma, Yunxiao Wang, Zhe Sun, Ziqun Cao
Department of Ophthalmology, Affiliated Hospital of Qingdao University, Qingdao Shandong

Received: Mar. 5th, 2019; accepted: Mar. 19th, 2019; published: Mar. 26th, 2019

ABSTRACT
Objective: To investigate the effect of ART on the expression of ICAM-1 and MMP-9 in vascular endothelial cells under high glucose condition. Methods: Human Umbilical Vein Endothelial Cells (HUVEC) were divided into glucose (G) group, 40 mmol/L G + ART (G40 + ART) group, mannitol (M) control group, dimethyl sulfoxide (DMSO) control group. The concentration gradient of G group is 5.5 mmol/L G (G5.5), 25 mmol/L G (G25), 40 mmol/L G (G40); the concentration gradient of M control group is 5.5 mmol/L G + 19.5 mmol/L M (M25), 5.5 mmol/L G + 34.5 mmol/L M (M40); the concentration gradient of ART of G40 + ART group is G40 + 10 ug /ml ART (10A), G40 + 20 ug/ml ART (20A), G40 + 40 ug/ml ART (40A); the volume of DMSO in the DMSO control group is the same as it is in the 40A group. Western blot and cell immunofluorescence technique were used to detect the protein expression of Intercellular adhesion molecule-1 (ICAM-1) and Matrix metalloproteinase-9 (MMP-9) in each group. Results: The protein expression of ICAM-1 and MMP-9 in G25 group was higher than that in G5.5 group (P < 0.01), and it increased in G40 group compared with G25 group (P < 0.01); the protein expression of ICAM-1 and MMP-9 in G25 group was higher than that of M25 group (P < 0.01), and it increased in G40 group compared with M40 (P < 0.01); the protein expression of ICAM-1 and MMP-9 of G40 + ART group was lower than that of G40 group, in which it was lower in 20A group than that of 10A group (P < 0.01), and it was lower in 40A group compared with 20A group (P < 0.01). The DMSO control group showed that the protein expression of ICAM-1 and MMP-9 in G40 + ART was lower than that of G40 + DMSO group (P < 0.01). Conclusion: The expression of ICAM-1 and MMP-9 protein was increased under high glucose condition in a concentration-dependent manner. ART inhibited the expression of ICAM-1 and MMP-9 protein in vascular endothelial cells under high glucose condition in a concentration-dependent manner. This experiment lays the foundation for further study of the changes of ICAM-1 and MMP-9 expression in the mechanism of ART inhibiting retinal neovascularization and leakage.
