Advances in Clinical Medicine
Vol.
11
No.
03
(
2021
), Article ID:
41378
,
6
pages
10.12677/ACM.2021.113198
肺泡巨噬细胞线粒体活性氧触发IL-8释放在机械通气肺损伤中相关性研究
朱蔚琳,黄元巳,叶钰琼*,王亚峰,黄爱兰,胡彦艳,马利
广西壮族自治区人民医院麻醉科,广西 南宁
收稿日期:2021年2月25日;录用日期:2021年3月12日;发布日期:2021年3月31日

摘要
目的:研究大鼠机械通气肺损伤中,肺泡巨噬细胞活性氧与炎性因子IL-8变化及相关性,揭示机械通气肺损伤中,肺泡巨噬细胞线粒体活性氧是否通过触发肺组织IL-8释放,引起肺病理损伤。方法:健康雄性SD大鼠30只,体重250~300 g,6~8周龄,采用随机数字表法分为5组(n = 6):正常对照组(NC组)、高潮气量组 + 生理盐水组(HV + NS组)、高潮气量 + 去铁胺组(200 mg∙kg−1) (HV + DFO组)、低潮气量组 + 生理盐水组(LV + NS组)、低潮气量 + 去铁胺组(200 mg∙kg−1) (LV + DFO组)。NC组和HV + NS组、LV + NS组腹腔注射生理盐水2 ml;HV + DFO组、LV + DFO组腹腔注射去铁胺200 mg/kg (溶于2 ml生理盐水中)。15 min后HV + NS组、LV + NS组、HV + DFO组与LV + DFO组连接小动物呼吸机,行容量控制机械通气4 h。机械通气结束后处死大鼠,取左肺组织,光镜下观察病理学结果,并行病理损伤评分,计算肺湿/干(W/D)比值;行右肺灌洗,收集灌洗液,制备巨噬细胞悬液,采用流式细胞仪测定线粒体ROS水平,ELISA法检测肺组织IL-8。结果:与HV + NS组比较,LV + NS组肺组织病理损伤评分和W/D比值、肺泡巨噬细胞及线粒体ROS水平明显降低(P < 0.05);LV + DFO组较HV + DFO组肺组织病理损伤评分和W/D比值、肺泡巨噬细胞及线粒体ROS水平显著降低(P < 0.05)。其中LV + DFO组与对照组比较,差异无统计学意义(P < 0.05);线粒体ROS与肺组织IL-8具有正相关。结论:初步揭示机械通气肺损伤中,巨噬细胞活性氧释放可能触发了炎性反应,从而引起肺病理损伤。
关键词
呼吸机相关肺损伤,巨噬细胞,线粒体,ROS,IL-8
Study on the Correlation of IL-8 Release Triggered by Mitochondrial Reactive Oxygen Species of Alveolar Macrophages in Mechanically Ventilated Lung Injury
Weilin Zhu, Yuansi Huang, Yuqiong Ye*, Yafeng Wang, Ailan Huang, Yanyan Hu, Li Ma
Department of Anesthesia, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning Guangxi
Received: Feb. 25th, 2021; accepted: Mar. 12th, 2021; published: Mar. 31st, 2021

ABSTRACT
Objective: To study the changes and correlation between the reactive oxygen species of alveolar macrophages and the inflammatory factor IL-8 in mechanically ventilated lung injury in rats, and reveal whether the mitochondrial reactive oxygen species of alveolar macrophages trigger the release of IL-8 in the mechanically ventilated lung injury to cause lung pathological damage. Methods: Thirty healthy male SD rats, weighing 250~300 g and 6~8 weeks old, were divided into 5 groups (n = 6) by random number table: normal control group (NC group), high tidal volume group + physiological saline group (HV + NS group), high tidal volume + deferoxamine group (200 mg∙kg−1) (HV + DFO group), low tidal volume group + normal saline group (LV + NS group), low tidal volume + deferoxamine group (200 mg∙kg−1) (LV + DFO group). The NC group, HV + NS group and LV + NS group were intraperitoneally injected with 2 ml of normal saline; the HV + DFO group and LV + DFO group were intraperitoneally injected with 200 mg/kg deferoxamine (dissolved in 2 ml of normal saline). After 15 minutes, the HV + NS group, LV + NS group, HV + DFO group, and LV + DFO group were connected to a small animal ventilator, and volume-controlled mechanical ventilation was performed for 4 hours. After the mechanical ventilation, the rats were sacrificed, the left lung tissue was taken, the pathological results were observed under a light microscope, and the pathological damage score was concurrently calculated, and the wet weight/dry weight (W/D) ratio was calculated; the right lung was lavaged and the lavage fluid was collected. Right lung lavage was performed, lavage fluid was collected, macrophage suspension was prepared, mitochondrial ROS level was determined by flow cytometry, and IL-8 in lung tissue was detected by ELISA. Results: Compared with the HV + NS group, the lung tissue pathological damage score and W/D ratio, alveolar macrophages and mitochondrial ROS levels in the LV + NS group were significantly reduced (P < 0.05). The LV + NS group was still higher than the control group (P < 0.05); compared with HV + DFO group, lung tissue pathological damage score and W/D ratio, alveolar macrophages and mitochondrial ROS levels were significantly lower in the LV + DFO group than in the HV + DFO group (P < 0.05). Among them, there was no significant difference between the LV + DFO group and the control group (P < 0.05); there was a positive correlation between mitochondrial ROS and lung IL-8. Conclusion: In mechanically ventilated lung injury, the release of reactive oxygen species from macrophage mitochondria may trigger IL-8 inflammatory factors and cause lung pathological damage.
