Advances in Clinical Medicine
Vol.
11
No.
11
(
2021
), Article ID:
46682
,
6
pages
10.12677/ACM.2021.1111792
EGFR L858R突变合并EGFR 20外显子插入突变肺腺癌1例报告并文献复习
朱文婷,陈衍
空军军医大学第一附属医院肿瘤科,陕西 西安
收稿日期:2021年10月22日;录用日期:2021年11月17日;发布日期:2021年11月24日

摘要
尽管表皮生长因子受体(Epidermal Growth Factor Receptor, EGFR)——酪氨酸激酶抑制剂(tyrosine kinase inhibitor, TKI)极大地改善了EGFR突变患者的疗效及预后,但对于EGFR 20外显子插入突变疗效差,患者生存期短、预后差。本文结合文献回顾报道1例EGFR L858R突变合并EGFR 20外显子插入突变的肺腺癌患者,应用吉非替尼治疗后疗效不佳,接受阿法替尼联合化疗疾病得到良好控制,提示阿法替尼联合化疗对合并EGFR 20外显子插入突变的肺腺癌患者有不错的疗效,为阿法替尼联合化疗治疗EGFR L858R突变合并EGFR 20外显子插入突变的肺腺癌患者提供临床依据。
关键词
EGFR 20外显子插入突变,EGFR复合突变,肺腺癌,阿法替尼,化疗

EGFR L858R Mutation Combined with EGFR Exon 20 Insertion Mutation in Lung Adenocarcinoma: A Case Report and Literature Review
Wenting Zhu, Yan Chen
Department of Oncology, First Affiliated Hospital of Air Force Military Medical University, Xi’an Shaanxi
Received: Oct. 22nd, 2021; accepted: Nov. 17th, 2021; published: Nov. 24th, 2021

ABSTRACT
Although Epidermal Growth Factor Receptor (EGFR)—Tyrosine kinase inhibitor (TKI) has greatly improved the efficacy and prognosis of patients with EGFR mutations, EGFR exon 20 insertion mutation has poor efficacy, short survival and poor prognosis. This article combined literature review to report a case of lung adenocarcinoma with EGFR L858R mutation combined with EGFR exon 20 insertion mutation. Gefitinib was not effective after treatment, and the disease was well controlled after receiving afatinib combined with chemotherapy, suggesting that afatinib combined with chemotherapy has a good effect on lung adenocarcinoma patients with EGFR exon 20 insertion mutation. It provides clinical evidence for afatinib combined with chemotherapy to treat lung adenocarcinoma patients with EGFR L858R mutation and EGFR exon 20 insertion mutation.
Keywords:EGFR Exon 20 Insertion Mutation, EGFR Complex Mutation, Lung Adenocarcinoma, Afatinib, Chemotherapy

Copyright © 2021 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
随着精准医学的发展,非小细胞肺癌(non-small cell lung cancer, NSCLC)的驱动基因逐渐被明确,EGFR作为突变发生率最高的基因被深入研究,同时开发出针对EGFR突变的多种靶向药物,其中一、二、三代EGFR-TKI对EGFR经典突变疗效确切,而针对发生率较低的其他EGFR位点突变或复合突变临床数据较少。本文报道1例EGFR L858R突变合并EGFR 20外显子插入突变的肺腺癌患者,应用吉非替尼治疗后疗效不佳,接受阿法替尼联合化疗疾病得到良好控制,提示阿法替尼联合化疗对合并EGFR 20外显子插入突变的肺腺癌患者有不错的疗效,为阿法替尼联合化疗治疗EGFR L858R突变合并EGFR 20外显子插入突变的肺腺癌患者提供临床依据。