Keywords:Artesunate, Intercellular Adhesion Molecule-1, Matrix Metalloproteinase-9, Vascular Endothelial Cells, Diabetic Retinopathy
青蒿琥酯对高糖条件下血管内皮细胞ICAM-1和MMP-9表达的影响
葛朋飞,姜涛*,宗瑶*,杨雪娇,马玉娜,王云霄,孙哲,曹子群
青岛大学附属医院眼科,山东 青岛

收稿日期:2019年3月5日;录用日期:2019年3月19日;发布日期:2019年3月26日

摘 要
目的:探讨ART对高糖条件下血管内皮细胞ICAM-1和MMP-9表达的影响。方法:将人脐静脉内皮细胞(Human Umbilical Vein Endothelial Cells, HUVEC)作为实验对象,分为葡萄糖(Glucose, G)组、40 mmol/L G + ART (G40 + ART)组,甘露醇(Mannitol, M)对照组、G40 + DMSO (DMSO)对照组,其中G组浓度梯度分为5.5 mmol/L G(G5.5)、25 mmol/L G(G25)、40 mmol/L G(G40);M对照组浓度梯度为5.5 mmol/L G + 19.5 mmol/L M(M25),5.5 mmol/L G + 34.5 mmol/L M(M40),G40 + ART组中依据ART浓度梯度分为G40 + 10 ug/ml ART(10A)、G40 + 20 ug/ml ART(20A)、G40 + 40 ug/ml ART(40A);DMSO对照组中DMSO用量与40A组中溶解ART所用DMSO体积相同。采用Western blot、细胞免疫荧光技术分别检测各组细胞间黏附分子-1(ICAM-1)、基质金属蛋白酶-9(MMP-9)蛋白表达情况。结果:1) ICAM-1、MMP-9蛋白在G25组较G5.5组表达升高(P < 0.01),且G40组较G25组表达升高(P < 0.01);ICAM-1、MMP-9蛋白在G40 + ART组较G40组表达下降,其中,10A组低于G40组(P < 0.01),20A组低于10A组(P < 0.01),40A组低于20A组(P < 0.01)。2) ICAM-1、MMP-9蛋白在G25组较M25组表达升高(P < 0.01);G40组较M40组表达升高(P < 0.01)。3) DMSO对照组显示G40 + ART组ICAM-1、MMP-9蛋白表达低于DMSO组(P < 0.01)。结论:ICAM-1和MMP-9蛋白在高糖条件下表达升高,且具有浓度依赖性,ART可抑制高糖条件下血管内皮细胞ICAM-1和MMP-9蛋白的表达,且具有浓度依赖性,为进一步研究ART抑制视网膜新生血管形成和渗漏的机制中ICAM-1和MMP-9表达变化奠定基础。
关键词 :青蒿琥酯,细胞间粘附分子-1,基质金属蛋白酶-9,血管内皮细胞,糖尿病性视网膜病变

Copyright © 2019 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
在糖尿病导致的微血管并发症中,DR是最常见的 [1] ,并成为全球工作年龄失明的主要原因 [2] 。DR的临床特征是血管通透性增加,微血管瘤形成,血管增生和黄斑水肿 [3] 。ART是一类新型抗疟药,近年来已经报道了其抗肿瘤作用,而且在肿瘤中拮抗血管生成的作用也引起了广泛的关注 [4] 。ART具有抑制血管内皮细胞增殖、迁移和管腔形成的作用,可以剂量依赖性地有效抑制肿瘤血管的形成和生长 [5] 。最近研究表明ART能抑制视网膜新生血管(NV)的生长和渗漏 [6] 。新生血管形成早期阶段细胞间粘附分子-1(ICAM1)及基质金属蛋白酶-9(MMP-9)至关重要。首先,MMP-9可以降解细胞外基质,此为新生血管形成的前提条件 [7] ,DR患者荧光素血管造影的临床证据表明,血-视网膜屏障是血管渗漏导致黄斑水肿的主要部位,MMP-9可促进血管渗透性增加 [8] 。其次,ICAM-1在白细胞与血管内皮细胞粘附 [9] [10] ,诱导毛细血管阻塞 [11] ,导致内皮细胞损伤和死亡 [12] ,进而导致视网膜毛细血管无灌注和新生血管形成 [13] [14] 。现阶段针对DR中新生血管形成和渗漏的主要治疗手段为抗VEGF治疗,但是,单纯抗VEGF治疗具有一定局限性,如抗VEGF药物靶点单一、半衰期短。因此,我们需要根据新生血管形成的分子机制阐明更多治疗靶点,应用一种能够多靶点、更加长久发挥治疗作用的药物,以预防和治疗这种疾病。之前研究表明,青蒿琥酯(Artesunate, ART)可通过多靶点抑制视网膜新生血管形成和渗漏,本实验应用ART处理高糖条件下HUVEC,并用Western blot、细胞免疫荧光检测ICAM-1、MMP-9蛋白表达情况,探讨ART对高糖条件下血管内皮细胞ICAM-1和MMP-9表达的影响,为进一步研究ART抑制视网膜新生血管形成和渗漏的机制中ICAM-1和MMP-9表达变化奠定基础。
2. 材料与方法
2.1. 材料
2.1.1. 细胞型号及来源
HUVEC-C(型号:ZQ0446,规格:5 × 105 cells/vial)来源于上海中乔新舟生物科技有限公司。
2.1.2. 主要试剂
DMEM低糖培养基(中国Solarbio),DMEM高糖培养基(中国Solarbio),胎牛血清(美国HyClone),青链霉素混合液(100X) (中国Solarbio),0.25%胰蛋白酶消化液(中国Solarbio),二甲基亚砜(美国Sigma),兔抗人MMP-9抗体(英国Abcam),兔抗人ICAM-1抗体(英国Abcam),兔抗人GAPDH抗体(中国Elabscience),羊抗兔Western blot二抗(中国Elabscience),羊抗兔荧光二抗(中国Elabscience),WB专用一抗二抗稀释液(美国 博士得),荧光抗体稀释液(中国Solarbio)。