Keywords:Ventilator-Related Lung Injury, Macrophages, Mitochondria, ROS, IL-8

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 前言
机械通气肺损伤(VILI)在临床上越来越引起重视。研究表明,大潮气量肺损伤早期导致肺内中性粒细胞浸润及促炎性因子的释放 [1],我们课题组前期研究:7 ml∙kg−1 PBW与传统的12 ml∙kg−1 PBW潮气量相比,明显降低老年患者的肺炎性因子IL-6、IL-8、TNF-a的释放,改善预后 [2]。炎性损伤是如何触发的呢?近年来研究发现,氧化应激参与了机械损伤 [3]。我们前期研究表明,大潮气量通气使肺泡巨噬细胞线粒体活性氧释放增加,同时肺病理损伤加重,该过程可以被螯合剂去铁胺抑制 [4] [5]。
肺泡巨噬细胞线粒体活性氧释放,是否与炎性因子具有相关性,并触发了炎性反应,还不清楚。
本课题将利用大鼠机械通气模型,研究大鼠肺泡巨噬细胞线粒体活性氧与机械通气肺损伤中炎性因子IL-8变化及相关性,初步揭示机械通气肺损伤中,巨噬细胞活性氧释放可能触发了炎性反应,从而引起肺病理损伤。
2. 实验方法
2.1. 实验分组
本研究是根据广西壮族自治区人民医院伦理与动物保护委员会批准的《实验动物的护理和使用指南》(中国南宁市,批准号2014-034)进行的。
将30只SD成年雄性大鼠,体重(285 ± 15 g),随机分为5组(每组6只):1) 正常对照组(NC组):腹腔注射10%水合氯醛5 ml∙kg−1麻醉后,气管插管保留自主呼吸4 h后取肺标本;2) 大潮气量 + 生理盐水(HV + NS组):麻醉后生理盐水4 ml∙kg−1腹腔注射,设潮气量40 ml∙kg−1,频率20~40次/分;3) 大潮气量 + 去铁胺组(HV + DFO组):去铁胺200 ml∙kg−1腹腔注射后,设潮气量40 ml∙kg−1,频率20~40次/分;4) 小潮气量组 + 生理盐水(LV + NS组):生理盐水4 ml∙kg−1腹腔注射,潮气量7 ml∙kg−1,频率40~60次/分;5) 小潮气量 + 去铁胺组(LV + DFO组):去铁胺200 ml∙kg−1腹腔注射,潮气量7 ml∙kg−1,频率40~60次/分。机械通气I:E均为1:1,控制呼吸4 h。
2.2. 麻醉、通气模型的建立及取材
麻醉:SD大鼠禁食8小时,10%水合氯醛5 ml∙kg−1行腹腔注射麻醉后电子秤称重,每1h追加首剂的1/3。
机械通气:按照前期研究建立大鼠机械通气模型,连接呼吸机(ALC-V9动物呼吸机)。大潮气量组:设潮气量40 ml∙kg−1;小潮气量通气组:设潮气量7 ml∙kg−1;加去铁胺组:连接呼吸机后,腹腔注射去铁胺200 ml∙kg−1 [3];加生理盐水组,连接呼吸机后腹腔注射生理盐水4 ml∙kg−1。呼吸机参数为:I:E为1:1,控制呼吸4 h,股动脉穿刺置管,测血气维持机械通气期间动脉血二氧化碳分压(PaCO2)为35~45 mmHg。
取材:机械通气4 h后,用组织剪刀从肋骨下方沿前正中线,向上打开胸腔,放血处死大鼠,整体取出气管、心脏及双肺。
2.3. 肺W/D比重
取新鲜肺组织,置于预先测得重量A的载物片上,电子天平称总湿重后记录为W′,置80℃烘箱中烘干脱水48 h至恒重,称取总干重后记录为D′,计算W/D值,评估肺水肿程度。W/D = (W′ − A)/(D′ − A)。
2.4. 肺病理切片及肺组织病理评分
取新鲜肺组织约1 mm × 1 mm × 1 mm,用10%甲醛固定,送广西壮族自治区人民医院病理科做石蜡包埋切片,光镜下观察病理切片。并参照文献 [3],进行病理损伤评分。
2.5. 巨噬细胞线粒体ROS检测
方法略,同前期研究 [3]。
2.6. 采用ELISA检测肺组织IL-8
按照ELISA试剂盒说明操作。
2.7. 统计分析
活性氧采用及Flowjo 7.6.2软件监测及分析,其它计量资料采用SPSS 24.0软件分析。计量资料以 ± s表示,组间比较采用单因素方差分析,活性氧与IL-8相关性采用Pearson双变量相关分析,P < 0.05为差异有统计学意义。
3. 结果
3.1. 各组肺W/D及肺病理评分比较