2. 临床资料
患者47岁,女性,无吸烟史及恶性肿瘤家族史。患者2019年1月因“感冒后持续咳嗽、气短”就诊外院,行胸部CT平扫提示右侧胸腔积液,心包积液。于外院引流胸腔积液,并行胸腔积液细胞病理学检查提示查见异型细胞。进一步于外院行PET-CT提示右肺门旁软组织影并右肺门侵犯,考虑恶性病变;右肺下叶部分肺不张,双侧胸膜局限性增厚,广泛淋巴结转移,右肺多发转移,左髋臼骨转移,子宫密度不均伴宫腔内、双侧附件区结节状葡萄糖代谢增高,建议进一步检查除外恶性病变。患者2019年1月18就诊我院,行胸腔穿刺置管引流胸腔积液,引流后咳嗽、气短症状逐渐缓解。并留置胸腔积液行细胞病理学检查提示:(胸水)查见癌细胞,细胞学倾向于腺癌;胸水离心沉淀物石蜡包埋切片行免疫细胞化学,结果示:肿瘤细胞AE1/AE3、CK7、TTF-1阳性,Napsin A少数细胞弱阳性,Vim (±),CR、D2-40、P40、Syn、PAX-8、WT-1阴性,免疫表型支持腺癌,建议临床首先查肺是否为原发。胸水离心沉淀物石蜡包埋、切片,进一步应用荧光PCR技术行肺癌相关三基因检测,提示:EGFR 21外显子L858R突变,ALK、ROS-1未见融合突变。头颅、盆腔MRI提示:双侧脑室旁及半卵圆区脱髓鞘改变;子宫右侧宫壁占位,考虑子宫肌瘤可能。骨扫描、SPECT/CT提示:左髋臼后柱肿瘤骨转移。胸腹部增强CT提示:右肺门软组织结节影并右肺少许软组织结节,纵隔散在肿大淋巴结,双侧胸腔积液并右肺部分膨胀不全,余两肺散在小结节。患者诊断为:肺癌(右侧,腺癌,EGFR L858R突变,IV期);右肺转移癌;右侧胸膜转移癌,恶性胸腔积液;左髋臼骨转移癌;淋巴结转移癌;子宫肌瘤。美国东部肿瘤协作组体力状况评分(Eastern Cooperative Oncology Group performance status, ECOG PS) 1分。于2019-2-5至2019-3-14口服吉非替尼250 mg 1次/日靶向治疗,治疗后无皮疹、腹泻等不良反应。治疗期间再次出现咳嗽、气短症状,胸腔积液B超探查提示右侧胸腔中–大量胸腔积液,因此于2019-2-27行胸腔穿刺置管,引流胸腔积液缓解症状并送二代基因测序,结果提示:EGFR 21外显子L858R突变,突变丰度20.39%;EGFR 20外显子插入突变,突变丰度1.89%。引流胸腔积液后行基线胸腹盆CT检查(图1)。因患者治疗前影像学检查见右侧胸腔大量积液,压迫肺组织且导致右肺部分膨胀不全,影响右肺病变观察,难以从影像学角度准确评估疗效,结合患者治疗后右侧胸腔积液控制不佳,再次出现咳嗽、气短症状,考虑患者吉非替尼治疗疗效欠佳。二代基因测序结果提示患者并非单纯EGFR L858R突变,还合并EGFR 20外显子插入突变,一代EGFR-TKI吉非替尼对该复合突变疗效差,因此与患者沟通后,于2019-3-15更换为阿法替尼40 mg 1次/日 靶向治疗联合培美曲塞 + 顺铂化疗6周期(2019-3-16至2019-7-14),治疗后出现颜面部、胸部、背部皮肤轻度皮疹伴瘙痒,口腔黏膜溃疡、腹泻,对症给予尿素软膏外用、生理盐水漱口、蒙脱石散止泻等治疗后好转,且在持续口服阿法替尼1月后以上不良反应发生率及严重程度逐渐降低、减轻,患者耐受性提高;化疗后出现轻度恶心、腹胀。期间多次复查胸腹盆增强CT,疗效评估为部分缓解(图2)。6周期化疗结束后,患者未再返院化疗,仅口服阿法替尼40 mg 1次/日继续靶向治疗,2019-11-17复查胸腹盆增强CT提示:右肺门、右肺下叶及斜裂旁软组织较前增大,右侧心膈角结节较前略增大;疗效评价为疾病进展。之后患者未再于我院住院治疗。该病例已获得病人的知情同意。

Figure 1. Before afatinib combined with chemotherapy
图1. 阿法替尼联合化疗治疗前
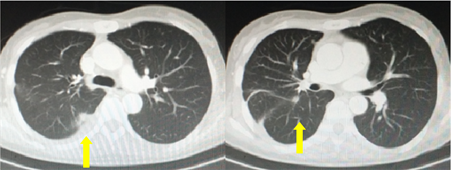
Figure 2. After afatinib combined with chemotherapy
图2. 阿法替尼联合化疗治疗后
3. 讨论
肺癌作为全球发病率最高的恶性肿瘤严重威胁人们的生命健康。针对肺癌的诊断、治疗成为肿瘤科医生必须积极面对的严峻问题,病理学诊断是肺癌诊断的金标准,其病理学亚型可分为小细胞肺癌和非小细胞肺癌,其中非小细胞肺癌最为常见,约占到所有肺癌的80%~85% [1]。随着精准医学的发展,发现了一些驱动非小细胞肺癌发生、发展的基因,其中最为常见、研究最为深入的就是EGFR基因,该基因在亚裔人群中突变率高达50%,但在高加索人群中突变率仅为12% [1] [2]。EGFR基因突变主要发生在其18~21号外显子区,其中最为常见的是EGFR 19外显子缺失突变和L858R点突变,这2个位点的突变也称为EGFR基因经典突变。随着对该基因的深入研究及二代测序技术的发展,我们还发现了许多其他位点的突变,由于这些位点突变率较低,统称为非经典突变,其中EGFR 20外显子插入突变最为常见,在NSCLC人群中突变率约为2%~3%,占所有EGFR突变NSCLC患者的4%~12%,有意思的是EGFR 20外显子插入突变的发生率在种族间无明显差异 [3] [4] [5] [6]。随着基因检测范围及深度的增加,越来越多的EGFR 20外显子插入突变的复合突变被发现,可以是EGFR基因其他位点的突变,也可以是其他基因的突变。在西班牙裔患者中发现 [5],多达三分之一的EGFR 20外显子插入突变NSCLC合并了EGFR经典突变,这部分患者的预后相较于单纯EGFR 20外显子插入突变患者更好。但在EGFR 20外显子插入突变的中国NSCLC患者中,只有不到1%的患者同时出现了常见的EGFR经典突变 [7]。还有一些研究报道了EGFR 20外显子插入突变的常见复合突变,其中最为常见的是合并TP53基因突变,复合突变发生率高达65% [4] [7];其次为复合CDKN2A基因,CDKN2B基因,NKX2-1基因和RB1基因的突变,复合突变发生率分别为22%、16%、14%和11% [4]。