2.1.3. 主要仪器设备
洁净工作台(中国 苏州安泰空气技术有限公司),CO2恒温培养箱(德国Heraeus),倒置显微镜(日本OLYMPUS),倒置荧光显微镜(日本OLYMPUS),多功能成像系统(法国Vilber),低速台式离心机(中国 北京时代北利离心机有限公司),台式低温高速离心机(美国Sigma),电子天平(美国METTLER TOLEDO)。
2.2. 方法
2.2.1. 细胞培养
将冻存管内的HUVEC复苏,加入含10% FBS的低糖(5.5 mmol/L葡萄糖) DMEM培养液,调整细胞密度为1 × 105/mL,以每瓶4 ml接种于25 cm2培养瓶,放于37℃、5% CO2恒温培养箱恒温培养。
2.2.2. Western Blotting检测ICAM-1和MMP-9蛋白表达
采用SDS-PAGE进行蛋白质印迹分析,将蛋白质样品与5XSDS-PAGE蛋白上样缓冲液按比例(4:1)混匀,依据目的蛋白分子量,配制10%分离胶,5%浓缩胶进行电泳。将蛋白转移至PVDF膜上,用配制好的奶粉封闭液阻断膜上非特异性结合位点。用兔抗人MMP-9抗体(英国Abcam),兔抗人ICAM-1抗体(英国Abcam)进行一抗孵育,和兔抗人GAPDH抗体(中国Elabscience)作为内参对照。然后以羊抗兔Western blot二抗(中国Elabscience)进行二抗孵育。使用ECL发光液用凝胶成像分析仪显影;使用Image J软件定量分析条带灰度值,将各目的条带灰度值与内参GAPDH条带灰度值之比作为该蛋白的相对表达含量,重复三次实验的平均值作为最后统计值。
2.2.3. 细胞免疫荧光检测ICAM-1和MMP-9蛋白表达
根据实验需要将无菌盖玻片置于24孔板的12孔或10孔中。将HUVEC以1 × 105/ml的密度接种于盖玻片上37℃培养24 h。然后去除处理液,用PBS洗涤,4%多聚甲醛固定10分钟,并在室温下用0.1% Triton X 100通透1分钟。将细胞在含有10%封闭用正常山羊血清PBS中封闭1小时。用兔抗人ICAM-1荧光抗体(英国Abcam)在4℃孵育过夜,同时对应一孔不加一抗作为阴性对照。用羊抗兔荧光二抗(中国Elabscience)在室温下孵育1小时。使用DAPI复染核10 min。使用抗荧光衰减封片剂封片,通过放大倍数为200倍的荧光显微镜观察并拍摄结果。
2.3. 统计学方法
运用SPSS 17.0对数据进行统计学分析,各组数据经Shapiro-Wilk检验均呈正态分布,数据以均数±标准差表示,两组数据之间比较采用t检验,以P < 0.01或P < 0.05为差异具有统计学意义。
3. 结果
3.1. 高糖对HUVEC ICAM-1和MMP-9蛋白表达的影响及ART对高糖条件下HUVEC ICAM-1和MMP-9蛋白表达的影响
应用G5.5,G25,G40的培养液处理细胞,运用Western blot、细胞免疫荧光检测ICAM-1、MMP-9蛋白表达。结果表明ICAM-1、MMP-9蛋白在G25组较G5.5组表达升高,且G40组较G25组表达升高,差异有统计学意义(t = 4.796、17.31、3.430、2.987,均为P < 0.01)。应用10 A,20 A,40 A三种药物浓度对细胞进行处理,运用Western blot、细胞免疫荧光检测ICAM-1、MMP-9蛋白表达,结果表明ICAM-1、MMP-9蛋白在G40 + ART组较G40组表达下降,其中,10 A组低于G40组,差异有统计学意义(t = 3.846、8.887,均为P < 0.01),20A组低10A组,差异有统计学意义(t = 6.536、5.329,P < 0.01),40 A组低于20 A组,差异有统计学意义(t = 6.169、3.947,P < 0.01) (如图1A~图1D)。
3.2. 高渗透压对HUVEC ICAM-1、MMP-9蛋白表达的影响
对于M对照组,应用Western blot、细胞免疫荧光检测HUVEC ICAM-1、MMP-9蛋白表达情况,结果显示ICAM-1、MMP-9蛋白表达在G25组较M25组表达升高,G40组较M40组表达升高,差异具有统计学意义(t = 11.84、3.845、8.803、15.30,均为P < 0.01) (如图2A~图2D)。
3.3. 为进一步证明ART对高糖条件下HUVEC ICAM-1、MMP-9蛋白表达的影响,设置DMSO对照组
DMSO对照组中DMSO用量与40A组中溶解ART所用DMSO体积相同,采用Western blot、细胞免疫荧光检测ICAM-1、MMP-9蛋白表达,结果显示G40 + ART组ICAM-1、MMP-9蛋白表达低于DMSO组(t = 5.997、10.31、21.43、3.378、8.229、18.19,均为P < 0.01) (如图3A~图3D)。
 图C、D放大倍数(200×)
图C、D放大倍数(200×)