与NC组比较,大潮气量 + 生理盐水组(HV + NS组)肺湿/干重比值、肺病理损伤显著增加,差异有统计学意义(P < 0.05);与大潮气量 + 生理盐水组(HV + NS组)比较,小潮气量 + 生理盐水组(LV + NS组)、大潮气量 + 去铁胺组(HV + DFO组)、小潮气量 + 去铁胺组(LV + DFO组)肺湿/干重比值显著降低,差异统计学意义(P < 0.05);与LV + NS组比较,LV + DFO组的肺湿/干重比值显著降低,差异有明显差异(P < 0.05),见表1。
Table 1. Comparison of lung W/D in each group (n = 6, x ¯ ± s)
表1. 各组肺W/D的比较(n = 6, ± s)
注:与NS组比较,*P < 0.05;与LV + NS组比较,ΔP < 0.05。
3.2. 5组巨噬细胞线粒体ROS平均荧光强度的比较
与NC组比较,大潮气量+生理盐水组(HV + NS组)、小潮气量 + 生理盐水组(LV + NS组)巨噬细胞线粒体ROS平均荧光强度均显著增加(P < 0.05);与HV + NS组比较,大潮气量 + 去铁胺组(HV + DFO组)、小潮气量 + 生理盐水组(LV + NS组)、小潮气量 + 去铁胺组(LV + DFO组)巨噬细胞线粒体ROS平均荧光强度均显著降低(P > 0.05)。小潮气量 + 去铁胺组(LV + DFO组)显示出更显著的效果,见表2。
3.3. 5组肺组织IL-8的比较
与NC组比较,大潮气量 + 生理盐水组(HV + NS组)肺组织IL-8水平增加,大潮气量 + 去铁胺组(HV + DFO组)、小潮气量 + 去铁胺组(LV + DFO组)肺组织IL-8浓度显著降低,差异有统计学意义(P < 0.05);与HV + NS组比较,HV + DFO组、LV + DFO组的肺组织IL-8显著降低(P < 0.05);与LV + NS组比较,LV + DFO组、HV + DFO组的肺组织IL-8显著降低(P < 0.05),见表2。
3.4. 巨噬细胞线粒体ROS平均荧光强度与IL8的相关分析
作Pearson双变量相关分析,巨噬细胞线粒体ROS平均荧光强度与IL8相关系数为0.625,P = 0.000,有统计学意义。见图1。
Table 2. MFI of macrophage mitochondria was detected by flow cytometry (n = 6, x ¯ ± s)
表2. 流式检测巨噬细胞线粒体MFI (n = 6, ± s)
注:巨噬细胞线粒体MFI:与NC组比较,*P < 0.05;与HV + NS组比较,ΔP < 0.05;与LV + DFO组比较,#P < 0.05。
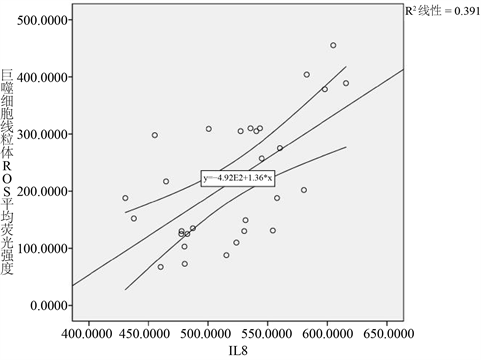
Figure 1. Macrophages cell mitochondria MFI and IL8 linear correlation diagram
图1. 巨噬细胞线粒体ROS平均荧光强度与IL8的线性相关图
4. 讨论
而VILI的特点是干扰肺泡毛细血管屏障,增加渗透性,造成水肿、出血、炎症性白细胞浸润(主要是中性粒细胞) [6],IL-8是嗜中性粒细胞和T淋巴细胞的主要趋化性细胞因子,在肺病理损伤的发生中起重要作用。前期研究机械通气肺损伤巨噬细胞线粒体活性氧增加,同时肺病理损伤加重,炎性因子IL-8增加 [2]。肺泡巨噬细胞活性氧与肺组织炎性因子IL-8释放是否具有相关性,还不清楚。为了进一步阐明巨噬细胞活性氧在机械通气肺损伤中、发展相关的肺病理学中的作用,我们研究了巨噬细胞活性氧对肺组织中IL-8表达的相关性研究。
本研究发现,巨噬细胞线粒体活性氧、肺组织炎性因子IL-8在大潮气量组显著增加,病理损伤加重。去铁胺是重要的抗氧化剂,可以抑制ROS产生 [5] [7]。随着去铁胺抑制肺泡巨噬细胞线粒体ROS产生,肺组织炎性因子IL-8水平也随着降低,肺病理损伤减轻。肺泡巨噬细胞线粒体ROS与肺组织IL-8水平变化显著相关(r = 0.625, P = 0.000)。提示:肺泡线粒体ROS可能激发了肺炎性因子IL-8释放,导致肺病理损伤。
总之,机械通气肺损伤中,巨噬细胞线粒体活性氧释放可能触发了炎性反应,引起了肺病理损伤。