EGFR-TKIs面市后极大地改善了EGFR突变患者的疗效及预后,但也发现不同的EGFR突变位点对EGFR-TKIs疗效不同,EGFR经典突变的患者无论是接受一代、二代还是三代EGFR-TKIs相较于化疗均显著延长患者中位无进展生存期(progress free survival,PFS)及中位总生存期(median overall survival, OS),出现EGFR T790M突变患者接受三代EGFR-TKIs也可获得不错的疗效,但EGFR 20外显子插入突变患者对EGFR-TKIs治疗不敏感,预后差 [8] [9]。这可能与EGFR 20外显子插入突变诱导药物结合部位形成空间位阻,阻止了EGFR-TKIs的结合有关。临床前模型也证实EGFR 20外显子插入突变对所有已知的第一代(厄洛替尼和吉非替尼)和第二代(阿法替尼和达克替尼) EGFR-TKIs的反应都很差 [10] [11]。同样,回顾性临床研究证实,与EGFR经典突变的NSCLC患者(N = 129)相比,厄洛替尼、吉非替尼或阿法替尼治疗EGFR 20外显子插入突变的NSCLC患者(N = 9)的PFS显著缩短(2个月vs. 14个月,p < 0.0001) [12]。这一结论在其他临床研究中也得到证实,EGFR 20外显子插入突变的NSCLC患者治疗的客观缓解率(objective response rate, ORR)为0%~28%,PFS不超过3个月,因此一代、二代EGFR-TKIs无法作为EGFR 20外显子插入突变的NSCLC患者的最佳选择 [3] [5] [7] [8] [9] [13] [14] [15] [16]。基于此,化疗仍被认为是EGFR 20外显子插入突变NSCLC的标准一线治疗 [8]。中国一项回顾性临床研究(N = 165)探索了EGFR 20外显子插入突变NSCLC患者接受任意一种EGFR-TKI对比含铂双药化疗的疗效,结果显示化疗疗效显著优于EGFR-TKI疗效(6.4个月vs. 2.9个月,p < 0.001) [7]。另一项研究纳入84例EGFR 20外显子插入突变NSCLC患者,结果提示含培美曲塞的一线化疗方案相较于不含培美曲塞的方案,显著延长了PFS (p < 0.001)和OS (28.0个月vs. 15.4个月,p = 0.009) [17]。
本例患者起初仅发现EGFR L858R突变,为经典突变,接受吉非替尼靶向治疗,但治疗后再次出现咳嗽、气短症状、右侧胸腔中–大量胸腔积液,考虑治疗后胸膜转移病变控制不佳,再次行胸水二代基因测序结果提示患者并非单纯EGFR L858R突变,还合并EGFR 20外显子插入突变,考虑患者前期疗效不佳可能与合并突变有关。根据临床研究的结果,目前针对EGFR 20外显子插入突变NSCLC患者仍首选培美曲塞联合铂类药物化疗;既往研究表明阿法替尼除对EGFR经典突变敏感外,对部分非经典突变也有一定疗效,因此在为本例患者进行个体化方案制定时我们选择了阿法替尼联合培美曲塞 + 顺铂方案治疗,最佳疗效为部分缓解,PFS为8个月,且联合治疗不良反应可控、可耐受,未出现3~4级不良反应,未因不良反应进行药物减量或停药,疗效优于既往临床研究结果。
目前也有一些针对EGFR 20外显子插入突变NSCLC的药物正在进行临床试验。日本武田Mobocertinib (TAK-788)治疗含铂化疗后进展的EGFR 20外显子插入突变NSCLC的I/II期临床研究,结果显示其中位PFS 7.3个月,ORR为43%且安全性可控 [18]。2020年WCLC公布的另一项CHRYSALIS研究显示EGFR/MET双特异性抗体Amivantamab用于治疗EGFR 20外显子插入突变的局部晚期、转移性NSCLC,ORR为40%,PFS为8.3个月,OS为22.8个月 [19]。基于此,2021年FDA批准Amivantamab上市,用于含铂化疗进展后的EGFR 20外显子插入突变NSCLC,但目前国内尚未上市。我们期待以上药物扩大样本量的III、IV期临床研究结果公布,也期待针对EGFR 20外显子插入突变这个位点能有更多有效的药物出现,为EGFR 20外显子插入突变患者带来生存获益。
文章引用
朱文婷,陈 衍. EGFR L858R突变合并EGFR 20外显子插入突变肺腺癌1例报告并文献复习
EGFR L858R Mutation Combined with EGFR Exon 20 Insertion Mutation in Lung Adenocarcinoma: A Case Report and Literature Review[J]. 临床医学进展, 2021, 11(11): 5358-5363. https://doi.org/10.12677/ACM.2021.1111792