Figure 1. A, B, C, D The results of Western blot and cell Immunofluorescence showed that the expression of HUVEC ICAM-1 and MMP-9 increased under high glucose condition in a concentration-dependent manner. ART inhibits the expression of HUCAMC ICAM1 and MMP-9 proteins under high glucose conditions in a concentration-dependent manner. (*indicates P < 0.01 compared with G5.5 group; **indicates P < 0.01 compared with G25 group, # indicates P < 0.01 compared with G40 group, ##indicates P < 0.01 compared with 10A group, ####indicates P < 0.01 compared with 20A group)
图1. A、B、C、D Western blot、细胞免疫荧光结果显示HUVEC ICAM-1、MMP-9在高糖条件下表达升高,且具有浓度依赖性;ART可抑制高糖条件下HUVEC ICAM1、MMP-9蛋白表达,且具有浓度依赖性(*表示与G5.5组相比P < 0.01;**表示与G25组相比P < 0.01,#表示与G40相比P < 0.01,##表示与10A组相比P < 0.01,####表示与20A组相比P < 0.01)
 图C、D放大倍数(200×)
图C、D放大倍数(200×)
Figure 2. A, B, C, D Western blot and cell Immunofluorescence showed that high osmotic pressure had no significant effect on ICAM-1 and MMP-9 protein expression. (*indicates P < 0.01 compared with G25, **indicates P < 0.01 compared with G40)
图2. A、B、C、D Western blot、细胞免疫荧光结果显示高渗透压对ICAM-1、MMP-9蛋白表达无显著影响。(*表示与G25相比P < 0.01,**表示与G40相比P < 0.01)
4. 讨论
本研究在细胞水平证实了ICAM-1和MMP-9蛋白在高糖条件下表达升高,且具有浓度依赖性,ART可抑制高糖条件下血管内皮细胞ICAM-1和MMP-9蛋白的表达,且具有浓度依赖性。
有越来越多的证据表明炎症在DR的发病机制中起着重要作用,DR始于低度慢性炎症性疾病 [15] ,
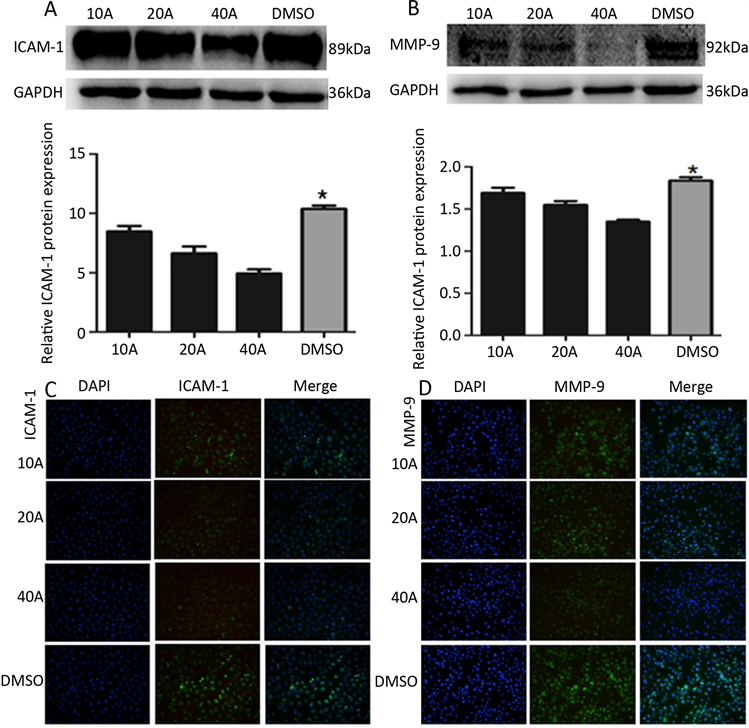
Figure 3. A, B, C, D Western blot and cell Immunofluorescence showed that the expression of ICAM-1 and MMP-9 in ART group was significantly lower than that in DMSO group. (*indicates P < 0.01 compared with the 10 A, 20 A, and 40 A groups)
图3. A、B、C、D Western blot、细胞免疫荧光结果显示ART组ICAM-1、MMP-9蛋白表达显著低于DMSO组(*表示与10 A、20 A、40 A组相比均为P < 0.01)
在DR发病过程中促炎因子、趋化因子、白细胞粘附增加 [16] ,白细胞瘀滞是炎症过程的主要组成部分 [17] ,内皮细胞表达的ICAM-1不仅可以调节白细胞与内皮细胞的粘附,而且在血视网膜屏障破坏、血管通透性的调节中也起着重要作用 [18] [19] [20] ,可诱导视网膜毛细血管无灌注和新生血管形成 [13] [14] 。MMP-9可促进白细胞瘀 [21] ,其机制与基底膜降解,白细胞聚集于受损组织处有关 [22] 。
此外,DR新生血管生成的阶段包括:发生基底膜降解,内皮细胞迁移和增殖,随后毛细血管形成。这种组织的迁移和重塑受基质金属蛋白酶的调节 [17] 。MMP-9是基质金属蛋白酶家族中最大的成员 [23] ,其可促进血管渗透性增加 [24] ,降解毛细血管基底膜,毛细血管基底膜是细胞外基质的一部分 [7] ,这是内皮细胞在内皮下基质中渗透和形成新腔的必要条件 [8] 。而且,MMP-9可降解BRB内皮细胞紧密连接蛋白组分,增加血管渗漏 [24] 。
研究表明高糖条件下血管内皮细胞及DR患者中ICAM-1、MMP-9表达升高 [8] [25] [26] [27] ,且血管内皮细胞ICAM-1的升高呈葡萄糖浓度依赖性 [28] ,本实验结果与之前研究结果一致,此外,本实验还进一步证明随着葡萄糖浓度升高,血管内皮细胞MMP-9表达会随之升高,提示更高的血糖浓度可能加重糖尿病性视网膜病严重程度。本研究结果显示ART可抑制高糖条件下血管内皮细胞ICAM-1,MMP-9表达,并且呈现浓度依赖性。ART通过下调VEGFR2,PKCα和PDGFR的表达来抑制兔的虹膜和视网膜新生血管,并缓解猴子的黄斑水肿 [6] 。本实验为ART抑制视网膜新生血管的形成和渗漏提供了新的作用靶点和理论支持。糖尿病患者不仅ICAM-1水平高,而且其配体CD11a/CD18和CD11b/CD18的水平也增加 [29] 。阻断ICAM-1或CD18的表达减弱了糖尿病动物的视网膜血管中的白细胞瘀滞、内皮细胞死亡和血管渗漏 [30] 。在敲除ICAM-1和CD18基因的DR小鼠模型中视网膜血管粘附的白细胞数量减少,血管内皮细胞损伤数目减少,血–视网膜屏障破坏降低,视网膜血管组织病理学改变减轻 [16] 。通过抑制MMP-9的活性可抑制角膜新生血管形成并保护BRB功能的完整性,减少视网膜血管渗漏 [31] [32] 。因此,结合本实验在细胞水平研究结果,ICAM-1、MMP-9可能成为ART治疗DR的新作用位点,并发挥治疗作用。