文章引用
朱蔚琳,黄元巳,叶钰琼,王亚峰,黄爱兰,胡彦艳,马 利. 肺泡巨噬细胞线粒体活性氧触发IL-8释放在机械通气肺损伤中相关性研究
Study on the Correlation of IL-8 Release Triggered by Mitochondrial Reactive Oxygen Species of Alveolar Macrophages in Mechanically Ventilated Lung Injury[J]. 临床医学进展, 2021, 11(03): 1387-1392. https://doi.org/10.12677/ACM.2021.113198
参考文献
- 1. Wilson, M.R., Choudhury, S., Goddard, M.E., et al. (2003) High Tidal Volume Upregulates Intrapulmonary Cytokines in an In Vivo Mouse Model of Ventilator-Induced Lung Injury. Journal of Applied Physiology, 95, 1385-1393. https://doi.org/10.1152/japplphysiol.00213.2003
- 2. 朱蔚琳, 何并文, 胡彦艳, 秦丹丹. 不同通气模式对老年患者肺炎性因子的影响[J]. 临床麻醉学杂志, 2013, 29(8): 755-757.
- 3. 朱蔚琳, 黄元巳, 叶钰琼, 等. 去铁胺对大鼠呼吸机相关肺损伤的影响[J]. 中华麻醉学杂志, 2018, 38(12): 1453-1455.
- 4. Takeyoshi, I., Iwanami, K., Kamoshita, N., et al. (2001) Effect of Lazaroid U-74389G on Pulmonary Ischemia-Reperfusion Injury in Dogs. Journal of Investigative Surgery, 14, 83-92. https://doi.org/10.1080/08941930152024200
- 5. Mousavi, S., Abdollahi, M., Ahmadi, A., et al. (2011) The Dilemma of Hyperoxia Following Positive Pressure Mechanical Ventilation: Role of Iron and the Benefit of Iron Chelation with Deferasirox. European Review for Medical and Pharmacological Sciences, 15, 1141-1148.
- 6. Wilson, M.R., Choudhury, S., Goddard, M.E., et al. (2003) High Tidal Volume Upregulates Intrapulmonary Cytokines in an In Vivo Mouse Model of Ventilator-Induced Lung Injury. Journal of Applied Physiology, 95, 1385-1393. https://doi.org/10.1152/japplphysiol.00213.2003
- 7. Zhu, W., Huang, Y., Ye, Y. and Wang, Y. (2018) Deferoxamine Preconditioning Ameliorates Mechanical Ventilation-Induced Lung Injury in Rat Model via ROS in Alveolar Macrophages: A Randomized Controlled Study. BMC Anesthesiology, 18, 116. https://doi.org/10.1186/s12871-018-0576-7
NOTES
*通讯作者。