参考文献
- 1. Chen, W., Zheng, R., Baade, P.D., Zhang, S., Zeng, H., Bray, F., et al. (2016) Cancer Statistics in China, 2015. CA: A Cancer Journal for Clinicians, 66, 115-132.
https://doi.org/10.3322/caac.21338 - 2. Midha, A., Dearden, S. and McCormack, R. (2015) EGFR Mutation Incidence in Non-Small-Cell Lung Cancer of Adenocarcinoma Histology: A Systematic Review and Global Map by Ethnicity (mutMapII). American Journal of Cancer Research, 5, 2892-2911.
- 3. Oxnard, G.R., Lo, P.C., Nishino, M., Dahlberg, S.E., Lindeman, N.I., Butaney, M., et al. (2013) Natural History and Molecular Characteristics of Lung Cancers Harboring EGFR Exon 20 Insertions. Journal of Thoracic Oncology, 8, 179-184.
https://doi.org/10.1097/JTO.0b013e3182779d18 - 4. Riess, J.W., Gandara, D.R., Frampton, G.M., Madison, R., Peled, N., Bufill, J.A., et al. (2018) Diverse EGFR Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of NSCLC. Journal of Thoracic Oncology, 13, 1560-1568.
https://doi.org/10.1016/j.jtho.2018.06.019 - 5. Cardona, A.F., Rojas, L., Zatarain-Barrón, Z.L., Freitas, H.C., Granados, S.T., Castillo, O., et al. (2018) EGFR Exon 20 Insertion in Lung Adenocarcinomas among Hispanics (Geno1.2-CLICaP). Lung Cancer, 125, 265-272.
https://doi.org/10.1016/j.lungcan.2018.10.007 - 6. Arcila, M.E., Nafa, K., Chaft, J.E., Rekhtman, N., Lau, C., Reva, B.A., et al. (2013) EGFR Exon 20 Insertion Mutations in Lung Adenocarcinomas: Prevalence, Molecular Heterogeneity, and Clinicopathologic Characteristics. Molecular Cancer Therapeutics, 12, 220-229.
https://doi.org/10.1158/1535-7163.MCT-12-0620 - 7. Yang, G., Li, J., Xu, H., Yang, Y., Yang, L., Xu, F., et al. (2020) EGFR Exon 20 Insertion Mutations in Chinese Advanced Non-Small Cell Lung Cancer Patients: Molecular Heterogeneity and Treatment Outcome from Nationwide Real-World Study. Lung Cancer, 145, 186-194.
https://doi.org/10.1016/j.lungcan.2020.03.014 - 8. Leduc, C., Merlio, J.P., Besse, B., Blons, H., Debieuvre, D., Bringuier, P.P., et al. (2017) Clinical and molecular characteristics of non-small-cell lung cancer (NSCLC) harboring EGFR mutation: results of the nationwide French Cooperative Thoracic Intergroup (IFCT) Program. Annals of Oncology, 28, 2715-2724.