DR发病过程中,血管内皮细胞生长因子(VEGF)为促使新生血管和黄斑水肿形成的主要因子 [33] ,但是,其它一些因子也具有同样作用,如ICAM-1、MMP-9,HMGB-1 [34] [35] [36] ,TNFα [37] [38] 等,因此,VEGF并非新生血管形成和渗漏的唯一因素。目前针对新生血管及黄斑水肿的治疗主要为抗VEGF药物的应用。抗VEGF药物可抑制新生血管形成和渗漏,并提升黄斑水肿患者的视力 [33] [39] 。但是,抗VEGF药物存在一定局限性,如半衰期短 [40] ,药物作用持续时间短,例如,目前广泛应用的雷珠单抗需每月玻璃体腔注射以维持药物在眼内组织的治疗水平 [41] ;靶点单一,仅能以VEGF某些亚型为治疗靶点,仅Aflibercept和Conbercept能额外以胎盘生长因子为治疗靶点 [40] ;最重要的是,只有少于50%的患者接受抗VEGF治疗后获得视力的提升 [42] [43] [44] [45] 。研究认为严格控制血糖可降低DR致盲率 [46] ,但临床上维持正常血糖水平是困难的,有时甚至是不可能的,需要根据其发展的分子机制阐明更多治疗靶点以预防和治疗这种疾病。由于ART可以通过作用于VEGFR2,PKCα,PDGFR三个靶点抑制视网膜新生血管的发生、发展和渗漏,药物作用时间长达6个月 [6] 。而且本实验在细胞水平证明ICAM-1、MMP-9可作为ART两个新作用位点。此外,ART耐受性较好 [47] ,青蒿素及其衍生物抗新生血管的作用浓度仅为临床用于疟疾治疗剂量千分之一 [48] ,在非大剂量(10 mg/L)全身长期应用条件下没有额外副作用 [46] [49] [50] [51] [52] [53] ,因此,ART应用于DR的早期和晚期治疗成为可能。
总之,本实验在细胞模型中证明ICAM-1和MMP-9的升高具有葡萄糖浓度依赖性,根据之前研究在一定程度上提示DR患者应严格控制血糖水平;此外,ART可抑制高糖条件下血管内皮细胞ICAM-1和MMP-9蛋白的表达,且具有浓度依赖性,ICAM-1和MMP-9可能作为ART治疗DR新作用位点,为其成为治疗DR的新型药物及ART抑制视网膜新生血管形成和渗漏的机制中ICAM-1和MMP-9表达变化、发现其更多潜在治疗价值提供帮助。但是,本实验仅局限于细胞水平ART对ICAM-1和MMP-9的影响,对于ART抑制视网膜新生血管形成和渗漏的机制中ICAM-1和MMP-9表达变化有待进一步实验研究。
基金项目
山东省自然科学基金(编号:ZR2018BH013)。
文章引用
葛朋飞,姜 涛,宗 瑶,杨雪娇,马玉娜,王云霄,孙 哲,曹子群. 青蒿琥酯对高糖条件下血管内皮细胞ICAM-1和MMP-9表达的影响
Effect of Artesunate on the Expression of ICAM-1 and MMP-9 in Vascular Endothelial Cells under High Glucose Condition[J]. 眼科学, 2019, 08(01): 41-51. https://doi.org/10.12677/HJO.2019.81008
参考文献
- 1. Rubsam, A., Parikh, S. and Fort, P.E. (2018) Role of Inflammation in Diabetic Retinopathy. International Journal of Molecular Sciences, 19, 942.
https://doi.org/10.3390/ijms19040942 - 2. Stefanini, F.R., Badaro, E., Falabella, P., et al. (2014) Anti-VEGF for the Management of Diabetic Macular Edema. Journal of Immunology Research, 2014, Article ID: 632307.
https://doi.org/10.1155/2014/632307 - 3. Barber, A.J. (2003) A New View of Diabetic Ret-inopathy: A Neurodegenerative Disease of the Eye. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 27, 283-290.
https://doi.org/10.1016/S0278-5846(03)00023-X - 4. He, R.R. and Zhou, H.J. (2008) Progress in Research on the Anti-Tumor Effect of Artesunate. Chinese Journal of Integrative Medicine, 14, 312-316.
https://doi.org/10.1007/s11655-008-0312-0 - 5. Chen, H.H. and Zhou, H.J. (2004) Inhibitory Effects of Ar-tesunate on Angiogenesis. Acta Pharmaceutica Sinica, 39, 29-33.
- 6. Zong, Y., Yuan, Y., Qian, X., et al. (2016) Small Molecular-Sized Artesunate Attenuates Ocular Neovascularization via VEGFR2, PKCalpha, and PDGFR Targets. Scientific Reports, 6, Article No. 30843.
https://doi.org/10.1038/srep30843 - 7. Rocca, A., Tafuri, D., Paccone, M., et al. (2017) Cell Based Therapeutic Approach in Vascular Surgery: Application and Review. Open Medicine (Warsaw, Poland), 12, 308-322.
https://doi.org/10.1515/med-2017-0045 - 8. Kowluru, R.A., Zhong, Q. and Santos, J.M. (2012) Matrix Metallo-proteinases in Diabetic Retinopathy: Potential Role of MMP-9. Expert Opinion on Investigational Drugs, 21, 797-805.
https://doi.org/10.1517/13543784.2012.681043 - 9. Cronstein, B.N. and Weissmann, G. (1993) The Adhesion Molecules of Inflammation. Arthritis and Rheumatism, 36, 147-157.
https://doi.org/10.1002/art.1780360204 - 10. Mackay, C.R. and Imhof, B.A. (1993) Cell Adhesion in the Immune System. Immunology Today, 14, 99-102.
https://doi.org/10.1016/0167-5699(93)90205-Y - 11. Schroder, S., Palinski, W. and Schmid-Schonbein, G.W. (1991) Activated Monocytes and Granulocytes, Capillary Nonperfusion, and Neovascularization in Diabetic Retinopathy. The American Journal of Pathology, 139, 81-100.
- 12. Joussen, A.M., Murata, T., Tsujikawa, A., et al. (2001) Leukocyte-Mediated Endothelial Cell Injury and Death in the Diabetic Retina. The American Journal of Pathology, 158, 147-152.
https://doi.org/10.1016/S0002-9440(10)63952-1 - 13. Adamis, A.P., Shima, D.T., Tolentino, M.J., et al. (1996) Inhibition of Vascular Endothelial Growth Factor Prevents Retinal Ischemia-Associated Iris Neovascularization in a Nonhuman Primate. Archives of Ophthalmology, 114, 66-71.
https://doi.org/10.1001/archopht.1996.01100130062010 - 14. Aiello, L.P., Pierce, E.A., Foley, E.D., et al. (1995) Suppression of Retinal Neovascularization in Vivo by Inhibition of Vascular Endothelial Growth Factor (VEGF) Using Soluble VEGF-Receptor Chimeric Proteins. Proceedings of the National Academy of Sciences of the United States of America, 92, 10457-10461.
https://doi.org/10.1073/pnas.92.23.10457 - 15. Adamis, A.P. (2002) Is Diabetic Retinopathy an Inflammatory Disease? The British Journal of Ophthalmology, 86, 363-365.
https://doi.org/10.1136/bjo.86.4.363 - 16. Joussen, A.M., Poulaki, V., Le, M.L., et al. (2004) A Central Role for Inflammation in the Pathogenesis of Diabetic Retinopathy. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 18, 1450-1452.
https://doi.org/10.1096/fj.03-1476fje - 17. El-Asrar, A.M. (2012) Role of Inflammation in the Pathogenesis of Diabetic Retinopathy. Middle East African Journal of Ophthalmology, 19, 70-74.
https://doi.org/10.4103/0974-9233.92118 - 18. Steeber, D.A., Campbell, M.A., Basit, A., et al. (1998) Optimal Selectin-Mediated Rolling of Leukocytes during Inflammation in Vivo Requires Intercellular Adhesion Molecule-1 Expression. Proceedings of the National Academy of Sciences of the United States of America, 95, 7562-7567.
https://doi.org/10.1073/pnas.95.13.7562 - 19. Williams, M.R. and Luscinskas, F.W. (2011) Leukocyte Rolling and Adhesion via ICAM-1 Signals to Endothelial Permeability. Focus on “Leukocyte Rolling and Adhesion both Contribute to Regulation of Microvascular Permeability to Albumin via Ligation of ICAM-1”. American Journal of Physiology. Cell Physiology, 301, C777-C779.