https://doi.org/10.1093/annonc/mdx404 - 9. Beau-Faller, M., Prim, N., Ruppert, A.-M., Nanni-Metéllus, I., Lacave, R., Lacroix, L., et al. (2014) Rare EGFR Exon 18 and Exon 20 Mutations in Non-Small-Cell Lung Cancer on 10117 Patients: A Multicentre Observational Study by the French ERMETIC-IFCT Network. Annals of Oncology, 25, 126-131.
https://doi.org/10.1093/annonc/mdt418 - 10. Yasuda, H., Park, E., Yun, C.H., Sng, N.J., Lucena-Araujo, A.R., Yeo, W.L., et al. (2013) Structural, Biochemical, and Clinical Characterization of Epidermal Growth Factor Receptor (EGFR) Exon 20 Insertion Mutations in Lung Cancer. Science Translational Medicine, 5, Article No. 216ra177.
https://doi.org/10.1126/scitranslmed.3007205 - 11. Yang, M., Xu, X., Cai, J., Ning, J., Wery, J.P. and Li, Q.-X. (2016) NSCLC Harboring EGFR Exon-20 Insertions after the Regulatory C-Helix of Kinase Domain Responds Poorly to Known EGFR Inhibitors. International Journal of Cancer, 139, 171-176.
https://doi.org/10.1002/ijc.30047 - 12. Robichaux, J.P., Elamin, Y.Y., Tan, Z., Carter, B.W., Zhang, S., Liu, S., et al. (2018) Mechanisms and Clinical Activity of an EGFR and HER2 Exon 20-Selective Kinase Inhibitor in Non-Small Cell Lung Cancer. Nature Medicine, 24, 638-646.
https://doi.org/10.1038/s41591-018-0007-9 - 13. Tu, H.Y., Ke, E.E., Yang, J.J., Sun, Y.L., Yan, H.H., Zheng, M.Y., et al. (2017) A Comprehensive Review of Uncommon EGFR Mutations in Patients with Non-Small Cell Lung Cancer. Lung Cancer, 114, 96-102.
https://doi.org/10.1016/j.lungcan.2017.11.005 - 14. Yang, J.C., Sequist, L.V., Geater, S.L., Tsai, C.M., Mok, T.S., Schuler, M., et al. (2015) Clinical Activity of Afatinib in Patients with Advanced Non-Small-Cell Lung Cancer Harbouring Uncommon EGFR Mutations: A Combined Post-Hoc Analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncology, 16, 830-838.
https://doi.org/10.1016/S1470-2045(15)00026-1 - 15. Kuiper, J.L., Hashemi, S.M.S., Thunnissen, E., Snijders, P.J., Grünberg, K., Bloemena, E., et al. (2016) Non-Classic EGFR Mutations in a Cohort of Dutch EGFR-Mutated NSCLC Patients and Outcomes Following EGFR-TKI Treatment. British Journal of Cancer, 115, 1504-1512.
https://doi.org/10.1038/bjc.2016.372 - 16. Naidoo J, Sima CS, Rodriguez K, Busby, N., Nafa, K., Ladanyi, M., et al. (2015) Epidermal Growth Factor Receptor Exon 20 Insertions in Advanced Lung Adenocarcinomas: Clinical Outcomes and Response to Erlotinib. Cancer, 121, 3212-3220.
https://doi.org/10.1002/cncr.29493 - 17. Wu, J.Y., Yu, C.J. and Shih, J.Y. (2019) Effectiveness of Treatments for Advanced Non-Small-Cell Lung Cancer with Exon 20 Insertion Epidermal Growth Factor Receptor Mutations. Clinical Lung Cancer, 20, e620-e630.
https://doi.org/10.1016/j.cllc.2019.06.018 - 18. Horn, L., Lin, H.M., Padda, S.K., Aggarwal, C., Elizabeth McCoach, C., Zhu, Y., et al. (2020) Indirect Comparison of TAK-788 VS. Real-World Data Outcomes In Refractory Non-Small Cell Lung Cancer (NSCLC) with EGFR Exon 20 Insertions. Journal of Clinical Oncology, 38, 9580.
https://doi.org/10.1200/JCO.2020.38.15_suppl.9580 - 19. Sabari, J.K., Shu, C.A., Park, K., Leighl, N., Mitchell, P., Kim, S., Lee, J., et al. (2021) Amivantamab in Post-Platinum EGFR Exon 20 Insertion Mutant Non-Small Cell Lung Cancer. Journal of Thoracic Oncology, 16, S108-S109.
https://doi.org/10.1016/j.jtho.2021.01.284