https://doi.org/10.1152/ajpcell.00250.2011 - 20. Joussen, A.M., Poulaki, V., Mitsiades, N., et al. (2003) Suppres-sion of Fas-FasL-Induced Endothelial Cell Apoptosis Prevents Diabetic Blood-Retinal Barrier Breakdown in a Model of Streptozotocin-Induced Diabetes. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 17, 76-78.
https://doi.org/10.1096/fj.02-0157fje - 21. Rangasamy, S., McGuire, P.G. and Das, A. (2012) Diabetic Retinopathy and Inflammation: Novel Therapeutic Targets. Middle East African journal of ophthalmology, 19, 52-59.
https://doi.org/10.4103/0974-9233.92116 - 22. Ram, M., Sherer, Y. and Shoenfeld, Y. (2006) Matrix Metallo-proteinase-9 and Autoimmune Diseases. Journal of Clinical Immunology, 26, 299-307.
https://doi.org/10.1007/s10875-006-9022-6 - 23. Malemud, C.J. (2006) Matrix Metalloproteinases (MMPs) in Health and Disease: An Overview. Frontiers in Bioscience: A Journal and Virtual Library, 11, 1696-1701.
- 24. Giebel, S.J., Menicucci, G., McGuire, P.G., et al. (2005) Matrix Metalloproteinases in Early Diabetic Retinopathy and Their Role in Alteration of the Blood-Retinal Barrier. Laboratory Investigation: A Journal of Technical Methods and Pathology, 85, 597-607.
https://doi.org/10.1038/labinvest.3700251 - 25. Matsumoto, K., Sera, Y., Ueki, Y., et al. (2002) Comparison of Serum Concentrations of Soluble Adhesion Molecules in Diabetic Microangiopathy and Macroangiopathy. Diabetic Medicine: A Journal of the British Diabetic Association, 19, 822-826.
https://doi.org/10.1046/j.1464-5491.2002.00799.x - 26. Limb, G.A., Hickman-Casey, J., Hollifield, R.D., et al. (1999) Vascular Adhesion Molecules in Vitreous from Eyes with Proliferative Diabetic Retinopathy. Investigative Ophthalmology & Visual Science, 40, 2453-2457.
- 27. Das, A., McGuire, P.G., Eriqat, C., et al. (1999) Human Dia-betic Neovascular Membranes Contain High Levels of Urokinase and Metalloproteinase Enzymes. Investigative Oph-thalmology & Visual Science, 40, 809-813.
- 28. Lee, C.H., Shieh, Y.S., Hsiao, F.C., et al. (2014) High Glucose In-duces Human Endothelial Dysfunction through an Axl-Dependent Mechanism. Cardiovascular Diabetology, 13, 53.
https://doi.org/10.1186/1475-2840-13-53 - 29. Song, H., Wang, L. and Hui, Y. (2007) Expression of CD18 on the Neutrophils of Patients with Diabetic Retinopathy. Graefe’s Archive for Clinical and Experimental Ophthalmology, 245, 24-31.
https://doi.org/10.1007/s00417-006-0379-2 - 30. Kociok, N., Radetzky, S., Krohne, T.U., et al. (2009) ICAM-1 Depletion Does Not Alter Retinal Vascular Development in a Model of Oxygen-Mediated Neovascularization. Exper-imental Eye Research, 89, 503-510.
https://doi.org/10.1016/j.exer.2009.05.005 - 31. Bhatt, L.K. and Addepalli, V. (2010) Attenuation of Diabetic Retinopathy by Enhanced Inhibition of MMP-2 and MMP-9 Using Aspirin and Minocycline in Streptozotocin-Diabetic Rats. American Journal of Translational Research, 2, 181-189.
- 32. Samtani, S., Amaral, J., Campos, M.M., et al. (2009) Doxycycline-Mediated Inhibition of Choroidal Neovascularization. Investigative Ophthalmology & Visual Science, 50, 5098-5106.
https://doi.org/10.1167/iovs.08-3174 - 33. (2015) VEGF Inhibitors for AMD and Diabetic Macular Edema. JAMA, 314, 2184-2185.
https://doi.org/10.1001/jama.2015.15427 - 34. Klaassen, I., Van Noorden, C.J. and Schlingemann, R.O. (2013) Molecular Basis of the Inner Blood-Retinal Barrier and Its Breakdown in Diabetic Macular Edema and Other Pathological Conditions. Progress in Retinal and Eye Research, 34, 19-48.
https://doi.org/10.1016/j.preteyeres.2013.02.001 - 35. Yu, Y., Yang, L., Lv, J., et al. (2015) The Role of High Mobility Group Box 1 (HMGB-1) in the Diabetic Retinopathy Inflammation and Apoptosis. International Journal of Clinical and Experimental Pathology, 8, 6807-6813.
- 36. Santos, A.R., Dvoriantchikova, G., Li, Y., et al. (2014) Cellular Mechanisms of High Mobility Group 1 (HMGB-1) Protein Action in the Diabetic Retinopathy. PLoS ONE, 9, e87574.
https://doi.org/10.1371/journal.pone.0087574 - 37. Clauss, M., Sunderkotter, C., Sveinbjornsson, B., et al. (2001) A Permissive Role for Tumor Necrosis Factor in Vascular Endothelial Growth Factor-Induced Vascular Permeability. Blood, 97, 1321-1329.
https://doi.org/10.1182/blood.V97.5.1321 - 38. Joussen, A.M., Poulaki, V., Mitsiades, N., et al. (2002) Non-steroidal Anti-Inflammatory Drugs Prevent Early Diabetic Retinopathy via TNF-Alpha Suppression. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 16, 438-440.
https://doi.org/10.1096/fj.01-0707fje - 39. Jampol, L.M., Bressler, N.M. and Glassman, A.R. (2014) Revolution to a New Standard Treatment of Diabetic Macular Edema. JAMA, 311, 2269-2270.
https://doi.org/10.1001/jama.2014.2536 - 40. Lu, X. and Sun, X. (2015) Profile of Conbercept in the Treatment of Neovascular Age-Related Macular Degeneration. Drug Design, Development and Therapy, 9, 2311-2320.
- 41. Wong, T.Y., Liew, G. and Mitchell, P. (2007) Clinical Update: New Treatments for Age-Related Macular Degeneration. The Lancet (London, England), 370, 204-206.
https://doi.org/10.1016/S0140-6736(07)61104-0 - 42. Nguyen, Q.D., Brown, D.M., Marcus, D.M., et al. (2012) Ranibizumab for Diabetic Macular Edema: Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology, 119, 789-801.
https://doi.org/10.1016/j.ophtha.2011.12.039 - 43. Rajendram, R., Fraser-Bell, S., Kaines, A., et al. (2012) A 2-Year Prospective Randomized Controlled Trial of Intravitreal Bevacizumab or Laser Therapy (BOLT) in the Man-agement of Diabetic Macular Edema: 24-Month Data: Report 3. Archives of Ophthalmology, 130, 972-979.
https://doi.org/10.1001/archophthalmol.2012.393 - 44. Brown, D.M., Schmidt-Erfurth, U., Do, D.V., et al. (2015) Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results from the VISTA and VIVID Studies. Oph-thalmology, 122, 2044-2052.
https://doi.org/10.1016/j.ophtha.2015.06.017 - 45. Michael, S. and Ip, M.D. (2008) A Randomized Trial Com-paring Intravitreal Triamcinolone Acetonide and Focal/Grid Photocoagulation for Diabetic Macular Edema. Ophthal-mology, 115, 1447-1449.
- 46. Ebneter, A. and Zinkernagel, M.S. (2016) Novelties in Diabetic Retinopathy. Endocrine Development, 31, 84-96.
https://doi.org/10.1159/000439391 - 47. Zang, M., Zhu, F., Zhao, L., et al. (2014) The Effect of UGTs Polymor-phism on the Auto-Induction Phase II Metabolism-Mediated Pharmacokinetics of Dihydroartemisinin in Healthy Chinese Subjects after Oral Administration of a Fixed Combination of Dihydroartemisinin-Piperaquine. Malaria Journal, 13, 478.
https://doi.org/10.1186/1475-2875-13-478 - 48. Efferth, T., Sauerbrey, A., Olbrich, A., et al. (2003) Molecular Modes of Action of Artesunate in Tumor Cell Lines. Molecular Pharmacology, 64, 382-394.
https://doi.org/10.1124/mol.64.2.382 - 49. Berger, T.G., Dieckmann, D., Efferth, T., et al. (2005) Artesunate in the Treatment of Metastatic Uveal Melanoma—First Experiences. Oncology Reports, 14, 1599-1603.
https://doi.org/10.3892/or.14.6.1599 - 50. White, N.J., Ashley, E.A. and Nosten, F. (2006) Toxic Brainstem En-cephalopathy after Artemisinin Treatment for Breast Cancer. Annals of Neurology, 59, 725-726.
https://doi.org/10.1002/ana.20815 - 51. Crespo-Ortiz, M.P. and Wei, M.Q. (2012) Antitumor Activity of Arte-misinin and Its Derivatives: From a Well-Known Antimalarial Agent to a Potential Anticancer Drug. Journal of Biomed-icine & Biotechnology, 2012, Article ID: 247597.
https://doi.org/10.1155/2012/247597 - 52. Ba, Q., Duan, J., Tian, J.Q., et al. (2013) Dihydroartemisinin Promotes Angiogenesis during the Early Embryonic Development of Zebrafish. Acta Pharmacologica Sinica, 34, 1101-1107.
https://doi.org/10.1038/aps.2013.48 - 53. D’Alessandro, S., Gelati, M., Basilico, N., et al. (2007) Differential Ef-fects on Angiogenesis of Two Antimalarial Compounds, Dihydroartemisinin and Artemisone: Implications for Embry-otoxicity. Toxicology, 241, 66-74.
https://doi.org/10.1016/j.tox.2007.08.084